Abstract
Introduction
Primary cardiovascular (CV) prevention may be achieved by lifestyle/nutrition changes, although a relevant role is now emerging for specific, functional foods and nutraceutical compounds (NCs). The aim of this study was to investigate the efficacy and safety of NCs in lowering blood pressure (BP) and improving lipid profile, when added to diet and lifestyle management versus diet alone in a group of patients with hypertension (HT) and hypercholesterolemia (HCh) with low CV risk.
Methods
Sixty-six patients with HT and HCh with grade 1 essential HT (mean age 56.0 ± 4.6 years) without history of CV diseases or organ damage were analyzed. These subjects were started on one tablet of an NC-containing red yeast rice, policosanol, berberine, folic acid and coenzyme Q10 once daily for 6 months and were age and gender matched with subjects following a diet program. Differences in clinic BP, 24-h ambulatory BP (24 h-ABPM), serum total cholesterol, low-density and high-density lipoprotein cholesterol (LDL-C and HDL-C) and triglyceride values were compared by analysis of variance.
Results
In the treatment group, a significant reduction of systolic 24 h-ABPM (141.6 ± 6.4 vs. 136.2 ± 4.8 mmHg; p < 0.05) and pulse pressure 24 h-ABPM (52.6 ± 7.2 vs. 47.3 ± 5.4 mmHg; p < 0.05) was found at the end of follow-up. A reduction of total cholesterol (−19.2%), LDL-C (−17.4%) and triglycerides (−16.3%) was observed (p < 0.001 for all); HDL-C remained unchanged. No difference was found in the control group.
Conclusions
The tested NCs was found to be safe, well tolerated and effective in reducing mean 24-h systolic and 24-h pulse pressure and in improving lipid pattern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although patients with hypertension (HT) and high cardiovascular (CV) risk have a higher chance of developing CV and, particularly, cerebrovascular events, they contribute to the overall number of events to a lesser extent as their absolute number is low [1]. In fact, the majority of CV events affect patients with HT with low-to-moderate risk, who are the most represented in the population and potentially give the main contribution to CV events [2]. However, as suggested in the 2013 Joint European Society of Hypertension (ESH) and European Society of Cardiology (ESC) arterial hypertension guidelines [3], the evidence in favor of anti-HT treatment in grade-1 HT patients is scant, as no controlled trial has addressed this condition. In addition, the risk of potential side effects due to antihypertensive agents could overcome the possible benefit of blood pressure (BP) reduction [3].
Arterial HT and hypercholesterolemia (HCh) are the main independent risk factors for CV and, particularly, cerebrovascular diseases. They are often detected in the same subject [4] and their interaction has a negative prognostic impact in terms of organ damage and clinical outcomes [5]; therefore, their management is mandatory for the prevention of global CV risk [2, 3]. Both HT and HCh are the result of the interaction between genetic and environmental factors. Dietary and behavioral factors have a predominant role in BP control and lipid homeostasis [6].
In the meantime, as discussed above, the available ways to reduce the gaps in scientific evidence is via the non-pharmacological treatment of grade-1 HT.
As suggested in the 2013 ESH/ESC guidelines [3], appropriate lifestyle changes safely and effectively delay antihypertensive therapy or make it unnecessary. This recommendation comes from clinical studies showing that targeted lifestyle modifications are often equivalent to drug monotherapy of HT [7], although the main drawback is the low level of adherence over time, which requires special action to be overcome [8].
As a consequence, in subjects with HT and HCh without target organ damage, appropriate lifestyle changes could be combined with an integrative and alternative approach using functional foods consisting of macro- and micronutrients of natural origin—namely nutraceutical compounds (NCs)—that have special properties affecting the CV system [9]. In addition, the NCs, in light of their properties, are free from relevant side effects. This approach is not completely new [10–12], as macro- and micronutrient deficiencies are very common in the general population and may be even more common in subjects with HT and HCh due to genetic or environmental causes and prescription drug use [13].
The aim of this study was to investigate the efficacy and safety of NCs added to diet and lifestyle management versus diet alone in lowering BP and in improving lipid profile in a group of subjects with HT and HCh at low CV risk.
Materials and Methods
Sixty-six subjects with HT and HCh (36 male, mean age 56.0 ± 4.6 years), age and gender matched using a case-to-case method with a control group of subjects with the same characteristics, were investigated. The study protocol was approved by a local ethics committee and institutional review boards, and the study was conducted in accordance with International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Harmonized Tripartite Guidelines for Good Clinical Practice. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Subjects were consecutively enrolled in our Hypertension Centre, and informed consent was obtained from all patients for being included in the study.
Subjects were free to discontinue or withdraw from the study at any time, for any reason, or if a serious adverse event occurred. Exclusion criteria were: severe HT (clinic systolic BP ≥180 mmHg or diastolic BP ≥110 mmHg), secondary HT, diabetes mellitus, presence of neoplastic or hepatic disease, chronic heart or renal failure, positive history or clinical signs of ischemic heart disease, severe obesity [i.e., body mass index (BMI) >35 kg/m2], disabling diseases such as dementia or inability to cooperate, pregnancy or breastfeeding, antihypertensive and/or lipid-lowering drug treatment, and organ damage (left ventricular hypertrophy diagnosed by electrocardiogram, carotid plaque or albuminuria) due to HT.
Data Collection
At baseline, clinic BP (diastolic Korotkoff phase 5) was taken in triplicate in a lying position using a mercury sphygmomanometer at 10-min intervals, taking special care to avoid any terminal digit preference. The average of the last two measurements was taken to minimize white-coat effects, if any; heart rate was also taken at the same time. Pulse pressure (PP) was the difference between systolic and diastolic BP. HT was defined as systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg. Clinic HT was confirmed with 24-h ambulatory BP monitoring (24 h-ABPM) using a 2430 oscillometric device (TM-2430, Takeda, Japan) applied to the non-dominant arm [14].
Fasting total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), serum glycemia, serum creatinine (SCr), creatine kinase (CK) and transaminase levels were analyzed by the enzymatic method. Low-density lipoprotein cholesterol (LDL-C) was estimated using Friedewald’s formula, according to the following algorithm: LDL-C = [(TC-HDL-C) − (TG/5)].
Subjects with TC >200 mg/dl or LDL-C >150 mg/dl were defined as hypercholesterolemic.
BMI was calculated as the ratio of weight (in kg) to squared height (in m). Subjects were classified as non-smokers and current smokers based on the number of cigarettes smoked per day (≥1 cigarette daily). Serum glycemia, SCr, CK and transaminases were measured as markers of biohumoral adverse effects. Albuminuria was also measured using the turbidimetric method (Cobas Mira Plus, Roche, Montclair, NJ) with a specimen of urine collected for 24 h. All subjects had normal SCr levels (<1.2 mg/dl for males and <0.9 mg/dl for females) and were normoalbuminuric (<30 mg/24 h).
Study Design
After a 2-week run-in period, subjects in the active treatment group (Group A) were assigned to receive the nutraceutical Armolipid Plus® (Rottapharm SpA, a MEDA Company), one tablet, once daily in the evening before bedtime, for 6 months, in addition to a specific dietary regimen (see below). ArmoLipid Plus is a food supplement combining natural ingredients containing red yeast rice (equivalent of 3 mg of monacolin K), 10 mg of policosanol, 500 mg of berberine, 0.2 mg of folic acid, 0.5 mg of astaxanthin and 2 mg of coenzyme Q10. Clinic BP, 24 h-ABPM and lipid values were evaluated compared to baseline at the end of the follow-up (Fig. 1). The control group (Group B) received a written prescription based on the standardized Mediterranean diet regimen, including a high intake of fish, fruits, vegetables, legumes, olive oil, unrefined whole grains and a moderate intake of lean meats and alcohol [15].
Plan of the study. A active treatment. B control group. Run-in: clinic blood pressure and 24 h-ABPM measurement; anthropometric measurements; blood tests for serum total cholesterol, HDL-C, triglycerides, glycemia, creatinine, aspartate aminotransferase, alanine aminotransferase, and creatine kinase. Follow-up (6 months): BP values and blood test measurement performed in the run-in period, and side effect assessment. 24 h-ABPM 24-h ambulatory blood pressure, BP blood pressure, HDL-C high-density lipoprotein cholesterol
Statistical Analysis
Average continuous variables were expressed as mean and standard deviation, and compared with analysis of covariance (ANCOVA) and the Bonferroni’s post hoc test [16]. Comparison between categorical variables was performed using the χ 2 test. Analysis of variance for repeated measure was used to compare mean changes of BP components and serum lipids at baseline and at the follow-up visits. Differences between continuous variables were evaluated in the two groups with the Tukey’s post hoc test [17] after adjustment for age, difference in BMI, BP and serum lipids at baseline and at follow-up. All statistical analyses were performed using the SPSS package version 17.0 for Windows (SPSS, Chicago, IL, USA). The null hypothesis was always rejected for values of p < 0.05.
Results
Whole Study Group
In the whole study group, the mean age was 56.0 ± 6.4 years. Although men were younger then women (54.4 ± 6.0 verus 58.0 ± 6.4, respectively; p < 0.001), all the components of clinic and monitored BP and serum lipids levels were not different between genders (data not shown). At baseline, PP was higher in subjects in the active treatment group than in the control group (60.5 ± 13.8 vs. 55.7 ± 11.3; p < 0.03). The general characteristics of the two groups at baseline are summarized in Table 1.
Active Treatment Group
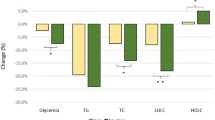
In actively treated patients, clinic systolic and diastolic BP and PP values were not significantly different at baseline or at follow-up (clinical systolic BP: 152.4 ± 11.8 mmHg at baseline vs. 150.1 ± 10.2 at follow-up; clinic diastolic BP: 91.8 ± 6.4 mmHg at baseline vs. 90.2 ± 5.1 mmHg at follow-up; and PP 60.5 ± 13.8 mmHg at baseline vs. 59.0 ± 11.2 mmHg at follow-up). Daytime 24 h-ABPM (systolic BP: 147.4 ± 8.1 mmHg at baseline vs. 145.8 ± 7.4 mmHg at follow-up; diastolic BP: 93.6 ± 30.6 mmHg at baseline vs. 92.8 ± 4.1 mmHg at follow-up; PP: 53.7 ± 9.0 mmHg at baseline vs. 53.0 ± 8.4 mmHg at follow-up) and night-time h-ABPM (systolic BP: 119.6 ± 4.4 mmHg at baseline vs. 119.1 ± 4.9 at follow-up; diastolic BP: 69.8 ± 5.7 at baseline vs. 70.0 ± 5.2 at follow-up; and PP: 49.7 ± 5.8 mmHg at baseline vs. 49.1 ± 6.2 mmHg at follow-up) were also not significantly different at baseline and at follow-up. In terms of 24 h-ABPM, a significant reduction of mean 24 h-systolic BP (141.6 ± 6.4 mmHg at baseline vs. 136.2 ± 4.8 mmHg at follow-up; p < 0.05) and mean 24 h-PP(52.6 ± 7.2 mmHg at baseline vs. 47.3 ± 5.4 mmHg at follow-up) levels was observed (Fig. 2). In addition, a significant improvement in lipid profile was observed in this group (TC: −19.2%; LDL-C: −17.4%; TG −16.3%; all p < 0.001). HDL-C in this group remained unchanged.
Changes from baseline to follow-up in both active treatment and control groups. *p < 0.05 versus baseline. 24 h-ABPM 24-h ambulatory blood pressure measurement, 24 h-DBP 24-h diastolic blood pressure, 24 h-PP 24 h pulse pressure, 24 h-SBP 24-h systolic blood pressure, FW follow-up, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoproteins cholesterol, NS non-significant, TC serum total cholesterol, TG triglycerides
Control Group
In the control group, no significant changes in 24 h-ABPM or in serum lipids values from baseline to the follow-up were observed (Fig. 2).
Safety Parameters
In the active treatment group, two patients were withdrawn from the study due to side effects (one due to a doubling in CK levels and one due to dyspepsia). Safety parameter monitoring values are shown in Table 2.
Discussion
The main results of the study show that in subjects with low-risk hypertension the addition of NCs to diet is effective in lowering 24 h-ABPM values and in improving serum lipid profile. The antihypertensive effect of NCs is not a completely new notation, but most previous studies have evaluated clinical values rather than 24 h-ABPM values. Our data are in agreement with those of Trimarco et al. [12], demonstrating that NCs were able to reduce mean 24 h BP values. The innovative result of our study is the effect of NCs in reducing 24 h-PP.
PP is the pulsatile component of the pressor curve, as opposed to mean arterial pressure, which is a steady component [18]. Increase of PP is an age-related phenomenon and the common belief is that it is an indicator of large artery stiffness and is considered to be an established independent CV risk in different clinical settings [19]. High PP may derive both from the rise of systolic BP (most commonly) and from decrease of diastolic BP. In this study, the systolic component of BP was prominent, as diastolic BP remained unchanged or even increased a little. From a pathophysiological point of view, PP reduction may be attributable to the mechanisms of action of the NCs tested in this study. As demonstrated in animal models, berberine is able to lead a reduction of systolic BP values and of sympathetic drive [20], and improves vascular reactivity and endothelial function [10]. We can also speculate that these actions on the CV system may have acted in a synergistic way with the positive indirect effect of berberine on lipid profile leading to an improvement of endothelial-dependent flow-mediated vasodilation [11]. Finally, some randomized placebo-controlled clinical trials support the antihypertensive effect of another component of NCs: coenzyme Q10 [21]. In the meta-analysis by Ho et al. [22], oral treatment with ≥100 mg/day of coenzyme Q10 decreased systolic BP by 11 mmHg and diastolic BP by 7 mmHg in subjects with HT. However in our experience, the dose of coenzyme Q10 tested is low (10 mg) and only in part explains its possible BP-lowering effect. Furthermore, the high costs of coenzyme Q10 and the high doses needed to achieve a significant BP reduction limit its current use as a drug in clinical practice [22]. Finally, it is well known that participation in a clinical study enhances adherence, persistence and effectiveness of antihypertensive agents, particularly in patients with HT taking active treatment rather than those in the control group [3]. This phenomenon is a possible explanation of the BP-lowering effect observed in the group treated with NCs compared to the control group.
On the other hand, the lack of effect on clinic BP is not surprising. In clinical practice, the most commonly used technique of BP measurement is the auscultatory method with mercury or mercury-like sphygmomanometer and a stethoscope [3]. Many criticisms of this method have been raised, including inter-observer and intra-observer variability and terminal digit preferences, all possibly biasing the accuracy of measurement [3]. 24 h-ABPM is superior to clinic measurement in evaluating the real pressor load in different clinical settings [3]. This also justifies the current use of 24 h-ABPM to assess antihypertensive treatment in clinical trials. Furthermore, the measurements derived from the slope of systolic and diastolic BP values obtained with 24 h-ABPM incorporate important clinical and prognostic information, and are considered to be vascular markers [3].
In recent years, there has been a growing interest on the putative lipid-lowering effect of the NCs [21, 24]. In particular, this effect was observed in a parallel, controlled randomized multicenter study [25] in subjects intolerant to statin treatment [26], in subjects with metabolic syndrome or who were overweight [10], and in elderly hypercholesterolemic subjects [27]. On the contrary, studies investigating the lipid-lowering effect of NCs in low-risk patients with HT and HCh are limited. Our results are in agreement with recent studies [23, 24] where NCs showed a significant effect in lowering TC, LDL-C and TG. However, in disagreement with Trimarco et al. [25], but in agreement with Gonelli et al. [28] and Li et al. [10], no significant increase of HDL-C was found after treatment with NCs. This finding may be related to higher levels of HDL-C at baseline of our subjects, intrinsic characteristics of the subjects enrolled, the doses of the NCs used, and to the duration of treatment.
Beyond their proven lipid-lowering effects, NCs also have a direct effect in improving endothelial dysfunction [11] and aortic stiffness [29]. We speculate this latter mechanism may have contributed in part to reduction of PP observed in our study.
Many pre-hypertensive patients and patients with HT at low CV risk are overweight or obese [5]. Guidelines recommend lifestyle changes, healthy diet and weight reduction as the first-line treatment for overweight- and obesity-associated HT and HCh [3, 6]. However, this approach is an elusive long-term endpoint for most overweight and obese subjects with HTs, where non-pharmacologic therapy is neither simple or consistently effective [30]. Dietary and behavioral changes are accompanied by high rate of weight regain, a relapse that reflects neurobiological and psychological adaptations to dietary restriction [6]. Furthermore, chronic BP-lowering effects of weight loss produced by lifestyle changes and/or dietary measures are not as sustained and pronounced as commonly thought [3]. Finally, there is evidence that dietary restriction, independent of weight loss, reduces sympathetic nervous system activity and might thereby contribute to reducing BP [3]. We speculate that this mechanism in part explains the failure of BP reduction observed in our control group. Although randomized clinical trials (RCTs) are the uncontested gold standard for establishing causation between a therapy and outcomes, their results are often not confirmed in reality [30]. In the same way, although effectiveness of dietary changes in treatment of dyslipidemia has been clearly demonstrated in RCTs [3, 6], a systematic review of 19 RCTs showed that dietary advice can result in reductions in TC in only 3–6% of cases, mainly because the dietary targets are not achieved by participants [31]. This data was also confirmed in general practice, where the lipid-lowering effects of diet and lifestyle changes were not as sustained and pronounced as commonly thought [32]. Many patients do not make durable changes, and responses to lifestyle changes vary among individuals. As a consequence, population-wide generalizations regarding diet are impractical, and individualized strategies are more likely to be successful in facilitating long-term benefits in improving BP and lipid level control.
The results should be interpreted in light of several limitations. The study group is small, monocentric and is not randomized.
In conclusion, although our findings are preliminary, we believe they represent an encouragement to further research, as NCs may provide an alternative to antihypertensive and lipid-lowering agents in patients with low-risk HT and HCh. Given the difficulties in designing and bringing forward a hard endpoint-based study in the contemporary scenario of primary prevention, larger, longer, more rigorous and better-powered studies are needed to evaluate the benefits of NCs on simple and validated surrogate endpoints of CV disease. Further clinical research is advisable to identify between the available active NCs and those with the best cost-effectiveness and risk–benefit ratio.
References
Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13.
Gaziano TA, Bitton A, Anand S, Weinstein MC. International society of hypertension. The global cost of non-optimal blood pressure. J Hypertens. 2009;27:1472–7.
ESH, ESC Task Force for the Management of Arterial Hypertension. Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC task force for the management of arterial hypertension. J Hypertens. 2013;2013(31):1925–38.
Filippidis FT, Gerovasili V, Majeed A. Association between cardiovascular risk factors and measurements of blood pressure and cholesterol in 27 European countries in 2009. Prev Med. 2014;67:71–4.
Kim MK, Ahn CW, Kang S, et al. Association between Apolipoprotein B/Apolipoprotein A-1 and arterial stiffness in metabolic syndrome. Clin Chim Acta. 2014;437:115–9.
European Association, for Cardiovascular Prevention and Rehabilitation, Reiner Z, Catapano AL, et al. ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European society of cardiology (ESC) and the European atherosclerosis society (EAS). Eur Heart J. 2011;32:1769–818.
Elmer PJ, Obarzanek E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;144:485–95.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97.
Li Y, Jiang L, Jia Z, et al. A meta-analysis of red yeast rice: an effective and relatively safe alternative approach for dyslipidemia. Plos. 2014;9:e98611.
Cicero AF, Tartagni E, Ertek S. Nutraceuticals for metabolic syndrome management: from laboratory to benchside. Curr Vasc Pharmacol. 2014;12:565–71.
Cicero AF, Ferroni A, Ertek S. Tolerability and safety of commonly used dietary supplements and nutraceuticals with lipid-lowering effects. Expert Opin Drug Saf. 2012;11:753–66.
Trimarco V, Cimmino CS, Santoro M, et al. Nutraceuticals for blood pressure control in patients with high-normal or grade 1 hypertension. High Blood Press Cardiovasc Prev. 2012;19:117–22.
Houston MC. The role of nutrition, nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Altern Ther Health Med. 2013;19(Suppl 1):32–49.
Palatini P, Frigo G, Bertolo O, Roman E, Da Cortà R, Winnicki M. Validation of the A&D TM-2430 device for ambulatory blood pressure monitoring and evaluation of performance according to subjects’ characteristics. Blood Press Monit. 1998;3:255–60.
Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med. 2013;69:676–7.
Tarone RE. A modified Bonferroni method for discrete data. Biometrics. 1990;46:515–22.
McHugh ML. Multiple comparison analysis testing in ANOVA. Biochem Med (Zagreb). 2011;21:203–9.
Safar ME, Boudier HS. Vascular development: pulse pressure, and the mechanisms of hypertension. Hypertension. 2005;46:205–9.
Mazza A, Pessina AC, Tikhonoff V, Pavei A, Privato G, Casiglia E. Pulse pressure: an independent predictor of coronary and stroke mortality in elderly females from the general population. Blood Press. 2001;10:205–11.
Hong Y, Hui SS, Chan BT, Hou J. Effect of berberine on catecholamine levels in rats with experimental cardiac hypertrophy. Life Sci. 2003;72:2499–507.
Banach M, Serban C, Sahebkar A, et al. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc. 2015;90:24–34.
Ho MJ, Bellusci A, Wright JM. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database Syst Rev. 2009;4:CD007435.
Patti AM, Katsiki N, Nikolic D, et al. Nutraceuticals in lipid-lowering treatment: a narrative review on the role of chitosan. Angiology. 2015;66:416–21.
Rizzo M, Giglio RV, Nikolic D, et al. Effects of chitosan on plasma lipids and lipoproteins: a 4-month prospective pilot study. Angiology. 2014;65:538–42.
Trimarco B, Benvenuti C, Rozza F, et al. Clinical evidence of efficacy of red yeast rice and berberine in a large controlled study versus diet. Med J Nutr Metab. 2011;4:133–9.
Cicero AFG, Derosa G, Bove M, et al. Long term effectiveness and safety of a natraceutical based approach to reduce cholesterolemia in statin intolerant subjects with and withouth metabolic syndrome. Curr Topics Nutr Res. 2009;7:1216.
Arazzi G, Cacciotti L, Pelliccia F, et al. Long term effects of nutraceuticals (berberine, red yeast rice, policosanol) in elderly hypercholesterolemic patients. Adv Ther. 2011;28:1105–13.
Gonnelli S, Caffarelli C, Stolakis K, Cuda C, Giordano N, Nuti R. Efficacy and tolerability of a nutraceutical combination (red yeast rice, policosanols and berberine) in low-moderate risk hypercholesterolemic patients: a double-blind, placebo controlled study. Curr Ther Res. 2015;77:1–6.
Pirro M, Lupattelli G, Giorno Del, et al. Nutraceutical combination (red yeast rice, berberine and policosanols) improves aortic stiffness in low moderate risk hypercholesterolemic patients. Pharma Nutr. 2013;1:73–7.
Mark AL. Weight reduction for treatment of obesity-associated hypertension: nuances and challenges. Curr Hypertens Rep. 2007;9:368–72.
Tang JL, Armitage JM, Lancaster T, Silagy CA, Fowler GH, Neil HA. Systematic review of dietary intervention trials to lower blood total cholesterol in free-living subjects. BMJ. 1998;316:1213–20.
Willett WC. The Mediterranean diet: science and practice. Public Health Nutr. 2006;9:105–10.
Acknowledgments
Article processing charges for this study were funded by Rottapharm SpA, a MEDA Company, Monza, Italy. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Mazza, S. Lenti, L. Schiavon, M. Zuin, M. D’Avino, E. Ramazzina and E. Casiglia declare no conflict of interest.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mazza, A., Lenti, S., Schiavon, L. et al. Nutraceuticals for Serum Lipid and Blood Pressure Control in Hypertensive and Hypercholesterolemic Subjects at Low Cardiovascular Risk. Adv Ther 32, 680–690 (2015). https://doi.org/10.1007/s12325-015-0229-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-015-0229-x