Abstract
Cerebellar transcranial direct current stimulation (ctDCS) modulates the primary motor cortex (M1) via cerebellar brain inhibition (CBI), which affects motor control in humans. However, the effects of ctDCS on motor control are inconsistent because of an incomplete understanding of the real-time changes in the M1 excitability that occur during ctDCS, which determines motor output under regulation by the cerebellum. This study investigated changes in corticospinal excitability and motor control during ctDCS in healthy individuals. In total, 37 healthy individuals participated in three separate experiments. ctDCS (2 mA) was applied to the cerebellar hemisphere during the rest condition or a pinch force–tracking task. Motor-evoked potential (MEP) amplitude and the F-wave were assessed before, during, and after ctDCS, and pinch force control was assessed before and during ctDCS. The MEP amplitudes were significantly decreased during anodal ctDCS from 13 min after the onset of stimulation, whereas the F-wave was not changed. No significant changes in MEP amplitudes were observed during cathodal and sham ctDCS conditions. The MEP amplitudes were decreased during anodal ctDCS when combined with the pinch force–tracking task, and pinch force control was impaired during anodal ctDCS relative to sham ctDCS. The MEP amplitudes were not significantly changed before and after all ctDCS conditions. Motor cortical excitability was suppressed during anodal ctDCS, and motor control was unskilled during anodal ctDCS when combined with a motor task in healthy individuals. Our findings provided a basic understanding of the clinical application of ctDCS to neurorehabilitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebellar functions play a crucial role in motor control, contribute to timing and sensory acquisition, and are involved in the prediction of the sensory consequences of motor performance [1]. Dysfunction of the cerebellum and other relevant pathways results in a decreased ability to control the upper extremities (e.g., adjusting the pinch force during writing and grasping) in patients with stroke and neurodegenerative disorders [2]. These impairments cause poor abilities of daily living and reduce the quality of life of patients [3, 4]. Therefore, a more effective rehabilitation approach is needed to improve motor control ability in patients with stroke and neurodegenerative disorders.
Cerebellar transcranial direct current stimulation (ctDCS) is a noninvasive approach that is able to alter cerebellar activation. Animal studies have shown that the current flowing from the cortical surface of the cerebellum excites the cell bodies and proximal dendrites of Purkinje cells [5, 6]. This cerebellar activation induced by ctDCS modulates cerebellar brain inhibition (CBI), which inhibits or disinhibits the primary motor cortex (M1) via the cerebellothalamocortical pathway in a polarity-specific manner [7]. Anodal ctDCS leads to neuronal membrane depolarization in Purkinje cells, resulting in enhanced CBI to the contralateral M1, whereas cathodal ctDCS decreases CBI via the hyperpolarization of Purkinje cells [7, 8]. CBI changes are associated with motor learning and skill acquisition [9, 10]. Thus, ctDCS may be an adjunctive tool to facilitate conventional rehabilitation in patients with impaired cerebellar function.
However, human studies of ctDCS for motor performance paint a mixed picture: some evidence suggests that ctDCS improves the maximum isometric voluntary contraction force in compound movements [11] and the motor learning of overhand throwing [12], whereas others have found that ctDCS impairs postural control learning [10] and the choice reaction time task [13]. There is increasing evidence that ctDCS changes the brain network after stimulation; however, the exact manner in which the neural signals are changed during ctDCS remains unknown. Understanding such real-time changes in neural dynamics, in particular the excitability of the M1, which determines the motor output under regulation from the cerebellum, is vital for understanding the mechanism underlying the effects of ctDCS on motor performance.
The most straightforward question regarding the neural effect of ctDCS, i.e., whether the brain network is changed during ctDCS, remains largely unanswered. A single report showed that no changes in corticospinal excitability during ctDCS were observed at 5 min after the onset of ctDCS [14], whereas no investigation has been conducted beyond 5 min of ctDCS. The effects of ctDCS are strongly time dependent [15, 16], as it induces membrane potential shifts via the modulation of voltage-dependent Na+ and Ca2+ channels over time. In this study, we explored the question of how neural signals and motor performance are affected during ctDCS as follows: (1) Do corticospinal and spinal excitabilities change during ctDCS in the resting state? (2) How does motor control change during ctDCS?
Materials and Methods
Subjects
Thirty-seven healthy adults participated in this study, with 15 participants in experiment 1, 10 participants in experiment 2, and 30 participants in experiment 3. The sample size was determined on the basis of previous studies that investigated the effects of ctDCS on neurophysiological changes and motor learning [10, 17] via the application of G-power [18]. All participants gave written, informed consent to participate in this study. This study was approved by the ethics committee of the Tokyo Bay Rehabilitation Hospital (approval number: 251–3). This study was performed in accordance with the ethical standards established by the Declaration of Helsinki.
General Experimental Procedure
This study used a randomized, double-blind (participants and outcome assessor), sham-controlled experimental design to minimize the biased assessment of the ctDCS intervention effects. Three experiments were conducted to investigate the changes in cortical excitability, spinal excitability, and behavioral learning that occur during ctDCS. In the first experiment, we explored the changes in corticospinal excitability using transcranial magnetic stimulation (TMS) to the M1 during ctDCS. In the second experiment, we investigated the changes in spinal excitability using electrical stimulation of the ulnar nerve during anodal ctDCS. In the third experiment, we investigated the changes in skill acquisition on a pinch force–tracking task and cortical excitability during anodal ctDCS. Some subjects participated in two or three experiments. To prevent carryover effects from previous experiments, washout intervals of at least 2 weeks were scheduled between experiments. The methods used for each experiment are described in detail in the following sections.
Experiment 1: Changes in Corticospinal Excitability During ctDCS
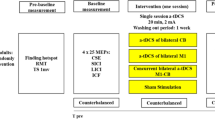
Fifteen volunteers (seven women; 24 ± 1 years of age) participated in the following three interventions: anodal, cathodal, and sham ctDCS for 20 min while resting in three different days. The order of the interventions was randomized across participants using a computer-generated list. The motor-evoked potentials (MEPs) were assessed before the stimulation (baseline); at 3 (T3) and 13 (T13) min after the onset of the stimulation (online effect); and at 3 min after the end of the stimulation (offline effect) (Fig. 1). To prevent carryover effects from the previous intervention, washout intervals of at least 3 days were applied between sessions.
Procedure used in experiments 1, 2, and 3. The motor-evoked potential (MEP) amplitudes in experiment 1 and F-waves in experiment 2 were measured a total of four times; before ctDCS (baseline), at 3 (T3) and 13 (T13) min after the onset of stimulation during 20 min of ctDCS, and at 3 min after the end of the stimulation (post), respectively. In experiment 3, the participants perform a tracking task (TASK) using controlled pinch force during ctDCS. MEP amplitudes and root mean square error (RMSE) were measured. MEP amplitudes were assessed six times: before ctDCS (baseline); at 3 (T3), 8 (T8), 13 (T13), and 18 (T18) min after the onset of stimulation during the 20 min of ctDCS; and at 3 min after the end of the stimulation (post). RMSE was assessed before ctDCS (baseline) and at 0 (T0), 5 (T5), 10 (T10), and 15 (T15) min after the onset of the stimulation during the 20 min of ctDCS
Cerebellar Transcranial Direct Current Stimulation
ctDCS (2 mA, 20 min) was delivered by a Compact-DC Stimulator GD-800 (OG Wellness, Okayama, Japan) connected to a pair of saline-soaked sponge-surface electrodes, which each had a surface area of 35 cm2 (5 cm × 7 cm). One electrode was placed on the skin over the right cerebellar hemisphere (3 cm lateral to the inion of the occipital bone) [19]. Another electrode was placed on the left supraorbital region. Electrical field simulations (SimNIBS2 [20]: www.simnibs.org) with a current strength of 2 mA revealed that this electrode arrangement induced an electrical field covering the medial part of the right cerebellum (mean ± SD, 0.36 ± 0.13 V/m), as assessed using T1- and T2-weighted magnetic resonance imaging brain images of 10 Japanese healthy subjects (five women; 28 ± 5 years of age) (Fig. 2). This mean electrical field value in the cerebellar region might exceed 0.2 V/m, which is necessary for the activation of neurons in the cerebellum [21]. The current was ramped up to 2 mA over a 15-s period, and a descending current ramp was used at the end of the stimulation period. For sham ctDCS, the current was turned off after 15 s, to mimic the transient skin sensation felt at the beginning of direct current delivery.
Electromyography (EMG)
Participants sat comfortably in a chair with their forearms and hands relaxed on a pillow that was placed on their thigh. EMG was recorded via Ag/AgCl-plated surface electrodes (diameter, 1 cm). The active electrode was positioned on the muscle belly of the right first dorsal interosseous (FDI) muscle. The reference electrode was placed on the first metacarpophalangeal joint. EMG signals were recorded at a sampling rate of 5 kHz using a Neuropack MEB-2200 instrument (Nihon Kohden, Tokyo, Japan) through filters set at 10 Hz and 1 kHz. EMG signals were stored on a computer for later analysis using the LabVIEW software (National Instruments Inc., Austin, TX, USA).
TMS
TMS was delivered through a figure-eight-shaped coil (9-cm diameter loops) connected to a Magstim 200 stimulator (Magstim Company, Whitland, Dyfed, UK). The TMS coil was positioned over the right FDI hotspot, at 45° away from the midline, to induce a posterior-to-anterior-directed current in the underlying M1. The hotspot was determined on the basis of the induction of the largest MEP amplitudes in the right FDI at rest. The stimulus intensity was set to produce 1-mV MEP amplitudes during the resting condition at the beginning of the experiment [7]. This intensity was maintained throughout the experiment. Fifteen stimuli were delivered every 5 ± 0.5 s at each testing time point.
Experiment 2: Changes in Spinal Excitability During Anodal ctDCS
Ten volunteers (five women; 24 ± 2 years of age), four of whom had participated in experiment 1, participated in both the anodal and sham ctDCS intervention for 20 min while resting on two different days. The order of the interventions was randomly decided across participants using a computer-generated list. The parameters for ctDCS applied to the cerebellum were the same as those described in experiment 1. The F-wave was assessed before the stimulation (baseline), at 3 (T3) and 13 (T13) min after the onset of the stimulation (online effect), and at 3 min after the end of the stimulation (offline effect), which was the same protocol as that described in experiment 1 (Fig. 1).
F-wave
Electrical stimulation (0.1 ms) was delivered to the ulnar nerve on the wrist via a pair of disk electrodes [22]. The cathode electrode was placed proximally [23]. The stimulus intensity was adjusted to 120% of the maximal muscle response (M-max) of the FDI muscle [22]. Twenty stimuli were delivered every 1 s at each time point. To normalize the amplitude of the F-wave, the F/M amplitude ratio was calculated by dividing by the peak-to-peak amplitude of the M wave when the maximal stimulation was delivered to the motor nerve [24, 25]. F-wave persistence, which represents the percentage of F-wave responses out of 20 stimulations, was also calculated. The F-wave was considered present when the response had a peak-to-peak amplitude of at least 20 μV [26]. Electrical stimulation and recording and the analysis of the F-wave were conducted using a Neuropack MEB-2200 instrument.
Experiment 3: Changes in Skill Acquisition on a Pinch Force–Tracking Task and Cortical Excitability During Anodal ctDCS
Thirty volunteers (15 women; 24 ± 2 years of age; eight of whom had participated in experiment 1 only, three of whom had participated in experiment 2 only, and three of whom had participated in experiments 1 and 2) were randomly allocated to the anodal or sham ctDCS group using a computer-generated list. The settings of ctDCS were the same as those described in experiments 1 and 2. The participants performed the tracking task during anodal or sham ctDCS. Moreover, they were instructed to perform a 2-min trial tracking task (total of five sets) before the stimulation (baseline) and at 0 (T0), 5 (T5), 10 (T10), and 15 (T15) min after the onset of the stimulation. In the assessment of cortical excitability, we increased the testing time point compared with that in experiment 1 because we predicted that the changes in MEP amplitudes would occur earlier when a motor skill task was combined with ctDCS [27, 28]. Thus, MEP amplitudes were assessed while resting before the stimulation (baseline); at 3 (T3), 8 (T8), 13 (T13), and 18 (T18) min after the onset of the stimulation (online effect); and at 3 min after the end of the stimulation (offline effect) (Fig. 1).
Tracking Task
Participants were comfortably seated on a chair with their forearms relaxed on a pillow that was placed on their thigh. They performed a pinch force–tracking task using the thumb and index finger [29,30,31,32]. The participants were instructed to adjust the pinch force to match the cursor (a dot) with sinusoidal waves displayed on the monitor as accurately as possible by changing the pinch force (Fig. 3). The cursor moved automatically from left to right in the monitor. Moreover, the cursor moved upward on the monitor when the pinch force was increased and downward when the pinch force was decreased. The amplitude of the sinusoidal waves is set to a range from 0 to 10% of the maximal pinch force. It took 40 s for the cursor to move from the left to the right edge of the monitor (one set). The participants were instructed to perform three consecutive sets in a trial (total of 2 min). The pattern of sinusoidal waves displayed on the monitor was the same in all trials. The participants were provided with visual feedback showing their accuracy rates promptly after the completion of a trial. The root mean square error (RMSE) was used as an accuracy index corresponding to the differences between the target-tracking waveform and the position of the dot. To compare changes in the accuracy rates, the RMSE recorded during ctDCS was divided by the RMSE recorded at the baseline. A decrease in the RMSE ratio implies that the task was performed more accurately. The tracking tasks were performed using a custom-written computer program in the LabVIEW software ver. 7.1 (National Instruments Corp., Austin, TX, USA).
Pinch force–tracking task. Participants performed the task of matching the automatically moving cursor from left to right against the sigmoid curve using a pinch force transducer. They were instructed to control the vertical movement of the cursor by adjusting the pinch force. The tracking tasks consisted of five sets of 2-min trials (one time before ctDCS and four times during ctDCS)
Statistical Analysis
The Shapiro–Wilk test was used to assess whether all data were normally distributed.
For experiment 1, a two-way repeated-measures analysis of variance (ANOVA) using the factors of polarity of the stimulus (anodal, cathodal, or sham ctDCS) and time points (baseline, T3, and T13) was performed to investigate the real-time changes in MEP amplitudes during each polarity type of the stimulus. A two-way repeated-measures ANOVA using the factors of polarity of the stimulus (anodal, cathodal, or sham ctDCS) and time points (baseline and 3 min after the onset of the stimulation) was also performed to assess the after-effects of the polarity of the stimulus on MEP amplitudes.
For experiment 2, a two-way repeated-measures ANOVA using the factors of polarity of the stimulus (anodal or sham ctDCS) and time points (baseline, T3, and T13) was performed to investigate the changes in F/M amplitude ratio and F-wave persistence during each polarity of the stimulus. A two-way repeated-measures ANOVA using the factors of polarity of the stimulus (anodal or sham ctDCS) and time points (baseline and 3 min after the onset of the stimulation) was also performed to assess the after-effects of the polarity of the stimulus on two indicators of the F-wave.
For experiment 3, to investigate whether anodal ctDCS and the motor skill task affect the changes in MEP amplitudes that occur during the stimulation, a two-way ANOVA with a split-plot factorial design, including the factor of groups (anodal or sham ctDCS group) and time points (baseline, T3, T8, T13, and T18), was used. A two-way ANOVA with a split-plot factorial design to analyze the effects of group (anodal or sham ctDCS group) and time points (baseline and 3 min after the end of the stimulation) was also used to assess the after-effects of anodal ctDCS and the motor skill task on MEP amplitudes. To confirm the occurrence of motor learning after the tracking task (the change in RMSE ratio), a two-way ANOVA with a split-plot factorial design, including the groups (anodal or sham ctDCS group) and time points (T0, T5, T10, an T15), was used.
For post hoc comparisons, t-tests with Bonferroni adjustments for multiple comparisons were performed to analyze the results of all data gathered in experiments 1, 2, and 3. Results with P values < 0.05 were considered statistically significant for all analyses. Statistical analyses were performed using IBM SPSS 27.0 for Windows (IBM Corp., New York, NY, USA).
Results
Experiment 1: Changes in Corticospinal Excitability During ctDCS
The baseline values of the MEP amplitudes did not differ significantly among the conditions [F(2, 28) = 0.374, P = 0.691].
Anodal ctDCS decreased the MEP amplitudes during the stimulation, whereas cathodal and sham ctDCS had no effect on the MEP amplitudes (Fig. 4). These results were supported by a statistically significant interaction between polarity and time [F(4, 56) = 2.59, P = 0.046]. No significant main effect of polarity [F(2, 28) = 0.933, P = 0.405] and time [F(2, 28) = 0.248, P = 0.782] was observed. Post hoc analyses revealed that anodal ctDCS significantly decreased MEP amplitudes at T13 compared with the baseline (P = 0.008). Regarding the comparison between before and after the stimulation, no significant interaction was observed between polarity and time [F(2, 28) = 2.635, P = 0.089]. Moreover, there was no significant main effect of polarity [F(2, 28) = 0.712, P = 0.499] and time [F(1, 14) = 0.079, P = 0.783].
Individual plots of MEP amplitudes before (baseline) and during anodal, cathodal, and sham ctDCS (T3, T13) in experiment 1. The horizontal lines represent the mean. The open circles indicate the anodal ctDCS condition, the filled circles indicate the cathodal ctDCS condition, and the filled triangles indicate the sham ctDCS condition. The asterisks indicate significant differences between time courses (P < 0.05)
Experiment 2: Changes in Spinal Excitability During Anodal ctDCS
Anodal and sham ctDCS had no effect on the F/M amplitude ratio and F-wave persistence (Table 1). No significant interactions [F/M amplitude ratio: F(2, 18) = 1.116, P = 0.349; F-wave persistence: F(2, 18) = 0.612, P = 0.553] were observed during the stimulation. There were no significant main effects of polarity [F/M amplitudes: F(1, 9) = 0.486, P = 0.503; F-wave persistence: F(1, 9) = 0.357, P = 0.565] and time [F/M amplitude: F(2, 18) = 3.278, P = 0.061; F-wave persistence: F(2, 18) = 0.055, P = 0.946] during the stimulation.
Regarding the comparison between before and after the stimulation, we did not find significant interactions between the F/M amplitude ratio [F(1, 9) = 0.493, P = 0.500] and the F-wave persistence [F(1, 9) = 0.086, P = 0.777]. Moreover, there were no significant main effects of polarity [F/M amplitude: F(1, 9) = 0.388, P = 0.549; F-wave persistence: F(1, 9) = 0.433, P = 0.527] and time [F/M amplitude: F(1, 9) = 2.919, P = 0.122] before and after the stimulation. A significant main effect of time was found on the F-wave persistence [F(1, 9) = 9.553, P = 0.013].
Experiment 3: Changes in Cortical Excitability and Skill Acquisition on the Pinch Force–Tracking Task During Anodal ctDCS
No significant difference between the anodal and sham ctDCS groups was observed regarding the MEP amplitudes at the baseline (P = 0.255).
Anodal ctDCS decreased the MEP amplitudes relative to sham ctDCS during the stimulation (Fig. 5). These results were qualified by a statistically significant interaction between group and time [F(4, 112) = 3.356, P = 0.012]. A significant main effect of group was found [F(1, 28) = 11.197, P = 0.002], whereas no main effect of time was observed [F(4, 112) = 0.054, P = 0.994]. Post hoc analyses revealed that the MEP amplitudes in the anodal ctDCS group were significantly lower at T8, T13, and T18 compared with those in the sham ctDCS group (P = 0.018, P = 0.007, and P < 0.001, respectively). Regarding the MEP amplitude changes observed before and after the stimulation, no significant interactions [F(1, 28) = 0.314, P = 0.580] or main effects [group: F(1, 28) = 1.368, P = 0.252; time: F(1, 28) = 1.046, P = 0.315] were detected.
Individual plots of MEP amplitudes before (baseline) and during anodal and sham ctDCS with a tracking task (T3, T8, T13, and T18) in experiment 3. The MEP amplitudes were recorded at rest. The horizontal lines represent the mean. The open circles indicate the anodal ctDCS group, and the filled triangles indicate the sham ctDCS group. The asterisks indicate significant differences between two groups (P < 0.05)
There was no significant difference in RMSE between the two groups at the baseline (P = 0.362). Regarding the change in the RMSE ratio, no significant interactions between group and time were observed [F(3, 84) = 0.250, P = 0.861]. Conversely, significant main effects of group [F(1, 28) = 7.421, P = 0.011] and time [F(3, 84) = 50.749, P < 0.001] were identified. These results indicated that the skill acquisition on the pinch force–tracking task was decreased during anodal ctDCS, because no difference in RMSE was observed between the two groups at the baseline, whereas a significant main effect of the group on the RMSE ratio was detected (Fig. 6).
Individual plots of the RMSE ratio during the tracking task in experiment 3. The RMSE ratio was calculated using the RMSE recorded during ctDCS at T0, T5, T10, and T15 divided by the RMSE recorded at the baseline. The horizontal lines represent the mean. The open circles indicate the anodal ctDCS group, and the filled triangles indicate the sham ctDCS group. A main effect of polarity was observed (P < 0.05)
To elucidate the relationship between motor control ability changes and physiological changes in each group, Pearson correlation analysis was employed to analyze the relationship between the RMSE ratio at T15 and the change in MEP amplitude (T18/baseline); however, no significant correlations between RMSE ratio and changes in MEP amplitudes were observed in the anodal ctDCS group (r = − 0.25, P = 0.37) or sham ctDCS group (r = − 0.18, P = 0.51).
Discussion
The present study was the first to reveal changes in corticospinal excitability and motor control during ctDCS in healthy persons. Our main findings were as follows: (1) corticospinal excitability was decreased during anodal ctDCS, whereas spinal excitability was not changed; (2) corticospinal excitability was decreased during anodal ctDCS combined with a motor task, and motor control ability was unskilled during anodal ctDCS relative to sham ctDCS; and (3) no changes in corticospinal and spinal excitabilities were observed after ctDCS. These results indicate that anodal ctDCS enhances the inhibition from the cerebellum to the M1 and of an unskilled motor control task during the simulation. Our findings provided important fundamental insights into the understanding of the mechanism and clinical application of ctDCS for neurorehabilitation.
tDCS can modify neuronal membrane polarity and, thus, the action potential generation through the activation of voltage-gated presynaptic and postsynaptic Na+ and Ca2+ channels, which causes increased presynaptic release of excitatory neurotransmitters and postsynaptic calcium influx [33, 34]. Thus, in this study, depolarization of the resting membrane potential of Purkinje cells in the cerebellum might have enhanced the inhibition from the cerebellum to the M1 via the CBI pathway [7, 8, 10, 35,36,37,38], whereas corticospinal excitability was decreased during anodal ctDCS. However, the activation of other brain regions (e.g., sensorimotor and dorsal attention networks) by anodal ctDCS may also be involved [39]. Conversely, our results showed that spinal excitability was not altered during anodal ctDCS. These results emphasize the contention that anodal ctDCS strongly modulates the corticospinal tract via cortical excitability changes afforded by the enhancement of the CBI pathway, rather than other pathways, such as the reticulospinal or rubrospinal tract or both.
No significant changes in corticospinal excitability were observed during cathodal ctDCS. The reason for this may be that the strength of the electric field generated in the brain varies with polarity [40,41,42]. It has been reported that anodal tDCS produces stronger electric fields around the stimulation site than cathodal tDCS [41, 42]. In fact, previous studies reported that anodal ctDCS, but not cathodal ctDCS, changes corticospinal excitability [10], pain threshold [43], and motor skill learning [44]. Therefore, it is possible that the electric field might not have been sufficient to modulate Purkinje cells during cathodal ctDCS in this study. Further studies are warranted to examine the effect of stimulus intensity on corticospinal excitability during ctDCS [45].
A decrease in cortical excitability was observed from 13 min after the onset of anodal ctDCS in experiment 1. This change in excitability over time may be time-dependent [15]. This may be induced by increased membrane potential shifts during anodal tDCS, which cause increased modulation of voltage-dependent Na+ and Ca2+ channels over time [15, 16, 27, 46, 47]. In contrast, a previous study reported that no significant change in corticospinal excitability was observed at 5 min after the onset of anodal ctDCS (online effect) [14]. Thus, the change in corticospinal excitability during observed ctDCS was induced at least 13 min after the onset of the stimulation. In contrast, no changes in corticospinal excitability were observed after anodal ctDCS. It has been reported that tDCS induces synaptic plasticity because of increased neurotransmitter efficiency by the modulation of glutamatergic and GABAergic neuron receptors [33, 48, 49]. In the present study, however, synaptic plasticity in the cerebellum might have been enhanced by anodal ctDCS, with no synaptic plasticity occurring in the CBI pathway [7, 8, 46]. Previous studies have found an increase in CBI without changes in corticospinal excitability after anodal ctDCS [7, 8]. Those results support our findings and speculations that M1 excitability was strongly modulated via CBI only during anodal ctDCS.
The modulation effect of ctDCS on physiological changes after an onset time of 13 min is a novel finding. A previous study examining changes in CBI after ctDCS reported that the CBI pathway was modulated by 15 min of ctDCS [8]. However, there are no existing reports on the effects of ctDCS on physiological changes with stimulation less than 15 min. In experiment 1 of the present study, MEP amplitudes were only assessed at 3 min and 13 min after the onset of ctDCS; thus, we cannot rule out that MEP amplitude changes might occur before 13 min. Future studies are thus needed to investigate the effects of ctDCS duration on physiological changes.
On the basis of the results of experiment 1, we hypothesized that the decrease in M1 during anodal ctDCS impairs fine motor skill training. In fact, the motor skill on the visuomotor force–tracking task was diminished in the anodal ctDCS group. The M1 excitability increases during the initial process of the motor skill learning of distal control [50,51,52]. In this process, motor skill learning proceeds by controlling the M1 overactivity via CBI [53, 54]. The results of experiment 3 showed that the corticospinal excitability was reduced in the anodal ctDCS combined with motor task group compared with the sham ctDCS group. Therefore, the excessive suppression of M1 excitability during anodal ctDCS might impair fine motor skill in young healthy individuals.
No significant correlations were observed between motor performance changes and M1 excitability changes in the anodal or sham ctDCS group. A previous study reported that motor control ability changes in postural control after ctDCS were positively correlated with changes in CBI [10]. Thus, changes in CBI may be related to motor performance, whereas changes in M1 excitability, which is an indirect indicator of ctDCS effects, might not be directly related to motor performance.
Considering our findings of suppressed motor cortical excitability, anodal ctDCS could be used to improve the symptoms of dystonia. Dystonia is a neurological disorder in which involuntary muscle contractions occur due to M1 overexcitability caused by impairment of CBI [55]. A previous study showed that anodal ctDCS improved CBI and motor control of the hands in patients with dystonia [37]. Therefore, our findings led us to speculate that anodal ctDCS during motor training could improve motor dysfunction in patients with dystonia. Similarly, other patients with impaired CBI (e.g., patients with spinocerebellar degeneration or cerebellar infarction) may benefit from anodal ctDCS.
Another widely used noninvasive brain stimulation technique termed repetitive TMS (rTMS) has been shown to modulate the cerebellum and CBI pathway. Although rTMS has better spatial and temporal resolution, ctDCS has some advantages over rTMS, such as longer-lasting modulatory effects on cortical excitability induced by the membrane potential shift [56]. In addition, ctDCS is cheaper, safer, and simpler to apply as a part of rehabilitation. Thus, ctDCS may serve as a potential tool to facilitate neurorehabilitation.
This study had several limitations. First, it was limited by its small sample size, even though the sample size was determined using a power analysis. Some less robust results should be interpreted carefully, especially within the boundaries of our small sample size. Second, it was unclear whether our findings on the disturbance of motor performance by ctDCS can be extended to other types of motor performance. Previous studies have reported that anodal ctDCS improves the adaptation of whole-arm reach movements in a visuomotor rotation task [17], whereas that of hand and finger movements was disrupted [13, 57] or unchanged [17, 58,59,60]. Thus, it is necessary to investigate the type of motor tasks that would be effective in combination with anodal ctDCS. Third, other ctDCS parameters (e.g., stimulus intensity) could make the results of this study more powerful. It was previously reported that 2 mA ctDCS, rather than 1 mA ctDCS, induced long-lasting plasticity in the CBI pathway [7]. Therefore, future studies are needed to investigate different ctDCS parameters (e.g., higher stimulus intensity). Fourth, the present study only investigated the effects observed during and immediately after a single session of ctDCS. It was reported that a single session of ctDCS with motor tasks enhances the transfer learning of fine motor sequences after 24 h in healthy individuals [61], and it was also noted that repeated sessions of ctDCS might yield greater and clinically relevant benefits. Thus, ctDCS combined with motor practice might produce long-term clinically relevant results. Further studies are necessary to identify the long-term effects of a single session or repeated sessions of ctDCS.
Conclusion
The present study showed that motor cortical excitability was decreased during anodal ctDCS and that the motor control ability was unskilled during anodal ctDCS when combined with a motor task. These results suggest that anodal ctDCS enhances the inhibition from the cerebellum to the M1 and affects the motor control ability during the simulation in healthy individuals. Our findings provide the basic understanding that is necessary for the clinical application of ctDCS to neurorehabilitation. Further studies are warranted to clarify the clinical application of ctDCS in the context of neurological disorders.
References
Ates MP, Alaydin HC, Cengiz B. The effect of the anodal transcranial direct current stimulation over the cerebellum on the motor cortex excitability. Brain Res Bull. 2018;140:114–9.
Kassavetis P, Hoffland BS, Saifee TA, et al. Cerebellar brain inhibition is decreased in active and surround muscles at the onset of voluntary movement. Exp Brain Res. 2011;209(3):437–42.
Chu CY, Patterson RM. Soft robotic devices for hand rehabilitation and assistance: a narrative review. J Neuroeng Rehabil. 2018;15(1):9.
Germanotta M, Gower V, Papadopoulou D, et al. Reliability, validity and discriminant ability of a robotic device for finger training in patients with subacute stroke. J Neuroeng Rehabil. 2020;17(1):1.
Chan CY, Nicholson C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol. 1986;371:89–114.
Chan CY, Hounsgaard J, Nicholson C. Effects of electric fields on transmembrane potential and excitability of turtle cerebellar Purkinje cells in vitro. J Physiol. 1988;402:751–71.
Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29(28):9115–22.
Batsikadze G, Rezaee Z, Chang DI, et al. Effects of cerebellar transcranial direct current stimulation on cerebellar-brain inhibition in humans: a systematic evaluation. Brain Stimul. 2019;12(5):1177–86.
Jayaram G, Galea JM, Bastian AJ, Celnik P. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex. 2011;21(8):1901–9.
Katagiri N, Kawakami S, Okuyama S, et al. Single-session cerebellar transcranial direct current stimulation affects postural control learning and cerebellar brain inhibition in healthy individuals. Cerebellum. 2021;20(2):203–11.
Kenville R, Maudrich T, Maudrich D, Villringer A, Ragert P. Cerebellar transcranial direct current stimulation improves maximum isometric force production during isometric barbell squats. Brain Sci. 2020;10(4):235.
Jackson AK, de Albuquerque LL, Pantovic M, et al. Cerebellar transcranial direct current stimulation enhances motor learning in a complex overhand throwing task. Cerebellum. 2019;18(4):813–6.
Jongkees BJ, Immink MA, Boer OD, Yavari F, Nitsche MA, Colzato LS. The effect of cerebellar tDCS on sequential motor response selection. Cerebellum. 2019;18(4):738–49.
Hamada M, Galea JM, Di Lazzaro V, Mazzone P, Ziemann U, Rothwell JC. Two distinct interneuron circuits in human motor cortex are linked to different subsets of physiological and behavioral plasticity. J Neurosci. 2014;34(38):12837–49.
Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(3)(Pt 3):633–9.
Puri R, Hinder MR, Fujiyama H, Gomez R, Carson RG, Summers JJ. Duration-dependent effects of the BDNF Val66Met polymorphism on anodal tDCS induced motor cortex plasticity in older adults: a group and individual perspective. Front Aging Neurosci. 2015;7:107.
Weightman M, Brittain JS, Punt D, Miall RC, Jenkinson N. Targeted tDCS selectively improves motor adaptation with the proximal and distal upper limb. Brain Stimul. 2020;13(3):707–16.
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91.
Grimaldi G, Manto M. Anodal transcranial direct current stimulation (tDCS) decreases the amplitudes of long-latency stretch reflexes in cerebellar ataxia. Ann Biomed Eng. 2013;41(11):2437–47.
Saturnino GB, et al. SimNIBS 2.1: A comprehensive pipeline for individualized electric field modelling for transcranial brain stimulation. In: Makarov S, Horner M, Noetscher G, editors. Brain and Human Body Modeling: Computational Human Modeling at EMBC 2018; vol 2019, The Author(s). Cham (CH); 2019, Springer Copyright:3–25.
Priori A, Ciocca M, Parazzini M, Vergari M, Ferrucci R. Transcranial cerebellar direct current stimulation and transcutaneous spinal cord direct current stimulation as innovative tools for neuroscientists. J Physiol. 2014;592(16):3345–69.
Hara M, Kimura J, Walker DD, et al. Effect of motor imagery and voluntary muscle contraction on the F wave. Muscle Nerve. 2010;42(2):208–12.
Vastano R, Perez MA. Changes in motoneuron excitability during voluntary muscle activity in humans with spinal cord injury. J Neurophysiol. 2020;123(2):454–61.
Eisen A, Fisher M. The F wave. The International Federation of clinical neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:255–57.
Puksa L, Stålberg E, Falck B. Reference values of F wave parameters in healthy subjects. Clin Neurophysiol. 2003;114(6):1079–90.
Perez MA, Rothwell JC. Distinct influence of hand posture on cortical activity during human grasping. J Neurosci. 2015;35(12):4882–9.
Fritsch B, Reis J, Martinowich K, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66(2):198–204.
Wang B, Xiao S, Yu C, Zhou J, Fu W. Effects of transcranial direct current stimulation combined with physical training on the excitability of the motor cortex, physical performance, and motor learning: a systematic review. Front Neurosci. 2021;15: 648354.
Kuhtz-Buschbeck JP, Gilster R, Wolff S, Ulmer S, Siebner H, Jansen O. Brain activity is similar during precision and power gripping with light force: an fMRI study. Neuroimage. 2008;40(4):1469–81.
Bonnard M, Galléa C, De Graaf JB, Pailhous J. Corticospinal control of the thumb-index grip depends on precision of force control: a transcranial magnetic stimulation and functional magnetic resonance imagery study in humans. Eur J Neurosci. 2007;25(3):872–80.
Abe M, Schambra H, Wassermann EM, Luckenbaugh D, Schweighofer N, Cohen LG. Reward improves long-term retention of a motor memory through induction of offline memory gains. Curr Biol. 2011;21(7):557–62.
Lopez-Alonso V, Liew SL, Fernández Del Olmo M, et al. A preliminary comparison of motor learning across different non-invasive brain stimulation paradigms shows no consistent modulations. Front Neurosci. 2018;12:253.
Nitsche MA, Fricke K, Henschke U, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553(1):293–301.
Nitsche MA, Seeber A, Frommann K, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568(1):291–303.
Pauly MG, Steinmeier A, Bolte C, et al. Cerebellar rTMS and PAS effectively induce cerebellar plasticity. Sci Rep. 2021;11(1):3070.
Doeltgen SH, Young J, Bradnam LV. Anodal direct current stimulation of the cerebellum reduces cerebellar brain inhibition but does not influence afferent input from the hand or face in healthy adults. Cerebellum. 2016;15(4):466–74.
Bradnam LV, Graetz LJ, McDonnell MN, Ridding MC. Anodal transcranial direct current stimulation to the cerebellum improves handwriting and cyclic drawing kinematics in focal hand dystonia. Front Hum Neurosci. 2015;9:286.
Kawakami S, Inukai Y, Ikarashi H, et al. Transcranial direct current stimulation and transcranial random noise stimulation over the cerebellum differentially affect the cerebellum and primary motor cortex pathway. J Clin Neurosci. 2022;100:59–65.
Grami F, de Marco G, Bodranghien F, Manto M, Habas C. Cerebellar transcranial direct current stimulation reconfigurates static and dynamic functional connectivity of the resting-state networks. Cerebellum Ataxias. 2021;8(1):7.
Laakso I, Tanaka S, Koyama S, De Santis V, Hirata A. Inter-subject variability in electric fields of motor cortical tDCS. Brain Stimul. 2015;8(5):906–13.
Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol. 2006;117(7):1623–9.
Jeffery DT, Norton JA, Roy FD, Gorassini MA. Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp Brain Res. 2007;182(2):281–7.
Pereira M, Rafiq B, Chowdhury E, et al. Anodal cerebellar tDCS modulates lower extremity pain perception. NeuroRehabilitation. 2017;40(2):195–200.
Cantarero G, Spampinato D, Reis J, et al. Cerebellar direct current stimulation enhances on-line motor skill acquisition through an effect on accuracy. J Neurosci. 2015;35(7):3285–90.
Evans C, Bachmann C, Lee JSA, Gregoriou E, Ward N, Bestmann S. Dose-controlled tDCS reduces electric field intensity variability at a cortical target site. Brain Stimul. 2020;13(1):125–36.
Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–85.
Roche N, Geiger M, Bussel B. Mechanisms underlying transcranial direct current stimulation in rehabilitation. Ann Phys Rehabil Med. 2015;58(4):214–9.
Nitsche MA, Liebetanz D, Schlitterlau A, et al. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci. 2004;19(10):2720–6.
Stagg CJ, Best JG, Stephenson MC, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29(16):5202–6.
Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136(4):431–8.
Bagce HF, Saleh S, Adamovich SV, Krakauer JW, Tunik E. Corticospinal excitability is enhanced after visuomotor adaptation and depends on learning rather than performance or error. J Neurophysiol. 2013;109(4):1097–106.
McDougle SD, Bond KM, Taylor JA. Explicit and implicit processes constitute the fast and slow processes of sensorimotor learning. J Neurosci. 2015;35(26):9568–79.
Rabe K, Livne O, Gizewski ER, et al. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol. 2009;101(4):1961–71.
Burciu RG, Fritsche N, Granert O, et al. Brain changes associated with postural training in patients with cerebellar degeneration: a voxel-based morphometry study. J Neurosci. 2013;33(10):4594–604.
Wilson BK, Hess EJ. Animal models for dystonia. Mov Disord. 2013;28(7):982–9.
Han T, Xu Z, Liu C, et al. Simultaneously applying cathodal tDCS with low frequency rTMS at the motor cortex boosts inhibitory aftereffects. J Neurosci Methods. 2019;324: 108308.
Weightman M, Brittain JS, Miall RC, Jenkinson N. Direct and indirect effects of cathodal cerebellar TDCS on visuomotor adaptation of hand and arm movements [sci rep]. Sci Rep. 2021;11(1):4464.
Nguemeni C, Stiehl A, Hiew S, Zeller D. No impact of cerebellar anodal transcranial direct current stimulation at three different timings on motor learning in a sequential finger-tapping task. Front Hum Neurosci. 2021;15: 631517.
Kinakool AN, Abualait TS. Online polarity-dependent effects of cerebellar transcranial direct current stimulation on motor speed and fine manual dexterity. A randomized controlled trial. Saudi Med J. 2020;41(1):18–24.
Panouillères MT, Joundi RA, Brittain JS, Jenkinson N. Reversing motor adaptation deficits in the ageing brain using non-invasive stimulation. J Physiol. 2015;593(16):3645–55.
Shimizu RE, Wu AD, Samra JK, Knowlton BJ. The impact of cerebellar transcranial direct current stimulation (tDCS) on learning fine-motor sequences. Philos Trans R Soc Lond B Biol Sci. 2017;372(1711):20160050.
Funding
Partial financial support was received from the Funds for a Grant-in-Aid for Young Scientists (18K17723) to Tomofumi Yamaguchi.
Author information
Authors and Affiliations
Contributions
Keita Takano: methodology, validation, formal analysis, investigation, data curation, and writing–original draft. Natsuki Katagiri: investigation, writing—review and editing, and discussion. Takatsugu Sato: investigation, writing—review and editing, and discussion. Masafumi Jin: investigation, writing—review and editing, and discussion. Tadaki Koseki: writing—review and editing and discussion. Daisuke Kudo: writing—review and editing and discussion. Kaito Yoshida: writing—review and editing and discussion. Shigeo Tanabe: software, resources, and writing—review and editing. Masahiro Tsujikawa: writing—review and editing. Kunitsugu Kondo: resources and writing—review and editing. Tomofumi Yamaguchi: conceptualization, methodology, resources, writing—review and editing, discussion, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of Tokyo Bay Rehabilitation Hospital (approval number: 251–3) and was performed in accordance with the ethical standards established by the Declaration of Helsinki. All participants provided their written, informed consent before participating in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takano, K., Katagiri, N., Sato, T. et al. Changes in Corticospinal Excitability and Motor Control During Cerebellar Transcranial Direct Current Stimulation in Healthy Individuals. Cerebellum 22, 905–914 (2023). https://doi.org/10.1007/s12311-022-01469-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-022-01469-2