Abstract
Chiari Malformation type I (CM-I) is a neurological disorder characterized by a displacement of the cerebellar tonsils through the foramen magnum into the spinal canal. Most research has focused on physical symptomatology but few studies include neuropsychological examinations. Moreover, although current research highlights the involvement of the cerebellum on higher cognitive functions, little is known about cognitive consequences associated with CM-I. The aim of this study is to analyze cognitive functioning between 39 CM-I patients and 39 healthy controls, matched by gender, age and years of education. Participants have been examined on a large battery of neuropsychological tests, including executive functioning, verbal fluency, spatial cognition, language, verbal memory, processing speed, facial recognition and theory of mind. Results show a poorer performance of the clinical group compared to the control group, even after controlling the effect of physical pain and anxious-depressive symptomatology. The findings suggest the presence of a generalized cognitive deficit associated with CM-I, which makes it necessary to focus attention not only on physical consequences, but also on cognitive ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Chiari malformation (CM) is a pathology, usually congenital, that is included in the malformations of the posterior fossa of the skull and whose main characteristic is an ectopy of the cerebellar tonsils [1]. It was initially described by John Cleland in 1883; however, its classification is attributed to the Austrian pathologist Hans von Chiari in 1891. In addition to the four typologies proposed by Chiari (I, II, III and IV), the current diagnosis of CM includes two more subtypes (0, 1.5) [2,3,4].
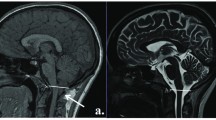
The present study evaluated a sample of patients with Chiari malformation type I (CM-I). CM-I is characterized by a herniation of the cerebellar tonsils greater than 5 mm through the foramen magnum, which invades the spinal canal and generates compression in the craniocervical area. It is usually associated to the presence of syringomyelia, although it is not frequently found with severe intracranial alterations [1].
Its prevalence rates are not clear, it is estimated to occur at a range of 1/1000–5000 cases [5] and with a higher prevalence among women [6]. Regarding the CM-I symptomatology, it is necessary to point out that there is great variability among patients, ranging from asymptomatic cases to cases with a severe clinical symptomatology. Typically, onset occurs at the age of 30, although it may fluctuate between periods when symptoms are accentuated and periods when symptoms subside [7]. The most frequent symptoms include headaches and pain, mainly in the cervical region, sensory alterations and limb weakness, visual impairment and balance problems, together with other symptoms derived from the compression of the cranio-cervical and brainstem area [8, 9]. In relation to treatment, if it is not controlled through pharmacological administration, a surgical intervention to decompress the posterior fossa is the most frequent choice [10]. Despite advances in the understanding of CM-I, most studies have focused on its description from a clinical or surgical perspective, and studies that include the cognitive symptomatology associated to CM-I are scarce.
In recent decades, there has been a growing interest in the consideration of the cerebellum as a structure whose function exceeds motor regulation and coordination. Ever since the publication of studies such as Leiner, Leiner and Dow [11], who proposed the involvement of the cerebellum in higher cognitive functions, this argument has become widely accepted [12, 13]. Anatomical data [14,15,16], neuroimaging studies [17, 18] and the presence of cognitive deficits in different disorders that occur with cerebellar abnormalities [19] support this idea.
Undoubtedly, the publication that marked a turning point in this respect was that of Schmahmann and Sherman [20], in which authors coined a new entity known as the “Cerebellar Cognitive Affective Syndrome” (CCAS). According to it, individuals affected by cerebellar pathologies present a clinical symptomatology characterized by alterations at four levels: (i) executive functioning; (ii) spatial cognition; (iii) changes in personality; and (iv) language impairment.
Schmahmann and Sherman’s [20] study was followed by numerous articles in which the connection between the cerebellum and different cognitive functions was highlighted, including verbal fluency [21], working memory [22], visuo-spatial processing [23], sequencing [24], emotional processing [25], executive function [26], attention [27], language [28], memory [29], and social cognition [30].
Although scientific research in this respect has increased, there are few studies that can be found in the literature on the cognitive symptomatology associated with CM-I. In one of the most recent and explicit works on this subject, Allen et al. [31] performed a neuropsychological evaluation on a sample of adults suffering from CM-I, in which they found that the deficit in the response inhibition ability was maintained, even after controlling the effect of anxious-depressive symptomatology. Another outstanding publication is that of Kumar et al. [32], as it is the first study to evaluate cognitive performance in a sample of patients suffering from CM-I and its correlation with measurements obtained through diffusion tensor imaging (DTI). In this study, the authors compared fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD) values in deep gray and white matter, between patients and controls. They found decreased FA together with increased MD in putamen, genu, splenium and fornix, comparing these results with a control group. Moreover, they also observed RD significantly increased in fornix and cingulum along with AD increased in putamen, thalamus and fornix. Based on their findings, they also posed the possibility that CM-I might present a deficit in myelination and, therefore, an abnormal development of the cerebellar white matter. This could be congruent with the presence of a lower cognitive performance in this group.
In other studies with children, evidence has also been found in favor of the presence of cognitive difficulties. Riva and colleagues [33] emphasized the importance of taking into account the cognitive symptomatology derived from CM and its influence in the developmental period. Novegno et al. [34] observed a deficit in language skills present in 50% of their sample with CM-I. Difficulties in executive functioning have also been reported in Lacy and colleagues’ [35] study, where the presence of these problems was assessed through self-reports completed by parents.
Despite these findings, there is still insufficient evidence to analyze the neuropsychological profile associated with CM-I from a global perspective. The main objective of this study is to compare the cognitive performance between two groups: CM-I patients and healthy controls. To this end, a large battery of neuropsychological tests was administered to assess whether congruence with CCAS exists, including executive functioning, verbal fluency, spatial cognition and language, in addition to analyzing performance on other cognitive domains considered in the literature about cerebellum such as verbal memory, processing speed, facial recognition and theory of mind. Unlike Allen et al. (2014), patients who had not undergone surgical decompression treatment of the posterior fossa were selected in order to avoid the possible influence of this variable on the results. As it is common for this pathology to present anxious-depressive symptomatology and pain symptoms [36], its effect on test performance has also been controlled for through statistical methods.
Method
Participants
The sample included a total of 78 participants divided into two groups. The clinical group was composed of 39 adults with CM-I of genetic origin (not surgically treated) recruited by convenience through the Friends of Arnold-Chiari National Association (Asociación Nacional de Amigos de Arnold-Chiari, ANAC) and the Chiari and Syringomyelia Association of Asturias (Asociación Chiari y Siringomielia del Principado de Asturias, ChySPA). They were not specifically candidates for surgery at the moment the study was carried out which does not mean that this condition would remain in the future. None of the patients of this group had hydrocephalus nor other specific craniocervical malformations. Regarding underlined symptomatology, patients reported headache (low frequency (LF): 17.9%, high frequency (HF): 82,1%), dizziness (no presence: 5.1%, LF: 33.3%, HF: 61.5%), muscle pain (LF: 15.4%, HF: 84.6%), muscle weakness (no presence: 2.6%, LF: 23.1%, HF: 74.3%) and trouble sleeping (no presence: 10.3%, LF: 25.6%, HF: 64.1%). The second group was composed of 39 healthy controls, recruited among volunteer participants (none of them were relatives of clinical group), and matched by gender, age, and years of education. Sample recruitment took place between 2014 and 2017. Socio-demographic and clinical data are shown in Table 1. There were no statistically significant differences between the two groups with respect to gender, χ2(1) = 0, p = 1; age, t = 0.066, p = 0.948; nor years of education, t = −0.578, p = 0.565.
The inclusion criteria for the study were being over 18 years of age, residing in Spain, using Spanish as their primary language of communication, having fulfilled the informed consent document prior to the assessment, having a diagnosis of Chiari malformation type I [Q07.01] confirmed by a neurosurgeon and having a magnetic resonance test confirming CM-I.
Exclusion criteria for this study included having any other neurological, psychological or psychiatric diagnosis not secondary to Chiari Malformation diagnosed according to ICD-10 criteria, suffering from illiteracy and/or having uncompensated sensory deficits that could impede the administration of the assessment protocol.
Despite not being considered an exclusion criterion, it was ensured that participants were not under any pharmacological treatment likely to affect their cognitive performance. Regarding the control group, the inclusion and exclusion criteria were the same, except for the specific CM-I-related criteria.
All members of the sample participated in the study voluntarily, signing an informed consent document, in compliance with the Organic Law 15/99 of 13th December of Spanish Law regarding the Protection of Personal Data and the Declaration of Helsinki (Edinburgh, 2000).
Instruments
Participants completed a large battery of neuropsychological tests, along with self-administered questionnaires to assess the presence of neuropsychiatric symptomatology. All tests fulfilled adequate reliability and validity criteria, as well as having a corresponding adaptation with Spanish samples.
Neuropsychological Assessment
Executive funtion: the Zoo Map subtest from the Behavioral Assessment of the Disexecutive Syndrome (BADS - [37]; spanish version [38]), the Backward Digit Span test from the Wechsler Adult Intelligence Scale-IV (WAIS-IV - [39]; spanish version [40]), and the color-word and interference score from the Stroop test [41] (spanish version [42]) were used. A composite with the total score of these tests was elaborated as an indicator for this cognitive domain.
Verbal fluency: the Controlled Oral Word Association Test (F-A-S) [43, 44] and a semantic verbal fluency test (“Cooking”-“Animals”) [43, 44] were administered. A computation of the total of the two tests was used as a global indicator of verbal fluency.
Spatial cognition: The Rey-Osterrieth Complex Figure test (ROCF - [45, 46]; spanish version [47]) was used as a measure of visuospatial, visuoconstructive and visual memory ability.
Language: The Boston Naming Test (BNT - [48]; spanish version [49]) was administered.
Verbal memory: the Spain-Complutense Verbal Learning Test (TAVEC) [50] was administered. As an indicator of the domain, the verbal learning, the short-term recall and the long-term recall scores were used. The recognition ability was assessed separately.
Processing speed: the Symbol Digit Modalities Test (SDMT - [51]; spanish version [52]) was used to assessed it.
Facial Recognition: The Benton Facial Recognition Test ([53]; adapted version [54]) was used for neutral faces; and the Facially Expressed Emotion Labeling test (FEEL - [55]; adapted version [56]) was used for emotionally expressive faces.
Theory of mind: it was assessed through the Happé’s Strange Stories test, which assesses the ability to comprehend nonliteral utterances ([57]; adapted version [58]).
The presence of anxious-depressive symptomatology was assessed through the Hospital Anxiety and Depression scale (HAD - [59]; adapted version [60]) and the presence of physical pain intensity was assessed through the Visual Analogue Scale from 0 (no pain) to 10 (pain as bad as it could be) (VAS - [61, 62]).
Procedure
The recruitment was carried out contacting by email and telephone with aforementioned organizations. The patients who were interested in participating were informed of the study. If they met the established criteria, they were included and called for the assessment. The protocol was administered in individual sessions guided by a neuropsychologist, with a duration of 1.5 h. All tests were administered in pencil and paper format, except for the FEEL test, which is computerized. After a brief interview in which sociodemographic data and clinical history were collected, each participant performed the neuropsychological tests indicated above, and ended the session by completing the self-administered questionnaires. Both, the clinical and the control group were administered the same tests.
Statistical Analysis
The statistical analyses were carried out using the Statistical Package for Social Sciences (SPSS), version 23.0. The normal distribution of the sample was tested with Kolmogorov-Smirnov test. Raw scores were converted into z scores to run the analyses. Differences between groups regarding socio-demographic data were tested with Student T test for quantitative variables, and chi-squared test for categorical variable. The associations between variables were analyzed using Pearson’s R test.
Differences between groups regarding cognitive tests were tested with Student T test for parametric variables and Mann-Whitney U test for non-parametric variables. Effect sizes were calculated according to Cohen’s d method. Analysis of variance (ANOVA) with post hoc multiple comparisons (Bonferroni test) was used to compare performance on cognitive tests between those cases of CM-I with syringomyelia and those without. In order to obtain more powerful measures of cognitive domains, composite scores were calculated for executive functioning, verbal fluency and verbal memory. These scores were calculated grouping the included indicators of each domain after raw scores were converted into z-values. Before this procedure, the positive correlation among included measures for each cognitive domain was confirmed. The internal consistency of each created composite was examined by the Cronbach’s alpha coefficient. Analysis of covariance (ANCOVA) was used to analyze if the physical pain intensity and anxious-depressive symptomatology could explain the differences between groups on cognitive tests. Effect sizes were established based on partial eta squared indicator. Significance levels are reported using false discovery rate (FDR) in order to maximize statistical power and report stringent p value correction (threshold: FDR-corrected p values <0.05).
Results
The scores obtained by both groups in the neuropsychological tests are shown in Table 2. As can be observed, there was a generalized cognitive deficit in the clinical group compared to the control group, except for the time in the Zoo Map task, coy time in the ROCF, verbal recognition and the FEEL Test, whose differences were not statistically significant after FDR correction. Furthermore, taking into account the effect size, the magnitude of the differences found between both groups ranged between moderate and high values.
Moreover, it is noteworthy that no correlation was found between the millimeters of amygdala descent (tested by a neurosurgeon) and performance on cognitive tests analyzing scores converted into z-values: executive functioning (r = 0.02, p = 0.935), verbal fluency (r = −0.17, p = 0.462), copy accuracy (r = 0.02, p = 0.930), copy time (r = 0.34, p = 0.052), visual memory (r = −0.06, p = 0.743), language (r = 0.01, p = 0.951), verbal memory (r = −0.03, p = 0.859), recognition (r = −0.26, p = 0.145), processing speed (r = 0.09, p = 0.625), facial recognition (r = 0.12, p = 0.506), emotional facial recognition (r = 0.32, p = 0.068) and theory of mind (r = 0.01, p = 0.985); nor with physical (physical pain: r = 0.02, p = 0.929) or psychological symptomatology (anxious-depressive symptomatology: r = 0.19, p = 0.283). In addition, no differences in performance were found in the clinical group between those cases of CM-I with syringomyelia and those without, analyzing scores converted into z-values: executive functioning (−0.16 [SE = 0.25] vs −0.49 [SE = 0.17], p = 0.969), verbal fluency (−0.67 [SE = 0.36] vs −0.34 [SE = 0.13], p = 1), copy accuracy (−0.48 [SE = 0.50] vs −0.26 [SE = 0.20], p = 1), copy time (0.30 [SE = 0.35] vs 0.22 [SE = 0.23], p = 1), visual memory (−0.46 [SE = 0.36] vs −0.59 [SE = 0.17], p = 1), language (0.22 [SE = 0.22] vs −0.60 [SE = 0.27], p = 0.215), verbal memory (−0.19 [SE = 0.27] vs −0.53 [SE = 0.18], p = 0.785), recognition (−0.29 [SE = 0.48] vs −0.32 [SE = 0.23], p = 1), processing speed (−0.55 [SE = 0.35] vs −0.49 [SE = 0.19], p = 1), facial recognition (−0.15 [SE = 0.29] vs −0.51 [SE = 0.23], p = 0.838), emotional facial recognition (0.03 [SE = 0.30] vs −0.24 [SE = 0.21], p = 1) and theory of mind (−0.86 [SE = 0.30] vs −0.63 [SE = 0.16], p = 1).
In relation to the anxious-depressive symptomatology, the differences between the clinical group (M = 19.62, SD = 8.18) and the control group (M = 9.54, SD = 5.43) were statistically significant (t = 6.408, p < 0.001). Taking into account physical pain, statistically significant differences were also found (U = 184.0, p < 0.001).
Since the latter two are variables that can influence cognitive performance, their effect was controlled through a covariance analysis (ANCOVA). This analysis was performed in the eight cognitive domains evaluated, having previously created a composite score with the tests described in the section “Instruments” for executive functioning (α = 0.73), verbal fluency (α = 0.85) and verbal memory (α = 0.97), which yielded good internal consistency. The composite score for spatial cognition was not created on account of its lack of proper internal consistency. The results are shown in Table 3.
According to Table 3, after controlling and eliminating the effect of the physical pain and the anxious-depressive symptomatology on performance on cognitive tests, the differences between groups obtained in the initial analysis were eliminated for the response accuracy in emotional facial recognition of sadness.
Therefore, despite eliminating the effect of possible covariates on the cognitive performance of both groups, differences between the CM-I group and the control group were observed in nine of the initially proposed variables (Fig. 1). The results are included in Table 3 along with the corresponding effect size for each variable. As an additional illustrative example, Fig. 2 shows the execution of two patients with CM-I in the ROCF. Some visuospatial disorganization is noticed in the precision of copy, as well as a poor execution in visual memory.
Discussion
In this study, the cognitive performance of a group of 39 adults with CM-I (not surgically treated) was compared to that of 39 healthy controls, matched by gender, age, and years of education. The findings indicate that people diagnosed with CM-I show a significantly lower cognitive performance, once the effect of physical pain and anxious-depressive symptomatology is controlled for, in the following domains: executive functioning, verbal fluency, copy accuracy, visual memory, naming ability, verbal memory, processing speed, facial recognition and theory of mind.
The findings of the present study coincide with the data provided by Kumar et al. [32]. These authors, using DTI and neuropsychological assessment tests, found that the group of adults with CM-I performed worse in executive functioning, visuospatial ability and visuomotor velocity, which was replicated in the present study’s results.
However, unlike Kumar et al.’s [32] article, the present study did not administer neuroimaging tests that would allow to associate the observed cognitive deficit with the cerebellar alteration of CM-I in a conclusive way. Nevertheless, although there has been no research investigating this pathology in this specific way, there are studies confirming the involvement of the cerebellum in higher cognitive functions, the main cause of which is attributed to cerebro-cerebellar circuitry [26].
The cognitive domains in which the evaluated CM-I group yielded a poorer performance require functions typically associated with the cerebral cortex. Thus, it is likely that CM-I implies a failure in the cortico-cerebellar connectivity. This fact was pointed out by Schmahmann and Sherman [20] as justification for the presence of CCAS, suggesting that there is a deficit in the connection with the prefrontal cortex, posterior parietal, temporal superior and limbic areas. At the same time, there are other evidences of the connection between the cerebellum and the prefrontal lobe (executive functioning), the frontal lobe (phonological verbal fluency), the temporal lobe, including the hippocampus and the amygdala (verbal fluency, verbal memory, facial recognition), the parietal lobe (visuospatial skills), mainly with the intraparietal area, from which a complex connective network is established that has links leading to the premotor cortex (visuoconstructive skills) and areas of the parahippocampal region [15, 25, 27, 63].
In the results obtained in the present study, while executive functioning, verbal fluency, spatial cognition, naming ability, processing speed and facial recognition show a clear deficit, the most significant differences between the clinical group and the control group were in its performance on verbal memory test and the theory of mind task (according to p values adjusted using FDR correction; p < 0.001). However, the highest effect size was found in the Happe’s Strange Stories task. This task involves a complex network of brain structures; however, the role played by the frontal lobe, with which the cerebellum has intense connections, is emphasized. To date, there are no studies evaluating this cognitive domain among the CM-I population, although difficulties have been reported in interpreting the mental states of others in cerebellar disorders such as spinocerebellar ataxia [64]. Moreover, pathologies such as autism or schizophrenia that have clear deficits in theory of mind, in turn, present structural abnormalities in the cerebellum [65,66,67]. Despite these findings, it is noted that a single test does not allow one to reach firm conclusions, however, it can establish indications for future research lines. Regarding emotional facial recognition, this domain seems to be spared in the CM-I patients because no differences were found between the two groups. However, studies such as Ferruci et al.’s [68], in which transcranial magnetic stimulation was applied to a sample of 21 healthy controls, conclude that the cerebellum is involved in the processing of negative facial expressions. Similarly, the results of the present study do not coincide with those found for a group of individuals suffering from spinocerebellar ataxia, which showed worse facial recognition of emotions, both for basic and social emotions [69]. In any case, further studies are needed to obtain conclusive results in this regard.
Although the evidence of publications that present cortical-cerebellar connectivity as a source involved in cognitive functioning is firm, it is not enough to attribute the cause of the cognitive profile associated with CM-I to this phenomenon. However, recent publications have led to the development of new hypotheses based on microstructural damages present in patients with CM-I. On the one hand, it could be due to the abnormalities in the white matter tracts demonstrated by Kumar et al. [32]. These findings suggest that cortical deafferences may occur as in other cerebellar lesions, thus generating difficulties in cognitive functioning [15]. In a study with children in which DTI was also used, alterations in the cerebellar tissue were found, concluding that the middle cerebellar peduncle is compromised in CM-I, with this structure giving rise to corticopontocerebellar afferents [70, 71]. On the other hand, it can also be considered to be caused by the pressure exerted by the descent of the cerebellar tonsils into the brainstem region [72]. In addition, two recent studies by Akar and colleagues [73, 74] showed that a group of patients with CM-I yielded differences in their white and gray matter of cerebellar tissue, as well as differences in the cerebrospinal fluid values compared to a group of healthy controls. This could also be related to the cognitive deficit observed, however, these authors did not include this variable in their study.
Regarding the shared variables between this study and the CCAS described by Schmahmann and Sherman [20], the results obtained for executive functioning, verbal fluency, spatial cognition (copy accuracy and visual memory) and naming ability could support the presence of this syndrome in CM-I patients. Both analyzing the intergroup differences and controlling for the effect of the covariates, CM-I patients show a lower cognitive performance on these domains. In fact, Kraan [75] included CM-I as one of the disorders in which CCAS can develop. According to Tedesco et al. [76], one of the possible reasons for this profile is that damage in the cerebellum is congruent with the appearance of a generalized negative effect over cognition which leads to a less efficient functioning. However, taking into account the topography of each particular pathology, the syndrome would manifest itself following different patterns but without eluding common aspects. These authors also add sequencing as a process transverse to other cognitive processes, and identify this function as something specific to cerebellar damage. In the present study, results regarding verbal memory, processing speed, facial recognition and theory of mind suggest that these domains could be included as areas also involved in the cerebellar cognitive profile.
Considering the evidence provided by the literature together with the results obtained in the present study, the implication of the cerebellum in cognitive functioning is undoubted. However, regarding what the role of the cerebellum is, there are still some issues that remain unresolved. The most widely accepted hypothesis in the literature is the one proposed by Schmahmann [77] as “dysmetria of thought”, according to which the cerebellum would play a role as modulator or coordinator, not only in motor functions, but also extending to cognitive processing. Thus, those individuals with cerebellar abnormalities, especially in the posterior lobe area, would have difficulties at this level [78, 79]. The findings obtained in the present study support this idea and also suggest the possibility that this “dysmetria of thought”, which would lead to the manifestation of CCAS, is present in CM-I.
Regarding the limitations of the study, it is necessary to point out that the included sample, besides not having been conformed by random sampling, does not have an equivalent gender distribution. There is little representation of males, which may compromise the generalizability of the results. However, it seems reasonable as, according to epidemiological data, this pathology has a higher prevalence among women. Another limitation about the sample concerns about the provenance of patients. They have been recruited from different organizations and it could be a bias, however, they are all supervised by neurosurgeons and neurologists.
Furthermore, only patients with CM-I who had not undergone decompression surgery were included in the present study, although the fact of having been exposed to this treatment could influence the conclusions. In addition, although the neuropsychological evaluation consists of a comprehensive test protocol, neither specific instruments have been included to assess sequencing, which is associated with the cerebellum, nor specific test to assess premorbid intellectual functioning. Another limitation is that this is a cross-sectional study, which does not offer the possibility of analyzing whether there is an evolution of cognitive symptoms during the course of the disease. These aspects should be addressed in future research in order to provide new evidence and reinforce these findings.
Conclusion
The results obtained in the present study suggest that CM-I courses with cognitive symptomatology once the effect of anxiety, depression and physical pain symptoms have been controlled. Moreover, these findings are congruent with the cognitive profile associated with cerebellar pathologies and provide evidence on the implication of this structure in cognitive functioning and the importance of cortical-cerebellar connectivity in this functioning. This fact makes it necessary to focus attention not only on surgical treatment or physical symptomatology, but also on the cognitive consequences associated with CM-I, leading to a global and interdisciplinary approach.
References
Manto M, Christian H. Chiari malformations. In: Manto M, Gruol DL, Schmahmann JD, Koibuchi N, Rossi F, editors. Handbook of the cerebellum and cerebellar disorders. Dordrecht: Springer; 2013. p. 1873–85. https://doi.org/10.1007/978-94-007-1333-8.
Mariwalla NR, Boydston WR, Chern JJ. Newer subsets: Chiari 0 and Chiari 1.5 malformations. In: Tubbs RS, Oakes WJ, editors. The Chiari malformations. New York: Springer; 2013. p. 241–6. https://doi.org/10.1007/978-1-4614-6369-6_2.
Tubbs RS, Oakes WJ. Introduction and classification of the Chiari malformations. In: Tubbs RS, Oakes WJ, editors. The Chiari malformations. New York: Springer; 2013. p. 1–3. https://doi.org/10.1007/978-1-4614-6369-6_2.
Tubbs RS, Oakes WJ. The Chiari malformations: a historical context. In: Tubbs RS, Oakes WJ, editors. The Chiari malformations. New York: Springer; 2013. p. 5–11. https://doi.org/10.1007/978-1-4614-6369-6_2.
Urbizu A, Toma C, Poca MA, Sahuquillo J, Cuenca-León E, Comand B, et al. Chiari malformation type I: a case-control association study of 58 developmental genes. PLoS One. 2013;8:e57241. https://doi.org/10.1371/journal.pone.0057241.
Öktem H, Dilli A, Kürkçüoglu A, Soysal H, Yazici C, Pelin C. Prevalence of Chiari type I malformation on cervical magnetic resonance imaging: a retrospective study. J Exp Clin Anat. 2016;10:40–5. https://doi.org/10.2399/ana.15.039.
Amado ME, Avellaneda A, Barrón J, Chesa E, De la Cruz J, Escribano M, et al. Malformaciones de la Unión Cráneo-Cervical (Chiari tipo I y Siringomielia). Documento de Consenso. Madrid: Editorial Médica A.W.W.W.E. S.A.; 2009.
deSouza RM, Zador Z, Frim DM. Chiari malformation type I: related conditions. Neurol Res. 2011;33:278–84. https://doi.org/10.1179/016164111X12962202723922.
Meadows J, Guarnieri M, Miller K, Haroun R, Kraut M, Carson BS. Type I Chiari malformation: a review of the literature. Neurosurg Q. 2001;11(3):220–9.
Chen J, Li Y, Wang T, Gao J, Xu J, Lai R, et al. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation type I in adult patients. Medicine. 2017;96:e5945. https://doi.org/10.1097/MD.0000000000005945.
Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behav Neurosci. 1986;100(4):443–54.
Bodranghien F, Bastian A, Casali C, Hallett M, Louis ED, Manto M, et al. Consensus paper: revisiting the symptoms and signs of cerebellar syndrome. Cerebellum. 2016;15:369–91. https://doi.org/10.1007/s12311-015-0687-3.
Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13:151–77. https://doi.org/10.1007/s12311-013-0511-x.
Habas C. Functional imaging of the deep cerebellar nuclei: a review. Cerebellum. 2010;9:22–8. https://doi.org/10.1007/s12311-009-0119-3.
Tirapu J, Luna P, Iglesias MD, Hernáez P. Contribución del cerebelo a los procesos cognitivos: avances actuales. Rev Neurol. 2011;53(5):301–15.
Voogd J. The human cerebellum. J Chem Neuroanat. 2003;26(4):243–52.
Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11:352–65. https://doi.org/10.1007/s12311-011-0260-7.
Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59:1560–70. https://doi.org/10.1016/j.neuroimage.2011.08.065.
Steinlin M, Wingeier K. Cerebellum and cognition. In: Manto M, Gruol DL, Schmahmann JD, Koibuchi N, Rossi F, editors. Handbook of the cerebellum and cerebellar disorders, vol. 2013. Dordrecht: Springer; 2013. p. 1687–99. https://doi.org/10.1007/978-94-007-1333-8.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;131(4):561–79.
Leggio MG, Silveri MC, Petrosini L, Molinari M. Phonological grouping is specifically affected in cerebellar patients: a verbal fluency study. J Neurol Neurosurg Psychiatry. 2000;69(1):102–6.
Gottwald B, Wilde B, Mihajlovic Z, Mehdorn HM. Evidence for distinct cognitive deficits after focal cerebellar lesions. J Neurol Neurosurg Psychiatry. 2004;75:1524–31. https://doi.org/10.1136/jnnp.2003.018093.
Molinari M, Leggio MG. Cerebellar information processing and visuospatial functions. Cerebellum. 2007;6:214–20. https://doi.org/10.1080/14734220701230870.
Leggio MG, Tedesco AM, Chiricozzi FR, Clausi S, Orsini A, Molinari M. Cognitive sequencing impairment in patients with focal or atrophic cerebellar damage. Brain. 2008;131:1332–43. https://doi.org/10.1093/brain/awn040.
Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. https://doi.org/10.1146/annurev.neuro.31.060407.125606.
Koziol LF, Barker LA. Hypotonia, jaundice, and Chiari malformations: relationships to executive functions. Appl Neuropsychol. 2013;2:141–9. https://doi.org/10.1080/21622965.2013.748390.
D’Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circ. 2013; https://doi.org/10.3389/fncir.2012.00116.
Mariën P, Ackermann H, Adamaszek M, Barwood CHS, Beaton A, Desmond J, et al. Consensus paper: language and the cerebellum: an ongoing enigma. Cerebellum. 2014; https://doi.org/10.1007/s12311-013-0540-5.
Nakamoto FK, Tsutsumiuchi M, Maeda MH, Uesaka Y, Takeda K. Memory impairment following right cerebelar infarction: a case study. Neurocase. 2015;21:660–4. https://doi.org/10.1080/13554794.2014.969277.
Van Overwalle F, Mariën P. Functional connectivity between the cerebrum and cerebellum in social cognition: a multi-study analysis. NeuroImage. 2016;124:248–55. https://doi.org/10.1016/j.neuroimage.2015.09.001.
Allen PA, Houston JR, Pollock JW, Buzzelli C, Li X, Harrington AK, et al. Task-specific and general cognitive effects in Chiari malformation type I. PLoS One. 2014;9(4):1–11. https://doi.org/10.1371/journal.pone.0094844.
Kumar M, Rathore RK, Srivastava A, Yadav SK, Behari S, Gupta RK. Correlation of diffusion tensor imaging metrics with neurocognitive function in Chiari I malformation. World Neurosurg. 2011;76:189–94. https://doi.org/10.1016/j.wneu.2011.02.022.
Riva D, Usilla A, Saletti V, Esposito S, Bulgheroni S. Can Chiari malformation negatively affect higher mental functioning in developmental age? Neurol Sci. 2011;32:307–9. https://doi.org/10.1007/s10072-011-0779-x.
Novegno F, Caldarelli M, Massa A, Chieffo D, Massimi L, Pettorini B, et al. The natural history of the Chiari type I anomaly. J Neurosurg Pediatr. 2008;2:179–87. https://doi.org/10.3171/PED/2008/2/9/179.
Lacy M, Ellefson SE, DeDios-Stern S, Frim DM. Parent-reported executive dysfunction in children and adolescents with Chiari malformation type 1. Pediatr Neurosurg. 2016;51:236–43. https://doi.org/10.1159/000445899.
Mestres O, Poca MA, Solana E, Radoi A, Quintana M, Force E, et al. Evaluación de la calidad de vida en los pacientes con una malformación de Chiari tipo I. Estudio piloto en una cohorte de 67 pacientes. Rev Neurol. 2012;55(3):148–56.
Wilson BA, Alderman N, Burgess PW, Emslie H, Evans JJ. Behavioral assessment of the dysexecutive syndrome. England: Thames Valley Test Company; 1996.
Vargas ML, Sanz JC, Marín JJ. Behavioral assessment of the dysexecutive syndrome battery (BADS) in schizophrenia. A pilot study in the Spanish population. Cogn Behav Neurol. 2009;22(2):95–100.
Wechsler D. Wechsler adult intelligence scale, 4th ed. WAIS-IV. San Antonio: Pearson; 2008.
Wechsler D. WAIS-IV. Escala de inteligencia de Wechsler para adultos-IV. Madrid: NCS Pearson; 2012.
Golden CJ. Stroop color and word test. Chicago: Stoelting; 1978.
Golden CJ. Stroop Test de Colores y Palabras. Madrid: TEA Ediciones; 2010.
Benton AL, Hamsher K. Multilingual aplasia examination. Iowa City: Department of Neurology and Psychology, The University of Iowa; 1989.
Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests. New York: Oxford University Press; 2006.
Osterrieth PA. Le test de copie d’une figure complexe: Contribution à l’étude de la perception et la mémoire. Arch Psychol. 1944;30:286–356.
Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique. Arch Psychol. 1941;28:286–340.
Rey A. Test de Copia de una Figura Compleja. Madrid: TEA Ediciones; 1980.
Kaplan E, Goodglass H, Weintraub S. Boston naming test. Philadelphia: Lippincott Williams and Wilkins; 2001.
Kaplan E, Goodglass H, Weintraub S. Test de Denominación de Boston. Madrid: Panamericana; 2005.
Benedet MJ, Alejandre MA. TAVEC Test de Aprendizaje Verbal España-Complutense. Madrid: TEA Ediciones, S.A; 1998.
Smith A. Symbol digits modalities test. Western Psyhological Services: Los Angeles; 1982.
Smith A. Test de símbolos y dígitos. Madrid: TEA Ediciones; 2002.
Benton AL, Sivan AB, Hamsher KS, Varney NR, Spreen O. Contributions to neuropsychological assessment. New York: Oxford University Press; 1994.
Escanilla A. Datos normativos piloto de una población española de tres pruebas visuales de Benton: reconocimiento facial, orientación de líneas y discriminación de formas. Psychiatry and Legal Medicine Department. Autonomous University of Barcelona; 2000.
Kessler H, Bayerl P, Deighton RM, Traue HC. Facially Expressed Emotion Labeling (FEEL): PC-gestützer Test zur Emotionserkennung. Verhaltenstherapie und Verhaltensmedizin. 2002;23(3):297–306.
Lázaro E, Amayra I, López-Paz JF, Martínez O, Pérez M, Berrocoso S, et al. Instrument for assessing the ability to identify emotional facial expressions in healthy children and in children with ADHD: the FEEL test. J Atten Disord. 2016; https://doi.org/10.1177/1087054716682335.
Happé F. An advanced test of theory of mind: understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. 1994;24:129–54.
Pousa E. Measurement of theory of mind in healthy adolescents: translation and cultural adaptation of F. Happé’s theory of mind stories (1999). Health Psychology and Social Psychology Department. Autonomous University of Barcelona; 2002.
Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
López-Roig S, Terol M, Pastor M, Neipp M, Massutí B, Rodríguez-Marín J, et al. Ansiedad y Depresión. Validación de la escala HAD en pacientes oncológicos. J Health Psychol. 2002;12(2):127–55.
Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37(4):378–81.
Haelferi M, Elfering A. Pain assessment. Eur Spine J. 2006;15:S17–24. https://doi.org/10.1007/s00586-005-1044-x.
Habas C, Kamdar N, Nguyen D, Prater K, Beckman CF, Menon V. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–94. https://doi.org/10.1523/JNEUROSCI.1868-09.2009.
Garrard P, Martin NH, Giunti P, Cipolotti L. Cognitive and social cognitive functioning in spinocerebellar ataxia. J Neurol. 2008;2008:398–405. https://doi.org/10.1007/s00415-008-0680-6.
Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318:1349–54. https://doi.org/10.1056/NEJM198805263182102.
Ozguven HD, Oner O, Baskak B, Oktem F, Olmez S, Munir K. Theory of mind in schizophrenia and Asperger’s syndrome: relationship with negative symptoms. Klinik Psikofarmakol Bulteni. 2010;20(1):5–13.
Tirapu J, Pérez G, Erekatxo M, Pelegrín C. Qué es la teoría de la mente? Rev Neurol. 2007;44(8):479–89.
Ferrucci R, Giannicola G, Rosa M, Fumagalli M, Boggio PS, Hallett M, et al. Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cognit Emot. 2012;26:786–99. https://doi.org/10.1080/02699931.2011.619520.
D’Agata F, Caroppo P, Baudino B, Caglio M, Croce M, Berqui M, et al. The recognition of facial emotions in spinocerebellar Ataxia patients. Cerebellum. 2011;10:600–10. https://doi.org/10.1007/s12311-011-0276-z.
Eshetu T, Meoded A, Jallo GI, Carson BS, Huisman TA, Poretti A. Diffusion tensor imaging in pediatric Chiari type I malformation. Dev Med Child Neurol. 2014;56:742–8. https://doi.org/10.1111/dmcn.12494.
Snell RS. El cerebelo y sus conexiones. In: Snell RS, editor. Neuroanatomía clínica. Madrid: Editorial Médica Panamericana; 2007. p. 243–65.
Krishna V, Sammartino F, Yee P, Mikulis D, Walker M, Elias G, et al. Diffusion tensor imaging assessment of microstructural brainstem integrity in Chiari malformation type I. J Neurosurg. 2016;125:1112–9. https://doi.org/10.3171/2015.9.JNS151196.
Akar E, Kara S, Akdemir H, Kɪrɪş A. Fractal dimension analysis of cerebellum in Chiari malformation type I. Comput Biol Med. 2015; https://doi.org/10.1016/j.compbiomed.2015.06.024.
Akar E, Kara S, Akdemir H, Kɪrɪş A. 3D structural complexity analysis of cerebellum in Chiari malformation type I. Med Biol Eng Comput. 2017;55:2169–82. https://doi.org/10.1007/s11517-017-1661-7.
Kraan C. Cerebellar cognitive affective syndrome. In: Rinehart N, Bradshaw J, Enticott P, editors. Developmental disorders of the brain. New York: Routledge; 2017. p. 25–43.
Tedesco AM, Chiricozzi FR, Clausi S, Lupo M, Molinari M, Leggio MG. The cerebelar cognitive profile. Brain. 2011;134:3672–86. https://doi.org/10.1093/brain/awr266.
Schmahmann JD. (1991). An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48(11):1178–87.
Noroozian M. The role of the cerebellum in cognition: beyond coordination in the central nervous system. Neurol Clin. 2014;32:1081–104. https://doi.org/10.1016/j.ncl.2014.07.005.
Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–78. https://doi.org/10.1176/jnp.16.3.367.
Acknowledgements
We thank ANAC, ChySPA, and all of participants for their involvement in the study.
Funding
This study was funded by a grant of the Education Department of the Basque Government’s “Programa Predoctoral de Formación de Personal Investigador No Doctor” [PRE_2016_1_0099 to Maitane García].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The co-authors declare that they have no conflict of interest.
Ethical Approval and Informed Consent
The study was developed in accordance with ethical standards and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
García, M., Lázaro, E., López-Paz, J.F. et al. Cognitive Functioning in Chiari Malformation Type I Without Posterior Fossa Surgery. Cerebellum 17, 564–574 (2018). https://doi.org/10.1007/s12311-018-0940-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-018-0940-7