Abstract
Purpose
In the last two decades, the non-motor functions of the cerebellum have become the centre of attention for researchers. Anecdotal observations of cognitive and psychiatric manifestations of cerebellar lesions have increased this interest. We aimed to investigate the executive function (EF), intellectual capacity, and comorbid psychiatric disorders in adolescents with Chiari malformation type 1 (CM1), which is a pathological manifestation of posterior cranial fossa structures include the cerebellum.
Methods
The Chiari group consisted of ten adolescents aged 12–18 years old, and the control group consisted of 13 healthy adolescents with similar age and sex with patients. Stroop test (ST), trail making test (TMT), and Behaviour Rating Inventory of Executive Function-Parent form (BRIEF) were used to evaluate EF; Kent EGY and Porteus Maze Test was used to measuring the intelligence quotient (IQ), and a semi-structured interview was used to determine the psychiatric disorders.
Results
EF test scores were found comparable between the two groups. IQ scores of the Chiari group were found in the normal range, but significantly lower than controls. No significant difference was revealed in terms of comorbid psychiatric disorders between the two groups.
Conclusion
In this study, we did not observe an impairing effect of CM1 on EF and intelligence. Also, we found that CM1 did not cause more psychiatric disorders compared to controls. Further studies need to support our findings in adolescents diagnosed with CM1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
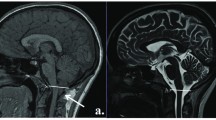
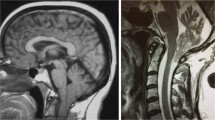
Chiari malformation (CM) is defined as the displacement of the posterior cranial fossa structures into the spinal canal [1]. Many theories have been put forward to explain the aetiology of CM; the most common view is that it develops as a result of genetic predisposition causing abnormal mesodermal development [2]. It is a congenital anomaly but may arise as a result of acquired reasons [3]. CM is classically divided into four subtypes, and classification depends on the degree of displacement. Type 1 is the mildest form of malformation and is described by the herniation of cerebellar tonsils at least 5 mm caudally through the foramen magnum. It often manifests in the 3rd and 4th decade and is named ‘adult-type’ [4].
Based on neuroimaging studies, the prevalence of CM1 was estimated at approximately 4% in the paediatric population [5]. CM1 is equal in females and males in paediatric cases, while females are more common in adult patients [6]. However, a certain paediatric population rate is unknown since many patients are asymptomatic and diagnosed incidentally. Saletti et al. [4] reported that about 59 % of children with CM1 were asymptomatic. The clinical presentation in CM1 is varied and includes symptoms such as severe headache, dizziness, dysphagia, extremity pain, nystagmus, motor, and sensory deficits [7]. With a rate of approximately 70%, the most common symptom is a suboccipital headache and neck pain in children and adults [8].
The traditional view suggests that the cerebellum does not play a role in cognition; however, over the last two decades, increasing evidence indicates that the cerebellum is not only associated with motor planning, balance, and coordination but also plays an essential role in many cognitive processes [9]. In particular, the posterior cerebellum is known to have comprehensive reciprocal cortical connections that affect and modulate several cognitive processes such as working memory, multitasking, executive functions, planning, shifting, and inhibition [10,11,12]. As a result of the displacement of the cerebellar tonsils in CM1 patients, the pressure on the cerebellum and brainstem can affect these regions’ functions, disrupt functional networks, and lead to cognitive impairment [13]. There are a few studies that investigated the cognitive functions of CM patients. Kumar et al. [14] and Allen et al. [15] showed in their studies that adults with CM1 performed significantly worse than healthy controls in executive function tasks. Also, Lacy et al. [11] found that children with CM1 exhibited executive function problems according to the parent-reported scale.
The association of CM1 and mental retardation (MR) is based on some authors’ case observations so far. As reported by these authors, MR accompanied 12% of the case series; however, these cases were mostly accompanied by meningomyelocele or supratentorial anomalies, which according to some authors should be classified as CM 2 [16, 17]. There is only one study that directly examines the relationship of CM1 with epilepsy and MR. In this study, MR, speech delay, and epilepsy were found in 25.7% of the cases [18].
A few case reports about the association between psychiatric comorbidities, anxiety disorder, panic disorder, and depressive disorder accompany CM patients in the literature [19,20,21]. Bakim et al. [22] evaluated adult patients with CM in terms of quality of life and psychiatric comorbidity. They found that 43.8% of the patients had a psychiatric disorder. They indicated that patients with psychiatric disorders had significantly high pain inventory scores, reflecting the poor quality of life. The most common psychiatric disorders in these patients were major depression and anxiety disorder, with a rate of 18.8% and 12.5%, respectively. To date, only one study has investigated psychiatric disorders in paediatric CM1 cases [23]. Eighty-six children diagnosed with CM1 were included in this study, and psychiatric disorders were found in 47% of the children. ADHD was the most common psychiatric disorder in these children, with a rate of 22%.
This study’s objective was to evaluate adolescents with CM1 from a psychiatric perspective and determine whether they have any comorbid psychiatric disorder. We also aimed to assess their intellectual capacities and executive functions by comparing them with healthy controls. Examining the literature, there is no study investigating the intellectual capacity, executive functions, and accompanying psychiatric conditions in adolescents with CM1.
Material and methods
Participants
Ten adolescents between 13 and 18 years of age who applied to the Department of Neurosurgery, Cumhuriyet University Faculty of Medicine, between September 2018 and December 2019 and diagnosed with Chiari malformation 1 (CM1) were included as a study group. All patients were asymptomatic and incidentally detected by MRI for a common symptom such as headache. Also, none of the patients underwent decompression surgery. The adolescents diagnosed with CM1 had no additional medical disease, such as epilepsy. The control group consisted of thirteen volunteer adolescents with similar age and gender as the study group, had no neurological disorder and mental retardation, and had no previous psychiatric admission.
Procedure
The researcher prepared a questionnaire form to ask the children’s sociodemographic data and their parents who participated in the study. Child and adolescent psychiatrist applied the Kent EGY and Porteus Maze Test to assess the intellectual capacity, and Stroop colour and word test (SCWT) and trail making test (TMT) to evaluate the executive functions of children’s and also conducted a semi-structured psychiatric interview with children and their parents to determine whether they have any psychiatric disorder.
Parents were also asked to complete the Conners’ Parent Rating Scale-Revised Short form (CPRS-R:S) to assess their children’s attention, impulsivity, mobility, behaviour, and the Behaviour Rating Inventory of Executive Function (BRIEF) to evaluate executive functions. Child and adolescent psychiatrist also evaluated all children with a semi-structured interview to determine whether they have any psychiatric disorder. Ethical approval was obtained from the ethics committee of the Sivas Cumhuriyet University for this study. Written consent was obtained from all children and their families included in the study.
Data collection tools
Questionnaire form
Prepared by the researcher. It consists of questions about sociodemographic data such as age, sex, residence, parental educational status, and monthly income. Also, there are questions about school notes and whether the child has a medical and psychiatric illness.
The Conners’ Parent Rating Scale-Revised short form (CPRS-R:S)
The parent form of the CPRS-R consists of 27 items appropriate for use with parents of children and adolescents ages 3–17. The items are collected in three sub-scales (Oppositional-O, Cognitive Problems-Inattention-CP-I, Hyperactivity-H) and an auxiliary scale (ADHD Index-ADHD). There are four answer options for each item with a score value ranging from 0 to 3: not true at all (never, seldom), 0 points; just a little true (occasionally), 1 point; pretty much true (often, quite a lot), 2 points; very much true (very often, very frequent), 3 points. The high score indicates that the child has more problems defined in the scale [24].
Behaviour Rating Inventory of Executive Function-Parent form (BRIEF)
The BRIEF was developed by Gioia et al. [25]. It is an 86-item scale designed to evaluate children’s executive functions 5–18 years completed by parents. It is a 3-point Likert scale, and parents complete each item with a score ranging from 1 to 3 (1: ‘never’, 2: ‘sometimes’, and 3: ‘often’) by taking into account the behaviour of the child or adolescent within the last 6 months. The scale is divided into eight subscales, including working memory, inhibiting, initiating, planning/organizing, organizing materials, monitoring, emotional control, and shift. These subscales load onto the two factors of the Behavioural Regulation Index (BRI), which consists of the sum of inhibiting, shift, and emotional control, and the Metacognition index consists of the sum of the remaining scales. This inventory is useful for assessing the executive functions in children and adolescents with neurological, psychiatric, and medical conditions, such as learning disabilities and attention disorders, traumatic brain injuries, lead exposure, pervasive developmental disorders, and depression. The Turkish validity and reliability study of the scale was conducted by Batan et al. [26].
The Kent EGY and Porteus Maze Test
The Kent EGY test is applied to evaluate the verbal intelligence skills of individuals based on knowledge and language; there is no time limit and is used individually in a single session. The Porteus Maze Test is a nonverbal intelligence test developed to measure planning and adaptation to new situations and is based on performance. It also measures some executive functions such as planning ability, judgement, foresight, impulsivity, and the ability to delay gratification [27].
Stroop Test (ST)
It is a neuropsychological test developed initially by Stroop in 1935 and used to measure executive functions including selective attention, cognitive flexibility, and the ability to inhibit cognitive interference (the function of sustained attention of individual by suppressing one of the two competing stimuli) [28]. Patterns of performance on the Stroop colour and word test in children with learning, attentional, and psychiatric disabilities. In this study, we used the Tubitak Basic Sciences Research Group version of the form, which was developed by combining the Stroop test with the Victoria form by Kilic et al. [28]. This form contains four cards. The first card contains colour names printed in black on a white background. The second card contains colour names printed in a different colour than the word. The third card contains circles printed in different colours, and the fourth card contains neutral words printed in different colours. The test consists of 5 sub-sections with an increasing level of difficulty. The first two sections require reading the words on the cards, and the last three sections require naming the colours of the words or shapes. The second card is used twice for reading in the second section and for naming colours in the fifth section. During the task, subjects are asked to read four different cards as fast as possible. Thus, the completion times, the number of errors, and corrections obtained from the five sections. The fifth section is the critical section, where the disturbing effect is measured [29].
Trail making test (TMT)
TMT is a neuropsychological test that requires visual-spatial processing and motor skills. It is used to measure working memory, complex attention, and executive functions such as planning and shifting sets [30]. It consists of two parts: TMT A and B. In TMA, the patient is asked to connect circles numbered 1–25 with a solid line in ascending order, and in TMB, the patient is asked to match circles numbered 1–13 with circles labelled A-L (A-1, B-2…L-13). Part A evaluates the processing speed based on visual scanning capability, while part B evaluates the ability to change the setup and follow sequencing between stimulus sets. Completion time for part B is more prolonged than that for part A and requires more visual-spatial processing due to its complex structure. Also, part B’s difficulty level is higher than that of part A because it requires more engine speed, agility, and attention [31].
Statistical analysis
IBM SPSS Statistics for Windows version 25 (IBM Corp., Armonk, NY, USA) was used to assess clinical data. We used nonparametric tests as statistical measurements, since the small sample size of the study. Mann-Whitney U and Fisher’s exact tests were used for numeric and categorical data analyses, respectively. Spearman correlation coefficients were obtained to evaluate the association between IQ and EF tests (SCWT, TMT, and BRIEF). Data were presented as median with range or interquartile range and number (%). A p value of less than 0.05 was considered statistically significant.
Results
The Chiari group and controls were similar in terms of age, gender, parental education, family income, and school note (p>0.05) (Table 1).
The mean age of the adolescents in the Chiari group was 13.9 ± 1.8 years, and the control group was 14.2 ±1.5 years. The patient group’s mean IQ score was 94.1 ± 10.7, while this score was 104.6 ± 5.6 in the control group. The control group’s intelligence level was found to be significantly higher than the patient group (p = 0.006).
Figure 1 presented the Stroop test results, including the completion time and numbers of error and correction obtained from sections 1–5. The completion time of the Chiari group from sections 1–5 was significantly higher than that of the controls (p=0.009, p=0.005, p=0.013, p=0.003, and p=0.007, respectively). In section 5, the number of error of the Chiari group was significantly higher than that of the controls (p=0.015). In sections 1–4, completion times of the Chiari group and controls were found comparable (p=1.000, p=1.000, p=0.099, and p=0.171, respectively). In section 5, the number of correction of the Chiari group was significantly higher than that of the controls (p=0.010). In sections 1–4, the number of corrections of the Chiari group and controls were found comparable (p=0.254, p=0.099, p=0.552, and p=0.151, respectively).
Data of the Chiari group and controls of the Stroop test, including completion time and numbers of error and correction obtained from sections 1–5. Data were expressed as median with range and analysed with the Mann-Whitney test. a,b,c,d,eCompletion time of the Chiari group was higher than controls (p<0.05). fNumbers of error of the Chiari group were higher than the controls. gNumbers of correction of the Chiari group were higher than those of the controls
Figure 2 presented the trail making test results, including the completion time and numbers of error obtained from sections A1–B. The completion time of the Chiari group from section A1 was significantly higher than that of the controls (p=0.006). In sections A2 and B, completion times of the Chiari group and controls were found comparable (p=0.051 and p=0.182, respectively). The number of errors of the Chiari group and controls from sections A1, A2, and B was found similar (p=0.254, p=0.808, and p=0.947, respectively).
The data of the BRIEF-Parent Form of the Chiari and the control groups were given in Table 2. As shown in the table, there was no significant difference found between the two groups in all sub-scales of BRIEF.
Table 3 displayed the CPRS-R:S scores of the Chiari and control groups. Accordingly, the scores of the Chiari group and controls were found comparable in all four sub-scales of CPRS-R:S (p>0.05).
Table 4 exhibited the comorbid psychiatric disorders of the Chiari and control groups. No significant difference was found between the two groups in terms of psychiatric disorders (p>0.05). According to our findings, half of the Chiari group adolescents had at least one comorbid psychiatric disorder. Three of them had two psychiatric disorders concurrently.
We examined whether there is a correlation between intelligence capacity and Stroop 5th section, which has the most disruptive effect on executive functions. Accordingly, there was a mild negative correlation between IQ and the completion time (r=− 0.414; p=0.05), and with the number of errors (r=− 0.430; p=0.041), and a moderate negative correlation with the number of corrections (r=− 0.883; p=0.000). When the correlation between IQ and TMT was examined, a moderate negative correlation with the completion time of TMT-A2 (r=− 0.582; p=0.004) and a mild negative correlation with the completion time of TMT-B (r=− 0.455; p=0.029) was found. Considering the correlation between Stroop subsections and BRIEF sub-scales, a significant correlation was found between only the 5th subsection of Stroop and the metacognition and GEC sub-scales of BRIEF. Accordingly, there was a moderate positive correlation between the completion time of the 5th subsection with metacognition and GEC (r=0.518, p=0.11; and r=0.476, p=0.22, respectively). We did not find a significant correlation between IQ values with the scores of BRIEF sub-scales and CPRS-R:S sub-scales (p>0.05). Analysing the correlation between BRIEF and CPRS-R:S sub-scales, we found positive moderate correlations between BRI and CP/I scores (r=0.526, p=0.010) and ADHD index (r=0.553, p=0.006), between metacognition and CP/I scores (r=0.705, p=0.000) and ADHD index (r=0.667, p=0.001), and between GEC and CP/I scores (r=0.686, p=0.000) and ADHD index (r=0.067, p=0.001). No meaningful association was found between Stroop and TMT with CPRS-R:S scores (p> 0.05).
Discussion
In the present study, we explored the executive functions, intellectual capacity, and accompanying psychiatric disorders in adolescents diagnosed with CM1 concurrently and compared them with their healthy peers in all these aspects for the first time in the literature.
Contrary to the traditional view, over the past 20 years, the cerebellum is associated with non-motor functions such as cognition, executive function, working memory, visuospatial abilities, expressive language, and affective behaviour as well as motor functions in experimental and clinical studies [12, 32, 33]. The findings obtained from these studies indicate that the cerebellum plays an essential role in monitoring the incoming sensory information and providing the adaptation of both motor and non-motor functions to perform related behaviours [32, 33]. Schmahmann and Sherman [34] described ‘cerebellar cognitive affect syndrome’ is characterized by impaired executive functions, impaired spatial cognition, language and speech problems, and personality changes, in 20 adult patients with acquired cerebellar lesions. Thus, they were amongst the first scientists to highlight the cerebellum’s role in planning, shifting, fluency, and working memory abilities. According to them, the posterior cerebellar lobe pathologies led to more cognitive problems [16, 34]. Levinsohn et al. [35] and Riva and Giorgi [36] found deficits in executive functions, visuospatial abilities, expressive language abilities, and verbal memory in children treated after surgery for a cerebellar tumour. Based on this information about cerebellar pathologies, some authors have examined cognitive functions in patients with CM1, which occurs due to the displacement of cerebellar tonsils. In one of these few studies, ten adults diagnosed with CM1 performed significantly worse in visual-motor coordination, concentration, mental speed, attention, and visual-spatial capacity tasks than controls with age and education matched [14]. In another study, 24 adult CM1 patients who underwent decompression surgery performed significantly worse in memory and executive function tasks than controls. They performed significantly worse in response inhibition tasks, even after surgery, compared to controls [15]. Lacy et al. [11] evaluated executive functions with the BRIEF-Parent Form in 77 children diagnosed with CM1. They found that these children exhibited executive dysfunction problems, and metacognitive problems related to working memory and initiation items were the most common in these children (38%).
Contrary to the literature, we could not find a significant difference between the Chiari and the control groups regarding executive functions in our study (p>0.05). Children in both groups received similar scores from the EF tests (Stroop and TMT) and the brief scale completed by the parents. This may be due to the small number of our samples, or to the fact that our cases do not require surgery or have no additional medical problems such as epilepsy and syringomyelia; that is, they are not clinically severe cases.
To the best of our knowledge, no comprehensive studies have been conducted to illuminate the relationship between mental retardation and Chiari I malformations or determine the prevalence of MR in CM1 patients. However, in the last decade, some authors have published their observations on CM1 patients with MR, based on data they obtained from patients hospitalized in neurological centres [16, 17, 37]. Elster and Chen [16] detected MR in 12% of the patients in their series of 68 CM1 cases. Likewise, Gabriella et al. [17] detected MR in 12% of the patients in their series of 50 asymptomatic CM1 cases. Brill et al. [37] reported eleven CM1 children, who had epilepsy and developmental delays in language and motor functions. We did not find MR in any of our CM1 cases. However, the Chiari group’s IQ scores were significantly lower than the controls; we found that the IQ scores of both groups are within the normal range (90–110).
To date, psychiatric disorders in CM1 cases have been presented mostly as case reports [19,20,21]. Bakim et al. [22] examined psychiatric disorders and their effects on quality of life in adult CM cases. In this study, 18 CM1 patients who underwent decompression surgery were included in the study 1 month after surgery. They found psychiatric disorders in 43.8% of all patients and half (50.0%) of those with syringomyelia. They also found that these patients’ quality of life scale scores were found to be lower than controls. To date, only Lacy et al. [23] have examined psychiatric disorders in juvenile CM1 cases. In this large cohort study, including 86 CM1 children, they determined that 47% of the children were accompanied by at least one psychiatric disorder. In this study, ADHD (22.1%), anxiety (12.8%), and depressive disorders (10.5%) were the most reported psychiatric conditions. In this study, the patients were accompanied by medical comorbidities such as pregnancy and/or delivery complications (43%), developmental delay (32.6%), syringomyelia (16.3), seizures (15.1%), and pseudotumour cerebri (11.6%). Also, there were no control groups in their study. None of our study cases was accompanied by medical comorbidities such as epilepsy and syringomyelia or mental retardation. Besides, none of our cases has undergone decompression surgery. In our study, while determining psychiatric disorders with a semi-structured psychiatric interview, we also used the CPRS-R:S to support the diagnosis of ADHD, which is the most closely related psychiatric disorder to EF and is more common in childhood. We found no difference in psychiatric comorbidity between the Chiari group and controls. The reason for this may be the low number of our cases or the fact that the cases do not require an operation, do not accompany syringomyelia, in short, without a severe clinical condition.
Our study has some limitations and strengths. The primary limitation is the small sample size of our study. We spread our research for over 1.5 years; however, we could only detect 10 CM1 cases during this period, since it is challenging to detect CM1 in children and adolescents. Secondly, we used only the Stroop and TMT to evaluate executive functions in these children. We could give other comprehensive tests such as the continuous performance test or the Wisconsin card sorting test. We could also use the Wechsler intelligence scale for children-IV (WISC-IV) to measure adolescents’ intelligence quotient, but we did not choose it. It takes approximately one and a half hours, so individuals have difficulty completing the test.
On the other hand, our study’s main strength is that this is the first study to examine executive functions and intelligence levels in adolescents with CM1 in addition to determining the psychiatric comorbidity. Reviewing the literature, there is no psychiatric study conducted on adolescents with CM1. For this reason, we believe that our findings will make significant contributions to the literature, albeit the small sample size of the study.
Conclusion
Our main purpose of this study was to evaluate EF in adolescents with CM1. We also aimed to measure these adolescents’ intellectual capacity, detect accompanying psychiatric disorders, and compare them with healthy controls. We found the EFs of adolescents with CM to be similar with the controls. Although we found a significant difference in intellectual capacity with controls, the IQ of adolescents with CM1 was within the normal range. We found at least one comorbid psychiatric disorder in half of the Chiari group, and ADHD was the most common disease with a rate of 40%. However, we could not find a significant difference between the two groups in terms of psychiatric disorders. These findings were in contrast with the literature but need to be supported by further studies.
Consequently, our study is like a preliminary study with a small sample size. Therefore, the data we obtained from this study are not sufficient to determine the treatment modality of CM1, including the decision of surgery. For this reason, future studies investigating similar variables in large sample groups should be conducted to reach a definitive conclusion on this matter.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
None
References
Loukas M, Noordeh N, Shoja MM, Pugh J, Oakes WJ, Tubbs RS (2008) Hans Chiari (1851–1916). Childs Nerv Syst 24(3):407–409
Abbott D, Brockmeyer D, Neklason DW, Teerlink C, Cannon-Albright LA (2017) Population-based description of familial clustering of Chiari malformation type I. J Neurosurg 128:460–465. https://doi.org/10.3171/2016.9.jns161274
Hadley DM (2002) The Chiari malformations. J Neurol Neurosurg Psychiatry 72 Suppl 2(Suppl 2):ii38–ii40. https://doi.org/10.1136/jnnp.72.suppl_2.ii38
Saletti V, Esposito S, Frittoli M, Valentini LG, Chiapparini L, Bulgheroni S, Riva D (2011) Neurological pictures in paediatric Chiari I malformation. Neurol Sci 32(Suppl. 3):295–298
Cesmebasi A, Loukas M, Hogan E, Kralovic S, Tubbs RS, Cohen-Gadol AA (2015) The Chiari malformations: a review with emphasis on anatomical traits. Clin Anat 28:184–194
Wilkinson DA, Johnson K, Garton HJ, Muraszko KM, Maher CO (2017) Trends in surgical treatment of Chiari malformation type I in the United States. J Neurosurg Pediatr 19(2):208–216. https://doi.org/10.3171/2016.8.peds16273
Durham SR, Fjeld-Olenec K (2008) Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation type I in pediatric patients: a meta-analysis. J Neurosurg Pediatr 2:42–49
McVige JW, Leonardo J (2015) Neuroimaging and the clinical manifestations of Chiari malformation type I (CMI). Curr Pain Headache Rep 19(6):1–6. https://doi.org/10.1007/s11916-015-0491-2
Rogers JM, Savage G, Stoodley MA (2018) A systematic review of cognition in Chiari I malformation. Neuropsychol Rev 28(2):176–187. https://doi.org/10.1007/s11065-018-9368-6
Bernard JA, Leopold DR, Calhoun VD, Mittal VA (2015) Regional cerebellar volume and cognitive function from adolescence to late middle age. Hum Brain Mapp 36(3):1102–1120. https://doi.org/10.1002/hbm.22690
Lacy M, Ellefson SE, DeDios-Stern S, Frim DM (2016) Parent-reported executive dysfunction in children and adolescents with Chiari malformation type 1. Pediatr Neurosurg 51(5):236–243. https://doi.org/10.1159/000445899
Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, Borgatti R (2007) Disorders of cognitive and affective development in cerebellar malformations. Brain 130(Pt 10):2646–2660. https://doi.org/10.1093/brain/awm201
Novegno F, Caldarelli M, Massa A, Chieffo D, Massimi L, Pettorini B, Tamburrini G, di Rocco C (2008) The natural history of the Chiari type Ianomaly. J Neurosurg Pediatr 2(3):179–187
Kumar M, Rathore RKS, Srivastava A, Kumar Yadav S, Behari S, Kumar Gupta R (2011) Correlation of diffusion tensor imaging metrics with neurocognitive function in Chiari I malformation. World Neurosurg 76:189–194
Allen PA, Houston JR, Pollock JW, Buzzelli C, Li X, Harrington AK, Martin BA, Loth F, Lien MC, Maleki J, Luciano MG (2014) Task-specific and general cognitive effects in Chiari malformation type I. PLoS One 9:e94844
Elster AD, Chen MYM (1992) Chiari I malformation: clinical and radiologic reappraisal. Radiology 183:347–353
Gabrielli O, Coppa GV, Manzoni M, Carloni I, Kantar A, Maricotti M, Salvolini U (1998) Minor cerebral alterations observed by magnetic resonance imaging in syndromic children with mental retardation. Eur J Radiol 27:139–144
Grosso S, Scattolini R, Paolo G, Di Bartolo RM, Morgese G, Balestri P (2001) Association of Chiari I malformation, mental retardation, speech delay, and epilepsy: a specific disorder? Neurosurgery 49(5):1099–1104. https://doi.org/10.1097/00006123-200111000-000158
Caykoylu A, Deniz O, Aygul R (2001) A case of Arnold-Chiari I malformation admitted depressive symptoms (in Turkish). MJAU 33:82–85
Chisholm BT, Velamoor R, Chandarana PC, Cochrane DK (1993) Anxiety disorder in a case of Arnold – Chiari malformation. J Psychiatry Neurosci 18:67–68
Kuloglu M, Caykoylu A, Ekinci O, Albayrak Y, Deniz O (2009) Comorbid panic disorder and Chiari I malformation: a case report. New Symp 47:120–122
Bakim B, Goksan Yavuz B, Yilmaz A, Karamustafalioglu O, Akbiyik M, Yayla S, Yuce I, Alpak G, Tankaya O (2013) The quality of life and psychiatric morbidity in patients operated for Arnold-Chiari malformation type I. Int J Psychiatry Clin Pract 17(4):259–263. https://doi.org/10.3109/13651501.2013.778295
Lacy M, DeDios-Stern S, Fredrickson S, Parikh S, Nader T, Frim DM (2018) Prevalence of psychiatric diagnoses in pediatric Chiari malformation type 1. Pediatr Neurosurg 53(6):371–378. https://doi.org/10.1159/000488460
Kumar G, Steer RA (2003) Factorial validity of the Conners’ Parent Rating Scale-revised: short form with psychiatric outpatients. J Pers Assess 80(3):252–259. https://doi.org/10.1207/S15327752JPA8003_04
Gioia GA, Isquith PK, Guy SC, Kenworthy L (2000b) Behavior Rating Inventory of Executive Function. Child Neuropsychol 6:235–238
Batan SN, Oktem-Tanor O, Kalem E (2011) Reliability and validity studies of Behavioral Rating Inventory of Executive Function (BRIEF) in a Turkish normative sample. Elem Educ Online 10(3):894–904
Porteus SD (1965) Porteus maze tests: fifty years’ application. Pacific Books, Oxford [Google Scholar] [Ref list]
Golden ZL, Golden CJ (2002) Patterns of performance on the Stroop color and word test in children with learning, attentional, and psychiatric disabilities. Psychol Sch 39(489):495
Kilic B, Kockar A, Irak M, Sener S, Karakas S (2002) Standardization study Stroop Test of BSRG Form for 6-11 year old children. J Child Youth Mental Health 2:86–99
Spreen O, Strauss E (1998) A compendium of neuropsychological tests: administration, norms and commentary, 2nd edn. Oxford University Press, New York
Lezak MD, Howiesen DB, Loring DW (2004) Neuropsychological assessment, 4th edn. Oxford University Press, New York
Bower JM (2002) The organization of cerebellar cortical circuitry revisited: implications for function. Ann N Y Acad Sci 978:135–155
Schmahmann JD (2004) Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatr Clin Neurosci 16:367–378
Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain 121:561–579
Levisohn L, Cronin-Golomb A, Schmahmann JD (2000) Neuropsychological consequences of cerebellar tumor resection in children. Cerebellar cognitive affective syndrome in a paediatric population. Brain 123:1041–1050
Riva D, Giorgi C (2000) The cerebellum contributes to higher functions during development. Evidence from a series of children surgically treated for posterior fossa tumors. Brain 123:1051–1061
Brill CB, Gutierrez J, Mishkin MM (1998) Chiari I malformation: association with seizures and development disabilities. J Child Neurol 12:101–106
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was obtained from the Ethics Committee of Sivas Cumhuriyet University.
Consent to participate
Informed consent was obtained from all individual participants included in the study. Also, written informed consent was obtained from the parents.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sari, S.A., Ozum, U. The executive functions, intellectual capacity, and psychiatric disorders in adolescents with Chiari malformation type 1. Childs Nerv Syst 37, 2269–2277 (2021). https://doi.org/10.1007/s00381-021-05085-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05085-z