Abstract
Purpose
The objective of this study is to evaluate the clinical and radiological results of reverse shoulder arthroplasty (RSA) with glenoid plating in a consecutive series of patients affected by cuff tear glenohumeral arthropathy with glenoid retroversion >15°. We hypothesized that autologous humeral head graft may be better stabilized between the baseplate and the native glenoid surface with the use of a glenoid plate.
Methods
Twenty consecutive patients affected by cuff tear arthropathy with glenoid retroversion >15° (B2 or C according to Walch classification) were enrolled in this study. To reconstruct the glenoid, a dedicated plate was used in addition to the standard reverse shoulder baseplate and the glenosphere. Clinical and radiological assessment was performed using constant score (CS), subjective shoulder value (SSV), X-rays and CT scan at 6, 12 and 24 months of follow-up. Healing and resorption of the graft and detection of the glenoid version were assessed.
Results
Sixteen patients were available for final follow-up. The mean preoperative retroversion of the glenoid was 24°, while the post-op was 2° (p = 0.002). At 24 months of follow-up, mean CS and SSV were 61 and 70. Respect to preoperative scores, the results were statistically significant (p < 0.001). The last CT scan revealed: a complete healing of the graft in 100% of cases; graft resorption less than 25% in two patients (12.5%); glenoid retroversion of 4°. A negative statistically significant correlation was found between final CS and preoperative glenoid retroversion (0.039).
Conclusions
The present study reports the favorable outcomes of retroverted glenoid reconstruction with glenoid plates in RSA, an alternative method to address severe glenoid deficiency.
Level of evidence
Level IV, case series with no comparison group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glenoid bone loss is a challenging preoperative variable in shoulder arthroplasty that is often inadequately addressed with current techniques and implants. Its presence has a negative effect on any type of adopted replacement [1, 2]. Moreover, asymmetric posterior glenoid erosion in osteoarthritis with resultant retroversion presents a more difficult scenario for surgical reconstruction. It is reported that posterior erosion is due to extended subluxation of the humeral head, even if it is not clear if osteoarthritis leads to altered kinematics and subluxation or altered kinematics with subluxation leads to osteoarthritis [3]. Walch et al. proposed a classification system for glenoid morphology based on the architecture and patterns of posterior wear in glenohumeral arthritis: A1, concentric; A2, concentric and centrally eroded; B1, posteriorly subluxated; B2, posteriorly eroded and subluxated, with resulting biconcavity; and C, with retroversion >25° (hypoplastic) [3]. The posterior erosion of type B2 leads to a biconcavity, resulting in 2 articular facets. The posterior facet is called the neoglenoid and is created by erosion from the posteriorly subluxated humeral head. The anterior facet, which is the remaining articular surface of the native glenoid, is called the paleoglenoid. In contrast, in type C glenoids there may not be a significant component of posterior wear or humeral head subluxation. This classification system has been recently modified by Chan et al. [4] that described the B3 glenoid, characterized by uniconcavity, absent paleoglenoid, medialization, retroversion, and subluxation.
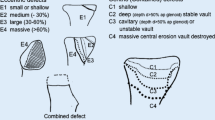
Erosion affects glenoid component fixation and contributes to failure. Several techniques are used to combat varying amounts of glenoid bone loss, including asymmetric reaming of the glenoid [5, 6], alteration of humeral component version, use of special glenoid components [7, 8], and glenoid bone grafting [7, 9, 10]. In reverse shoulder arthroplasty (RSA), complication related to the glenoid component (e.g., loosening, mechanical baseplate failure, dissociation) is reported in 4–16% of cases [11, 12], and scapular notching, that is an inferomedial impingement of the humeral insert against the pillar of the scapula during adduction and rotation of the arm responsible for bone erosion and polyethylene wear, is observed in 50–96% of postoperative radiographs [11,12,13,14]. For such reasons, Boileau et al. [13] have introduced the concept of bio-RSA, with a humeral head autologous bone grafting of the glenoid that lateralizes the center of rotation of RSA. The prosthesis is lateralized by an autogenous bone graft harvested from the humeral head placed on a specifically designed baseplate with a long central peg. Beyond the lateralization of the center of rotation, this technique has improved fixation of the glenoid baseplate, with a 0% rate of glenoid loosening and a much lower rate of scapular notching. At the same time, the attempt to restore the moderately to severely deficient glenoid by use of autologous bone grafting is technically difficult and characterized by an high rate of complications such as screw loosening and breakage, graft resorption, and early glenoid loosening [9, 15]. Currently, in RSA, glenoid grafting is mostly performed with dedicated glenoid baseplate [14, 16], with a cylinder of autologous cancellous bone graft harvested from the humeral head placed between the reamed glenoid surface and baseplate. Our hypothesis is that the autologous humeral head graft may be better stabilized between the baseplate and the native glenoid surface with the use of a pre-contoured glenoid plate. This plate may allow the surgeon to compress the graft to the glenoid bone without the risk of graft breakage and sliding. In fact, according to Scalise et al. [17] bone grafting of large glenoid defects results in high rates of graft subsidence, particularly when there is no supportive native glenoid vault. The use of the pre-contoured plates in cases of B2 or C glenoid may help the surgeon to recreate the native glenoid surface and to improve the fixation of the glenoid component (Fig. 1). The objective of this study is to evaluate the clinic and radiological results of RSA with glenoid plating in a consecutive series of patients affected by cuff tear glenohumeral arthropathy with glenoid retroversion >15° secondary or not to posterior erosion (B2 or C according to Walch classification) [3].
Drawing showing the concept of glenoid plate reconstruction in B2 and C glenoid (Walch classification) [3]
Methods
This is a prospective case-series study of 20 consecutive patients affected by cuff tear arthropathy with glenoid retroversion >15° secondary or not to posterior erosion (B2 or C according to Walch classification) [3] enrolled between January 2012 and December 2013, in a single shoulder surgery center. Surgeries were performed by a single surgeon (senior author) or under his direction.
Demographic data were collected on all the subjects. Data included age, sex, height, weight, body mass index (BMI), affected side, dominant side, comorbidities (ASA status). All the patients were non responders to at least 8 months of non-operative treatment including intraarticular steroids, NSAIDs, other local injective therapies and rehabilitation.
Inclusion criteria were:
-
Cuff tear arthropathy, with glenoid retroversion ≥15° secondary or not to posterior erosion (assessed on CT scan according to Friedman method); [18]
-
Glenoid B2 or C according to Walch classification; [3]
-
A minimum of 24 months of follow-up;
Exclusion criteria were:
-
previous shoulder replacement;
-
ASA status >3.
At the time of clinical evaluation, two researchers (two shoulder fellows) blinded evaluated shoulder function with the use of constant score (CS) [19] and subjective shoulder value (SSV) [20]. The researchers measured range of motion in the standing position using a goniometer and shoulder abduction strength by the MicroFET3 dynamometer (Hoggan Health Industries, West Jordan, UT). After a standard X-ray examination that included an anteroposterior, axillary and Y views of the shoulder, each patient was submitted to a CT scan of the shoulder (with bilateral shoulder axial images); the same two authors defined the glenoid retroversion preoperatively using the software Osirix (Pixmeo, Geneva, Switzerland) using the Friedman method [18]. In axial images, the transverse axis of the scapula was represented by a line drawn from the midpoint of the glenoid fossa to the medial end of the scapular body, with a perpendicular line considered as the neutral version. Rouleau et al. [21] presented 3 different reference lines to identify the glenoid version. In this study, we decided to use the neoglenoid line, considered as a line drawn from the anterior to the posterior margin of the glenoid. In our opinion, the use of neoglenoid line allows a better reproducibility of the measurements and is more representative of the pathologic condition.
The angle between the line of neutral version and the line connecting the anterior to posterior margin of the patient’s glenoid was measured and recorded to quantify the glenoid defect.
Surgical technique
The procedure was carried out under general anesthesia with an interscalene block in the beach chair position. Cefazolin 2 g was used as preoperative antibiotic therapy. A standard deltopectoral approach was used. If present, the subscapularis tendon was detached using a 2-cm medial to the bicipital groove tenotomy. The long head of the biceps tendon underwent simple sutures tenodesis. The procedure started with the humeral osteotomy and graft harvesting. After dislocation of the humeral head, a slight pre-cut was done to remove the remaining cartilage.
In order to define the bone landmarks of the graft, the Lima Glenoid Reconstruction Plate (Limacorporate Spa, Udine, Italy) was approached to the cancellous bone following a k-wire inserted in the center of humeral head perpendicular to the bone surface. Using a thin blade, the surgeon contoured the graft performing an hexagonal cut perpendicular to the bone surface, with thickness of the graft depending on the size of the glenoid defect (Fig. 2). After the graft was positioned into the glenoid plate, it was modeled depending on the shape of the defect using a burr. At this stage, the construct glenoid baseplate, glenoid reconstruction plate and glenoid graft were implanted on the glenoid surface, after reaming of the articular surface until cancellous bleeding bone. The fixation of the glenoid implant was achieved through the central keel and two cancellous screws to the native bone; then, the polyethylene glenosphere was applied to the metal back. The humeral steps were performed using the standard surgical technique described for implantation of Lima SMR reverse (Systema Multiplana Randelli, Udine, Italy) [22].
Operating time (min) and blood loss (ml) were recorded. Immediate postoperative CT scan was obtained in all cases, with detection of glenoid version (°) using Friedman method [18]. Patients followed a conventional postoperatory rehabilitation program, with: (a) 2 weeks of immobilization in a collar and cuff sling; (b) passive exercises until recovery of full range of motion; (c) active range of motion allowed at 4 weeks post-op. Return to full activities was allowed at 12 weeks after surgery. Clinical follow-up examination was prospectively done at 6, 12 and 24 months using CS and SSV. A CT scan was again performed at 24 months post-op to assess the healing of the graft, resorption of the graft (as suggested by Hoffelner et al. [23]: graft rate resorption <25, 25, 50, ≥75%) and detection of the glenoid version.
Complications were documented and categorized postoperatively and at follow-up visits. A major complication was considered to be any atraumatic event that involved neural injury, infection, reoperation or revision.
All patients were informed of the purpose and content of the study and signed a written consent to participate in the study, according to the Declaration of Helsinki.
Statistical analysis
Inter-tester reliability of the CT assessments (graft healing, graft resorption and glenoid version) was determined calculating intra-class correlation coefficients. Agreement was considered excellent if κ fell between 0.81 and 1.0, good if κ was between 0.61 and 0.80, moderate if κ was 0.41–0.60, fair if κ was 0.21–0.40, and poor if κ was 0.20 or less. Clinical measurement data are presented as means (with standard deviations) and were analyzed with the nonparametric Wilcoxon test. A multiple regression analysis was performed to identify potential associations between a dependent variable (final CS) and an independent variable (glenoid version). P < 0.05 was considered statistically significant.
Results
At the final 24-month follow-up, 16/20 (80%) patients were available for evaluation (study group). Of the four patients who were not available for final follow-up, one died for traumatic causes, two were not reachable and one had revision surgery for a peri-prosthetic fracture due to a fall with an hyper-stretched arm. Mean age was 75 (range 61–81, SD 5), with ten males and six females. Other demographic data are synthetized in Table 1.
Preoperative clinical evaluation of the study group revealed a mean CS of 31 (range 18–42, SD 7) and a mean SSV of 25 (range 0–40, SD 16). Inter-observer reliability of glenoid version (Friedman method) was rated as excellent (k value of 0.88); according to the senior author measurements, the mean preoperative retroversion of the glenoid was 24° (range 15°–38°, SD 7). According to Walch classification, there were 13 B2 and 3C glenoids. No intra- or perioperative complication was noted. Mean operation time was 54 min (range 42–84, SD 20), while mean blood loss was 269 ml (range 150–560, SD 66). No patient received postoperative blood transfusion. Postoperative CT scan revealed a mean glenoid retroversion of 2° (range 0°–7°, SD 2). Respect to the preoperative version, the result is statistically significant (p = 0.002). Mean discharge time from the hospital was 4.4 days (range 4–7, SD 1.05).
At the 24 months of follow-up, mean CS and SSV were 61 (range 45–70, SD 8) and 70 (range 35–90, SD 18). Respect to the preoperative scores, the results were statistically significant (p < 0.001). The scores of the intermediate follow-ups are synthetized in Table 2. At the latest CT examination, inter-observer reliability of glenoid version was rated as excellent (k value of 0.83), graft healing as excellent (k value of 0.81) and graft resorption as good (k value of 0.72). According to the senior author results, the last CT scan revealed: a complete healing of the graft in 100% of cases, with no radiolucency at the baseplate–bone interface; graft resorption less than 25% in two patients (12.5%); glenoid retroversion of 4° (0°–10°, SD 3) (Fig. 3). Respect to the postoperative glenoid retroversion, there was no significant difference (p = 0.73).
No major complication was recorded. A negative statistically significant correlation was found between final CS and preoperative glenoid retroversion (p = 0.039); no correlation was found between final CS and last follow-up glenoid retroversion (p = 0.097).
Discussion
This study addressed the clinical and radiographic results of RSA with glenoid reconstruction using humeral head autograft and glenoid reconstruction plates in a consecutive series of glenoid with a retroversion >15°. In the literature, the evidence of unsatisfactory results with glenoid erosion is high, because, if not correctly addressed, glenoid component retroversion decreases glenohumeral contact area, increases contact pressure, and may lead to eccentric loading with resultant glenoid component loosening [24, 25]. Several techniques are proposed to correct the glenoid version; in cases of severe retroversion (>15°), eccentric reaming of the anterior glenoid is limited by the amount of bone that can be removed safely without risking glenoid vault perforation [26]. Additionally, eccentric anterior reaming results in medialization and it may reduce the surface area of supportive bone where the glenoid component sits. The logic solution would be represented by an autologous graft that may correct the version of the glenoid and that should be stabilized in order to favor its healing and integration. Thanks to a modeled humeral head graft wedged between the glenoid metal back laterally, the glenoid reconstruction plate anteriorly and posteriorly and the glenoid surface medially, we have obtained a 100% of graft healing, a very low resorption (<25%) in two patients and a restored glenoid version in all cases. Respect to other studies where an autologous bone graft was used to restore the glenoid anatomy, the rate is encouraging. In particular, in a series of 24 patients who received structural bone grafting with a humeral head autograft and screw fixation, Klika et al. [15] had a large number of glenoid components radiographically at risk for loosening at a very long follow-up. They suggested that alternative treatment methods may well prove to be better to address this problem of substantial, asymmetric glenoid bone wear [15]. In patients with chronic anterior dislocation treated with bone grafting and RSA, Werner et al. [27] registered 2 cases of failure due to baseplate loosening and 1 collapse of the graft. As well as Klika et al. [15], they used screw fixation of the graft in addition to a long central peg of the glenoid baseplate, suggesting that a minimum anchorage length of 10–15 mm of the central peg in the native glenoid bone stock should be required to attain reliable baseplate stability [27]. In a different pathologic scenario of glenoid defect treated with iliac crest bone grafting, Hoffelner et al. [23] registered graft resorption less than 25% in one patient, 25% in three patients, 50% in five patients and 75% in two patients. While they noted a very high incidence of resorption, they affirmed that the implant was stable [23]. When lateralizing the center of rotation of RSA with humeral head bone graft, Boileau and colleagues showed the disk of cancellous bone graft healed to the native glenoid in 98% (41 of 42) of cases and no observed graft resorption or lysis under the baseplate [13]. We hypothesize that the very low rate of resorption we had is both due to the symmetric compression obtained with the reconstruction plate and to graft compression with the baseplate, the central keel and two cancellous screws to the native bone. Respect to the use of the humeral head graft to lateralize the center of rotation (with a not deficient glenoid surface) [13], we registered a higher percentage of resorption, probably because of the scant quantity of native bone supporting the graft.
In cases of severe glenoid deficiency, just as Levigne et al. [28] had in 6 of 34 cases of corticocancellous glenoid bone grafting for different etiologies, graft failure and resorption may be frequent, with rates up to 18% [28]. In addition, they noted a correlation between severe glenoid defects and both poorer outcome and the likelihood of glenoid failure [28]. This result reflects the negative correlation between final outcome and preoperative glenoid retroversion we have found in our study. Overall, it seems that bone grafting for glenoid deficiency in reverse shoulder arthroplasty has a favorable outcome, at least at short-term follow-up. In fact, our clinical results are comparable to those emerging from recent reports in the literature dealing with reverse shoulder arthroplasty and large glenoid bone deficiency [15, 27,28,29].
Assessment of graft resorption may be over or under estimated, as well as graft healing. We have evaluated the inter-observer reliability of both outcomes, with good and excellent results, respectively. Together with glenoid version, we believe these are the three fundamental parameters when performing glenoid reconstruction using bone grafts, both from humeral head or iliac crest.
This paper has several limitations. First, the study group is limited to only 16 cases, but these are represented by selected cases of patients with B2 or C glenoid with a retroversion ≥15° that underwent RSA with glenoid reconstruction using glenoid plates, an alternative technique that has never been reported in the literature. Second, the follow-up is limited to only 24 months, and so late radiological changes of the graft may not be noted. Third, there are some surgical difficulties due to the characteristics of the used hardware: (a) the planning and preparation of the graft is not supported by any dedicated instrumentation, and so our results may be not standardized and reproducible; (b) in small glenoids, the plate may overflow the neck of the scapula.
Conclusions
The present study reports the favorable outcomes of RSA with retroverted glenoid reconstruction using glenoid plates, an alternative method to address severe glenoid deficiency. Both clinical and radiological results at 24 months of follow-up are encouraging, even if some technical limitations relative to the glenoid implant should be improved.
References
Iannotti JP, Norris TR (2003) Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am 85:251–258
Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU (1997) Hemiarthroplasty for glenohumeral osteoarthritis: results correlated to degree of glenoid wear. J Shoulder Elbow Surg 6:449–454
Walch G, Badet R, Boulahia A, Khoury A (1999) Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 14:756–760
Chan K, Knowles NK, Chaoui J, Gauci MO, Ferreira LM, Walch G, Athwal GS (2017) Characterization of the Walch B3 glenoid in primary osteoarthritis. J Shoulder Elbow Surg 26:909–914. doi:10.1016/j.jse.2016.10.003
Gerber C, Costouros JG, Sukthankar A, Fucentese SF (2009) Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg 18:505–519. doi:10.1016/j.jse.2009.03.003
Walch G, Moraga C, Young A, Castellanos-Rosas J (2012) Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg 21:1526–1533. doi:10.1016/j.jse.2011.11.030
Neer CS 2nd, Morrison DS (1998) Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am 70:1154–1162
Radosky MW, Bigliani L (1996) Indications for glenoid resurfacing in shoulder arthroplasty. J Shoulder Elbow Surg 5:231–248
Hill JM, Norris TR (2001) Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am 83:877–883
Steinmann SP, Cofield RH (2000) Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg 9:361–367
Boileau P, Watkinson D, Hatzidakis AM, Hovorka I (2006) Neer award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, Glenoid reconstruction using glenoid plate and revision arthroplasty. J Shoulder Elbow Surg 15:527–540. doi:10.1016/j.jse.2006.01.003
Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G (2006) Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am 88:1742–1747. doi:10.2106/JBJS.E.00851
Boileau P, Moineau G, Roussanne Y, O’Shea K (2011) Bony increased-offset reversed shoulder arthroplasty. Minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res 469:2558–2567. doi:10.1007/s11999-011-1775-4
Nyffeler RW, Werner CM, Gerber C (2005) Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 14:524–528. doi:10.1016/j.jse.2004.09.010
Klika B, Wooten CW, Sperling JW, Steinmann SP, Schleck CD, Harmsen WS, Cofield RH (2014) Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg 23:1066–1072. doi:10.1016/j.jse.2013.09.017
Stephens SP, Paisley KC, Jeng J, Dutta AK, Wirth MA (2015) Shoulder arthroplasty in the presence of posterior glenoid bone loss. J Bone Joint Surg Am 97:251–259. doi:10.2106/JBJS.N.00566
Scalise JJ, Iannotti JP (2008) Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop Relat Res 466:139–145. doi:10.1007/s11999-007-0065-7
Friedman RJ, Hawthorne KB, Genez BM (1992) The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am 74:1032–1037
Constant CR, Murley AH (1987) A clinical method for functional assessment of the shoulder. Clin Orthop Relat Res 214:160–164
Gerber C, Fuchs B, Hodler J (2000) The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am 82:505–515
Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G (2010) Glenoid version: how to measure it? validity of different methods in twodimensional computed tomography scans. J Shoulder Elbow Surg 19:1230–1237. doi:10.1016/j.jse.2010.01.027
Young SW, Everts NM, Ball CM, Astley TM, Poon PC (2009) The SMR reverse shoulder prosthesis in the treatment of cuff deficient shoulder conditions. J Shoulder Elbow Surg 18:622–626. doi:10.1016/j.jse.2009.01.017
Hoffelner T, Moroder P, Auffarth A, Tauber M, Resch H (2014) Outcomes after shoulder arthroplasty revision with glenoid reconstruction and bone grafting. Int Orthop 38:775–782. doi:10.1007/s00264-013-2191-z
Farron A, Terrier A, Büchler P (2006) Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg 15:521–526. doi:10.1016/j.jse.2005.10.003
Shapiro TA, McGarry MH, Gupta R, Lee YS, Lee TQ (2007) Biomechanical effects of glenoid retroversion in total shoulder arthroplasty. J Shoulder Elbow Surg 16(3 suppl):S90–S95. doi:10.1016/j.jse.2006.07.010
Clavert P, Millett PJ, Warner JJ (2007) Glenoid resurfacing: what are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg 16:843–848. doi:10.1016/j.jse.2007.03.015
Werner BS, Böhm D, Abdelkawi A, Gohlke F (2014) Glenoid bone grafting in reverse shoulder arthroplasty for long-standing anterior shoulder dislocation. J Shoulder Elbow Surg 23:1655–1661. doi:10.1016/j.jse.2014.02.017
Lévigne C, Garret J, Grosclaude S (2010) Results of glenoid bone graft in primary reverse shoulder arthroplasty. In: Walch G, Boileau P, Mole D, Favard L, Lévigne C, Sirveaux F (eds) Shoulder concepts. The glenoid. Sauramps Medical, Montpellier, pp 403–409. ISBN 978-2-84023-676-4
Neyton L, Boileau P, Nove-Josserand L, Edwards TB, Walch G (2007) Glenoid bone grafting with a reverse design prosthesis. J Shoulder Elbow Surg 16(Suppl):S71–S78. doi:10.1016/j.jse.2006.02.002
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
IRB: The Health Director of Hopital privé La Châtaigneraie approved this study. Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Rights and permissions
About this article
Cite this article
Lanzone, R., Carbone, S., Albino, P. et al. Retroverted glenoid reconstruction using glenoid plate in reverse shoulder arthroplasty. Musculoskelet Surg 101 (Suppl 2), 121–127 (2017). https://doi.org/10.1007/s12306-017-0481-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-017-0481-0