Abstract
Background
The PERFORM™ pegged glenoid system has been used for shoulder arthroplasty since 2012. This system offers multiple backside curvatures per size to better match variable patient anatomy. As a result, less reaming is required and subchondral bone is preserved—a critical factor in preventing glenoid migration and loosening, thus enhancing implant longevity.
Purpose
The purpose of this study was to analyze all radiographic modifications around this new glenoid implant.
Method
Thirty-eight shoulders which received the PERFORM™ pegged glenoid component between June 2012 and January 2014 for primary or secondary osteoarthritis were reviewed at two-years minimum follow-up. There were 13 men and 22 women with an average age of 67 years. Humeral components were an uncemented short stem implant in nine (23%) and a resurfacing implant in 29 (77%).
Results
At 27-months average follow-up (24–41), Constant score improved from 30 to 65 points. Range of motion improved significantly at follow-up from 100° to 142° for the anterior elevation, and from 15 to 40° for the external rotation. Radiographic lucent lines (RLL) were observed post-operatively in eight cases (21%), and in 16 cases (42%) at the last follow-up with an increase of the RLL score from 0.36 ± 0.8 to 1.3 ± 2 (p < 0.001) without signs of loosening (RLL > 12). One revision has been performed after anterior shoulder dislocation, rotator cuff tear and glenoid component migration. RLL score was not correlated with dominant side, sex, age, or Constant score.
Discussion-Conclusion
The cemented pegged glenoid component with multiple backside curvatures gave satisfactory results at two-years minimum follow-up for up to three years with a low RLL score. Long-term studies are mandatory to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The common features of the arthritic glenoid are mainly an increase of the anteroposterior size because of osteophyte development and posterior wear causing glenoid retroversion. Furthermore, arthritic patients demonstrated that arthritic glenoid curvature is also much different than non-arthritic glenoid curvature, and this glenoid curvature can change from one patient to another [10].
Currently, all glenoid all-polyethylene components available are offered in one curvature, flat or convex, based upon the average non-arthritic curvature [3, 6, 12, 19, 26, 29]. Under these conditions, the glenoid bone surface must be reamed to be adapted to the implant geometry, and excessive reaming can be necessary in some cases compromising the subchondral bone thickness.
Many studies have shown that preserving the glenoid subchondral bone was of main importance to better resist to forces across the glenohumeral joint to provide long-term longevity of the glenoid component and resistance to glenoid migration [26, 27, 29].
A new all-polyethylene glenoid component design, the PERFORM™ pegged glenoid system, has been proposed recently that offers multiple backside curvatures per size to better match variable patient anatomy and preserve subchondral bone. The goal was to adapt the implant to the patient anatomy avoiding excessive reaming and preserving the subchondral bone.
Our hypothesis was that the PERFORM™ pegged glenoid component correctly matched the patient’s glenoid with no early failure and low incidence of lucent lines at two-year minimum follow-up. Our main objective was to analyze all radiographic modifications around this new glenoid implant. The second objective was to evaluate the clinical results at follow-up of the selected patients with a total shoulder arthroplasty.
Materials and methods
Patients demographics
A retrospective study was conducted in our Upper Limb Surgery Department of the University Hospital. Patients were followed prospectively, but results were evaluated retrospectively. All patients were informed about utilization of their personal data for the study and all approved.
The PERFORM™ pegged glenoid component (Wrigth-Tornier, Inc., Edina, MN, USA) has been used in our institution since 2012. This implant has a convex back, with a long central peg, and three peripheral pegs, one superiorly and two inferiorly, all for cement fixation (Fig. 1). Available sizes include five radii of curvature (30, 35, 40, 50, 60 degrees), and two sizes per radius (small/medium for 30°; small/medium for 35°; small/medium for 40°; large/extra-large for 40°; large/extra-large for 50°; and large/extra-large for 60°) or 12 components. The radial mismatch between the radius of curvature and the humeral head is variable according to the size of the humeral head and the glenoid component.
Inclusion criteria consisted of all patients that underwent total shoulder replacement with a PERFORM™ pegged glenoid component, and whose data were available at minimum two-year follow-up. Exclusion criteria consisted of all patients that underwent total shoulder replacement without a PERFORM™ pegged glenoid component, or whose data were not available at minimum two-year follow-up.
From June 2012 to January 2014, 38 total shoulder arthroplasties were performed on 35 patients with a PERFORM™ pegged glenoid component and were followed-up a minimum of two years. No patient has been lost for follow-up. Patients’ data are summarized in Table 1. A revision of a resurfacing shoulder arthroplasty because of a painful glenoid wear was performed in three cases, with removal of the resurfacing head, glenoid resurfacing with a PERFORM™ pegged glenoid component, and use of an anatomic humeral head with a short-stem prosthesis.
Surgical procedure
All patients were operated by the two senior authors (NB, PM). A delto-pectoral approach was used in all cases. A vertical tenotomy of the subscapularis was performed in 36 cases, and a tendon peeling directly on the lesser tuberosity in two. A complete circumferential capsulotomy was performed to expose the glenoid. After the humerus has been prepared for a resurfacing shoulder prosthesis or for an anatomic short-stem prosthesis, the glenoid was exposed. The glenoid curvature was then evaluated for each patient in the superior-inferior and in the anterior-posterior directions using six different radius gauges (Fig. 2). After the patient radius of curvature was identified (Fig. 3), trial glenoid implants were used to choose the adequate size. Nine different glenoid implants’ sizes were used and four different radii of curvature (Table 1). A reamer with the same size and the same radius of curvature as the trial was used to regularize the glenoid surface preserving the subchondral bone. After the different peg-holes have been performed, a trial pegged glenoid component was tested for perfect surface matching and stability. The peg-holes were cleaned with saline solution lavage with sponge drying, then low-viscosity cement with antibiotics was pressurized in the four holes with a small syringe. No cement was placed on the glenoid back-side surface. Then the definitive glenoid implant was impacted in the holes, and pressure was maintained until the cement hardened. The humerus was then gently dislocated and the humerus components were impacted without cement. The subscapularis tendon was then repaired by tendon-to-tendon sutures or with sutures through bone. The biceps tendon was tenodesed in 33 cases and tenotomised in five.
Post-operative management
Patients were protected in a sling for 45 days. Passive motion was allowed at day two after the drain has been removed. External rotation was protected until 45 days. Then active motion was started in all directions. Strengthening exercises were started at the end of the third month post-operatively. Usually six months of physiotherapy were necessary and then the patients were encouraged to make home exercises until the end of the first year after surgery.
Outcome measures
All patients were followed prospectively clinically and radiographically at three months, six months, one year, two years, and at the last follow-up. Clinical evaluation was performed using the Constant score and the Subjective Shoulder Value score (SSV). Range of motion was measured using a goniometer. Strength was analyzed with a dynamometer at 90 degrees of abduction of the shoulder, the value being the average of three measurements.
Radiographic analysis was performed for all patients pre-operatively, post-operatively, and at the last follow-up using an anterior-posterior view in neutral rotation and an axillary view. Preoperatively, CT-scan of the shoulder was systematically performed to analyse the type of glenoid wear, the trophicity of the rotator cuff muscles and the fatty infiltration index. According to Walch classification, there was concentric glenoid wear (type A) in 26 shoulders (8 A1 and 18 A2) and eccentric glenoid wear in 12 shoulders (10 B1 and 2 B2). The fatty infiltration of the four muscles of the rotator cuff averaged 1.3 (range, 1–1.6).
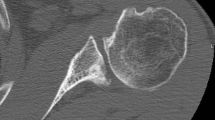
Post-operatively, specific attention was focused on the presence of radiographic lucent lines (RLL) around the glenoid component according to the six zones described around the component (Fig. 4). Using the system described by Molé et al. [13], score ranged from 0 point for no radiolucency, to 18 points for RLL exceeding 2 mm in the six zones (Table 2). Correct seating of the glenoid component on the glenoid surface was also evaluated according to the method of Lazarus et al. on a scale of A to E [9] (Table 3). Finally, the presence or absence of penetration of the pegs through the scapula cortex was also noted. A resident (FD) and a fellowship-trained shoulder surgeon (JL), who were familiar with the grading systems and who were not involved in the surgery or the post-operative care of these patients, evaluated the radiographs.
Statistical analysis
Statistical analyses were performed using SAS software version 9.3. Tests used were related to the type of variable analyzed. Variables of quantitative type were described by the average, the standard deviation, the minimum and the maximum. The Pearson test was used to evaluate the normal distribution of the values. Variables of qualitative type were described with the numbers and the percentage. Chi-square or Fisher's test was carried out to study the link between two variables of the qualitative type; the test of correlation of Pearson was used to study the link between two variables of the quantitative type; and the Student t test, the Fisher test or ANOVA to study the link between variables of a quantitative type and variable of a qualitative type. Statistical tests were considered significant at p < 0.05.
Results
Clinical evaluation
Patients were reviewed at 27-months average follow-up (range, 24–41). There were statistically significant improvements of all parameters of the Constant score as well as the Subjective Shoulder Value score (SSV). Clinical results are summarized in Table 4.
Radiographic analysis
Radiographic results are summarized in Table 4.
Radiolucent lines
Post-operatively, RLL were observed around the glenoid component in eight cases (21%). In all cases, there were of less than 1-mm thickness and very limited. Localization of the lucent lines were mainly in zone 6 in 62.5% of the cases, and in zone 3 in 50%. According to Molé [13], the average RLL score was 0.36 ± 0.8 points (0 to 3). At the last follow-up, RLL were observed around the glenoid component in 16 cases (42%). Localization were essentially in zone 3 in 75% of the cases, and in zone 6 in 56% of the cases. However, the RLL score was only 1.3 ± 2 (0 to 10) (p < 0.001). In one case, RLL score reached 10, but with an asymptomatic patient. Radiographic results are summarized in Table 3 (Fig. 5).
Glenoid seating
According to the Lazarus grading system, 29 components were staged grade A, two grade B, five grade C, and two grade D. There was no grade E in our study. No correlation could be found between glenoid seating and aetiology, pre-operative range of motion, type of glenoid wear, and type of humeral prosthesis used. However, it was correlated with the post-operative RLL score (p < 0.05) (Fig. 6).
Pegs penetration
In seven cases (18%), penetration of pegs through the posterior scapula cortex was noted on the axillary view. Occurrence of pegs penetration was correlated with the degree of pre-operative glenoid retroversion and the type of glenoid wear, with more posterior penetration for type B glenoid (71%) versus type A glenoid (29%) (p < 0.05).
Complications and revisions
Only one complication and one revision was observed in the same patient with an anatomic total shoulder arthroplasty and a stem implant. After a fall, she dislocated her shoulder, with glenoid component migration and tear of the subscapularis tendon. A revision was performed with conversion of the anatomic prosthesis into a reverse total shoulder arthroplasty.
Statistical analysis
No correlation was found between the increased number of RLL around the glenoid component at the last follow-up and the age of the patient, sex, dominant side, etiology, preoperative and postoperative Constant score, type of glenoid wear, type of humeral prosthesis, nor the presence of immediate RLL post-operatively.
Discussion
The PERFORM™ pegged glenoid component has matched correctly the patient’s glenoid by using four out of six different radius gauges and nine different glenoid implants’ sizes. No early failures were noted in our study, except in one patient because of anterior shoulder dislocation and glenoid component mobilization. However, some RLL were present immediately after surgery relating to the quality of glenoid surface preparation and to the cement technique. If the number of lucent lines increased slightly with follow-up, they were always very limited, with an RLL score always below 6, except in one patient whose score was 10.
The first all-polyethylene glenoid component was introduced by Charles Neer. It was a cemented keeled component rectangular, with conforming radius of curvature between the humeral head and the glenoid. The backside design was curved and convex. The survival of the original Neer glenoid component has been reported to be 83–93% at ten years, and to 73–87% at 15 years [5, 23].
Longevity of glenoid components depends mainly on bone preparation and glenoid component seating. To get a perfect glenoid surface matching of the glenoid implant, reamers are usually used. However, most modern reamers have a unique radius of curvature. The bone must be adapted to the prosthesis and sometimes efforts to achieve complete seating of the glenoid component necessitate removal of increased amounts of subchondral bone. In our series, there were 26 concentric and 12 nonconcentric cases of glenoid wear. Using four different radius gauges and nine different glenoid implants’ sizes, 29 out of 38 (76%) glenoid components were perfectly seated. However, no correlation could be found between glenoid seating and the type of the glenoid wear or the degree of glenoid retroversion.
Young et al. [29] have reported the long-term clinical and radiological outcomes of anatomic total shoulder replacements with a cemented all-polyethylene flat-back keeled glenoid component. Only two glenoid reamers were used to provide a flat surface for the glenoid component. The survivorship with radiological loosening as the end point using the Molé classification was 99.1% at five years, 80.3% at ten years, and 33.6% at 15 years. The mean glenoid RLL score was 11.3 ± 6.4 at 124 months follow-up meaning that glenoid bone was not sufficient to support glenoid component fixation with follow-up. The same results were published by Walch et al. [26] using a convex-back cemented keeled glenoid component with the survivorship with radiological loosening as the end point of 99.7% at five years and 51.5% at ten years. The authors outlined that the increased rates of glenoid component migration and radiologic loosening were related to the excessive use of a convex reamer. These results were confirmed by Walch et al. [27] who recommended preserving subchondral bone for long-term longevity of the glenoid component. This was the goal of the PERFORM™ glenoid component with various types of backside radius of curvatures adapted to the arthritic glenoid that may avoid excessive reaming and bone sacrifice by adapting the prosthesis to the bone.
Although it seems that osseous support is very important, the causes of glenoid component failure in total shoulder arthroplasty are multifactorial. The favourable effect of mismatch between the radius of curvature of the glenoid component surface and the humeral head has been outlined by various authors [17, 21, 25]. Several studies have also shown that using “modern cementing techniques” can decrease the rate of RLL around the component and increase the longevity of the glenoid component [1, 2, 4, 8, 14, 18, 28, 29]. The necessity to have cement present between the components and the glenoid face has been debatable. From the literature, it seems preferable to restrict cement to the keel slot or peg holes, even if a 1-mm cement layer will overflow from the slot or the holes [7]. Terrier et al. [20] demonstrated that an excessively thick cement mantle increased the rigidity of the cemented component causing increased interfacial stresses and micromotions. Risk of fatigue failure of cement between the component backside and the glenoid can induce instability of the component, lucent lines and loosening with follow-up [3]. In our series, cement was only injected into the peg holes, to get perfect contact between the glenoid component and the subchondral bone, and to minimize the cement interface between glenoid component and bone. However, minimal cement overflow from the holes could explain some immediate RLL behind the PERFORM™ glenoid pegged component.
Biomechanical studies have shown no difference of force transmission to the component–cement–bone interfaces between pegs and keel glenoid components [11, 16]. However, short to medium follow-up clinical and radiographic studies have shown that RLL at the cement–bone interface and incomplete component seating occurred more frequently with keeled components versus pegged components [4, 9, 24]. Cemented all-polyethylene keeled or in-line three-pegged glenoid components appear to have similar stability during the first two years after surgery [15, 22]. In 2017, McLendon et al. [12] found that at 7.2 years average follow-up, the rate of the Cofield II all-polyethylene in-line three-pegged component survival free from revision was of 99% at five years and 83% at ten years. Component survival rates free from radiographic failure at five and ten years were 92% and 43%.
This is the first study that reported the results of the pegged PERFORM™ glenoid component to validate the reliability of this component at more than two years follow-up. The radiologic reviewer was blinded to the patients’ clinical outcomes, and two surgeons participated in clinical examination and collection of outcome data as well as radiographic evaluation. However, the number of patients were limited, and follow-up was short limiting the power of the statistical analysis and the scope of the conclusion. Furthermore, there were different etiologies that could induce a bias in the analysis of RLL. The humeral head varied between a resurfacing shoulder prosthesis or an anatomic short-stem prosthesis that could influence glenoid component positioning. Finally, evaluation of the glenoid component was only performed using radiographic evaluation that was less accurate than CT evaluation.
Conclusion
The use of the pegged PERFORM™ glenoid components has produced encouraging results. At short to medium-term follow-up, all the components were stable, with a low RLL score and with only one revision due shoulder dislocation and glenoid component mobilization. Long-term follow-up of the same series of patients is mandatory to confirm the efficiency of this component.
References
Barwood S, Setter KJ, Blaine TA, Bigliani LU (2008) The incidence of early radiolucencies about a pegged glenoid component using cement pressurization. J Shoulder Elb Surg 17:703–708
Choi T, Horodyski M, Struk AM, Sahajpal DT, Wright TW (2013) Incidence of early radiolucent lines after glenoid component insertion for total shoulder arthroplasty: a radiographic study comparing pressurized and unpressurized cementing techniques. J Shoulder Elb Surg 22:403–408
Collin P, Tay AK, Melis B, Boileau P, Walch G (2011) A ten-year radiologic comparison of two-all polyethylene glenoid component designs: a prospective trial. J Shoulder Elb Surg 20:1217–1223
Edwards TB, Labriola JE, Stanley RJ, O'Connor DP, Elkousy HA, Gartsman GM (2010) Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elb Surg 19:251–257
Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH (2009) Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elb Surg 18:859–863
Fox TJ, Foruria AM, Klika BJ, Sperling JW, Schleck CD, Cofield RH (2013) Radiographic survival in total shoulder arthroplasty. J Shoulder Elb Surg 22:1221–1227
Kasten P, Pape G, Raiss P, Bruckner T, Rickert M, Zeifang F, Loew M (2010) Mid-term survivorship analysis of a shoulder replacement with a keeled glenoid and a modern cementing technique. J Bone Joint Surg (Br) 92:387–392
Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL (2005) Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res 435:118–125
Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd (2002) The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am 84-A:1174–1182
Mansat P, Bonnevialle N (2013) Morphology of the normal and arthritic glenoid. Eur J Orthop Surg Traumatol 23:287–299
Mansat P, Briot J, Mansat M, Swider P (2007) Evaluation of the glenoid implant survival using a biomechanical finite element analysis: influence of the implant design, bone properties, and loading location. J Shoulder Elb Surg 16(3 Suppl):S79–S83
McLendon PB, Schoch BS, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH (2017) Survival of the pegged glenoid component in shoulder arthroplasty: part II. J Shoulder Elb Surg 26:1469–1476
Molé D, Roche O, Riand N, Levigne C, Walch G (1999) Cemented glenoid component: results in osteoarthritis and rheumatoid arthritis. In: Walch G, Boileau P (eds) Shoulder arthroplasty. Springer, New York, pp 163–171
Nyffeler RW, Meyer D, Sheikh R, Koller BJ, Gerber C (2006) The effect of cementing technique on structural fixation of pegged glenoid components in total shoulder arthroplasty. J Shoulder Elb Surg 15:106–111
Rahme H, Mattsson P, Wikblad L, Nowak J, Larsson S (2009) Stability of cemented in-line pegged glenoid compared with keeled glenoid components in total shoulder arthroplasty. J Bone Joint Surg 91A:1965–1972
Roche C, Angibaud L, Flurin PH, Wright T, Zuckerman J (2006) Glenoid loosening in response to dynamic multi-axis eccentric loading. A comparison between keeled and pegged designs with an equivalent radial mismatch. Bull Hosp J Dis 63:88–92
Sabesan VJ, Ackerman J, Sharma V, Baker KC, Kurdziel MD, Wiater JM (2015) Glenohumeral mismatch affects micromotion of cemented glenoid components in total shoulder arthroplasty. J Shoulder Elb Surg 24:814–822
Szabo I, Buscayret F, Edwards TB, Nemoz C, O'Connor DP, Boileau P, Walch G (2005) Radiographic comparison of two glenoid preparation techniques in total shoulder arthroplasty. Clin Orthop Relat Res 431:104–110
Szabo I, Buscayret F, Edwards TB, Nemoz C, Boileau P, Walch G (2005) Radiographic comparison of flat-back and convex-back glenoid components in total shoulder arthroplasty. J Shoulder Elb Surg 14:636–642
Terrier A, Büchler P, Farron A (2005) Bone-cement interface of the glenoid component: stress analysis for varying cement thickness. Clin Biomech 20:710–717
Terrier A, Büchler P, Farron A (2006) Influence of glenohumeral conformity on glenoid stresses after total shoulder arthroplasty. J Shoulder Elb Surg 15:515–520
Throckmorton TW, Zarkadas PC, Sperling JW, Cofield RH (2010) Pegged versus keeled glenoid components in total shoulder arthroplasty. J Shoulder Elb Surg 19:726–733
Torchia ME, Cofield RH, Settergren CR (1997) Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elb Surg 6:495–505
Vavken P, Sadoghi P, von Keudell A, Rosso C, Valderrabano V, Müller AM (2013) Rates of radiolucency and loosening after total shoulder arthroplasty with pegged or keeled glenoid components. J Bone Joint Surg Am 95:215–221
Walch G, Edwards TB, Boulahia A, Boileau P, Mole D, Adeleine P (2002) The influence of glenohumeral prosthetic mismatch on glenoid radiolucent lines. Results of a multicenter study. J Bone Joint Surg Am 84:2186–2191
Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P (2011) Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elb Surg 20:385–394
Walch G, Young AA, Boileau P, Loew M, Gazielly D, Molé D (2012) Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis. J Bone Joint Surg Am 94:145–150
Young AA, Walch G (2010) Fixation of the glenoid component in total shoulder arthroplasty: what is “modern cementing technique?”. J Shoulder Elb Surg 19:1129–1136
Young AA, Walch G, Boileau P, Favard L, Gohlke F, Loew M, Molé D (2011) A multicentre study of the long-term results of using a flat-back polyethylene glenoid component in shoulder replacement for primary osteoarthritis. J Bone Joint Surg (Br) 93:210–216
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The implant manufacturer provided funding for the collection and entry of data.
Rights and permissions
About this article
Cite this article
Dauzère, F., Arboucalot, M., Lebon, J. et al. Evaluation of thirty eight cemented pegged glenoid components with variable backside curvature: two-year minimum follow-up. International Orthopaedics (SICOT) 41, 2353–2360 (2017). https://doi.org/10.1007/s00264-017-3635-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3635-7