Abstract
Coleus forskohlii, an Indian-origin medicinal plant is the sole natural source of the labdane terpenoid forskolin (C22H34O7), with growing demand. Forskolin emerged as an industrially important bioactive compound, with many therapeutic applications in human health. It has established potential effects in the treatment of various diseases and conditions such as glaucoma, asthma, obesity, allergies, skin conditions and cardiovascular diseases. Moreover, clinical trials against different types of cancers are progressing. The mechanism of action of forskolin mainly involves activating adenylyl cyclase and elevating cAMP, thereby regulating different cellular processes. For the extraction of forskolin, tuberous roots of C. forskohlii are used as they contain the highest concentration of this metabolite. Approximately 2500 tonnes of the plant are cultivated annually to produce a yield of 2000–2200 kg ha−1 of dry tubers. The forskolin content of the root is distributed in the range of 0.01–1%, which cannot meet the increasing commercial demands from industries such as pharmaceuticals, cosmetics, dietary supplements, food and beverages. Hence, various aspects of micropropagation with different culture methods that employ precursors or elicitors to improve the forskolin content have been explored. Different extraction and analytical methods are also introduced to examine the yield and purity of forskolin. This review discusses the significance, clinical importance, mechanism of action and different approaches used for mass production including tissue culture for the lead compound forskolin to meet market needs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coleus forskohlii Briq. (Syn. Plectranthus barbatus Andr.), a plant of Indian origin belonging to the mint family (Lamiaceae) has been used in traditional and modern medicines. Moreover, C. forskohlii (Fig. 1a) is the only known source of the active compound, forskolin with high demand. Forskolin [(3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-ethenyl-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-5,6,6a,8,9,10-hexahydro-2H-benzo[f]chromen-5-yl] acetate is a labdane diterpene with a molecular weight of 410.5 g mol−1. The molecule consists of tetrahydropyran ring with five oxidized positions and eight chiral centres (Fig. 1b). The plant contains a wide variety of phytoconstituents such as deactylforskolin, 9-deoxyforskolin, 1, 9-deoxyforskolin, forskoditerpenoside C, D, and E, labdane diterpene glycosides, labdane diterpene forskoditerpene A, and 1, 9-dideoxy-7-deacetylforskolin which are reported from the root extract. (Ammon and Kemper 1982; De Souza and Shah 1988). In addition, higher contents of polyphenols, flavones and flavonols, and antioxidants with free radical scavenging activity, contribute to its wide medicinal properties (Rasineni et al. 2008).
a Coleus forskohlii (Image courtesy: Dinesh Valke 2018). b Decalin core structure of forskolin (ref: Wikimedia commons. https://commons.wikimedia.org/wiki/File:Forskolin.svg)
In India, about 2500 tonnes of C. forskohlii is cultivated annually for the active ingredient forskollin (Pullaiah 2022a). The plant is cultivated through root cuttings or stem cuttings that are planted during the monsoon season (Paul et al. 2013). The crop is harvested after 4 to 5 months and the tuber yield ranges from 2000 to 2200 kg ha−1 (Rajamani and Vadivel 2009; Singh et al. 2011). The geographic and climatic factors affect the growth of the plant and the yield of forskolin as the differences in altitude, temperature, light intensity, etc., influence the yield (Rana et al. 2022). Although traces of forskolin are found all through the plant body, higher forskolin content is in roots (0.01to 1%) and stem (0.103%) (Saleem et al. 2005; Shukla et al. 2016; Srivastava et al. 2017).
The global forskolin market was valued at USD 457.18 million in 2021and is expected to grow by a compound annual growth rate (CAGR) of 8.7% from 2022 to 2030 owing to its multiple emerging applications (Grand View Research, Market Research Database). Forskolin is widely used in pharmaceuticals, health care products, skin care products, and food and beverages. The pharma industry faces challenges due to the variation in forskolin content in the cultivated plant and fails to satisfy the requirements (Pullaiah 2022b). Thus, an improved strategy of cultivation practices should be developed for the consistent yield of forskolin to meet the market demand.
Challenges in crop cultivation and disease management
C. forskohlii has emerged as an important medicinal cash crop and is broadly cultivated with utmost care to meet the growing demand for forskolin in international trade. The cultivation faces challenges due to the frequent infections by pathogens and parasites. This results in the reduction of growth and yield of root tubers leading to serious loss of crop affecting the commercial scale cultivation. The use of pesticides can leave residual amounts in the raw materials that may affect the quality and trade. Thus, sustainable management of diseases and pathogens is imperative in commercial cultivation.
Root rot or wilt disease, leaf spot disease, root knot disease and other fungal infections are some of the common diseases that affect the growth and yield of C. forskohlii. Root rot or wilt disease is a common soil-borne disease that is caused by fungi species such as Fusarium chlamydosporum, Macrophomina phaseolina and bacterium species such as Ralstonia solanacearum. This is a serious concern in tuber yield and a major threat to the cultivation of C. forskohlii (Shyla 1998; Kamalakannan et al. 2006; Thiribhuvanamala et al. 2020) as it causes a heavy loss (> 50%) of crop yield in South India. Fusarium wilt caused by F. oxysporum can result in plant death (Miao et al. 2021). Corynespora cassiicola and Botryodiplodia theobromae are reported to cause leaf spot disease (Fernandes and Barreto 2003; Ramprasad 2005Lokesh et al. 2018) whereas, Rhizoctonia solani and Phytophthora nicotianae var. nicotianae cause Blight disease in C. forskohlii (Singh et al. 2011). Root tubers are highly prone to the infestation of soil-inhabiting nematodes Meloidogyne species that causes root knot disease. And disease management using neem cake showed better activity in reducing nematode population (28.6–31.2%) with increased yield of root tuber (Seenivasan 2010). Biological control agents such as Trichoderma viride, Paecilomyces lilacinus, Glomus fasciculatum and neem oil seed cake treatment reduce the disease with an increase in the plant growth and yield of forskolin content (Goswami et al. 2012). Bacterial endophyte, Alcaligenes faecalis reduce the severity of nematode infection and root knot by 81% and 78% in C. forskohlii respectively and enhance the production of forskolin (Mastan et al. 2020).
The forskolin yield is also impacted by the abiotic stress that influences the habitat and thereby deteriorates the crop. Macronutrients such as nitrogen, phosphorus, potassium and calcium along with soil nutrients are required for the cultivation of C. forskohlii. Poor soil fertility, inefficient drainage, and soil texture are reported to reduce the yield of C. forskohlii (Balasubramanian et al. 2020). The growth and physiology are also affected by the salinity stress by reducing the photosynthetic effect, water status and increased electrolyte leakage (Kotagiri and Kolluru 2017). A better understanding of edaphic factors that favor cultivation and crop yield needs to be explored to improve forskolin production sustainably.
In vitro propagation of C. forskohlii
Forskolin production is mainly from the wild and cultivated C. forskohlii which results in major exploitation of plants. To prevent this, an alternative approach to plant regeneration is essential to sustain the availability of the plant metabolite. Vegetative cuttings and seeds are traditionally used to propagate C. forskohlii and these processes are time-consuming. Moreover, seeds with low germinating rates fail to produce a homogenous population bringing about changes in metabolite production. Plant tissue culture has the potential for consistent and higher yields of forskolin as it can be manipulated by altering the culture conditions (Chandran et al. 2020). Micropropagation involves (i) initiation of aseptic culture, (ii) shoot multiplication, (iii) rooting of in vitro raised shoots, (iv) hardening and field transfer (Nagpal et al. 2008). Explants like leaf, nodal segment and shoot tip are employed to achieve the regeneration of C. forskohlii (Rajasekharan et al. 2010; Sreedevi et al. 2013; Chandra et al. 2019). Shoot apex and internodal stem have a higher proportion of meristematic tissues and are more effective in callus culture compared to the leaves. Shoots showed higher callus (12 folds) biomass yield in every six weeks with nodal segment explants (Sivakumar et al. 2021). The shoot apex can serve as the best explant for the highest frequency of root per elongated shoot than the internodal segment (Chandra et al. 2019). In addition, stems have a higher proportion of meristematic tissues and are more effective in callus culture compared to the leaves. The apical shoot tip explant in Murashige and Skoog medium (MS) with growth regulators is found to be more robust compared to the nodal segment for shooting and rooting (Sharan et al. 2014). However, leaf-derived callus also generated high-frequency shoot organogenesis in C. forskohlii (Sairam Reddy et al. 2001; Krishna et al. 2010). Hence, it is explicated that explants such as leaf, nodal segment and shoot tip are desirable for the organogenesis and culturing of C. forskohlii.
Micropropagation methods for forskolin production
Forskolin production mainly depends on biomass yield and secondary metabolite synthesis which can be accomplished by different culture methods as detailed in the following sections.
Callus culture
Callus culture has gained popularity as it is a faster approach for the production of bioactive metabolites (Benjamin et al. 2019; Efferth 2019). Callus is a proliferating mass of undifferentiated cells derived from different explants and it grows under optimum conditions like light, temperature, humidity, and nutrients. Maximum callus growth and secondary metabolite production are indispensable to produce commercially important plant metabolites. The balanced concentrations of auxin and cytokinin facilitate callus induction to form a friable and compact callus. The yield parameters such as fresh weight, dry weight, and growth index are employed for the evaluation of the callus culture. Sustainable and large-scale production of the metabolite can be achieved by callus culture without the need to sacrifice the entire plant. It can also offer an advantage in extraction and is free from soil contaminants such as microplastics, pesticides and heavy metals. In vitro callus culture of C. forskohlii was established from shoot tip, root tip, and hypocotyl segments and forskolin was identified from shoot differentiating culture and micropropagated plants (Sen et al. 1992). The callus from the root is slow-growing compared to the leaf and stem. Moreover, forskolin content is higher in the callus from stem than in leaf callus. The callus-derived forskolin is present in the range of 0.002–0.01% throughout the roots, stems, and leaves (Malathy and Pai 1999). Studies have also shown that the root callus with each 0.5 ppm IAA and IBA, 5 ppm glycine, 200 ppm casein hydrolysate and 7% sucrose can produce 0.08% (w/v) forskolin (Tripathi et al. 1995).
Cell suspension culture
Cell suspension culture is one of the potential in vitro tissue culture model systems to produce highly valuable therapeutic compounds in less time and with higher yields. In this technique, callus is transferred into the liquid medium with growth hormones for suspension culture in a shake flask or a suitable bioreactor. Friable callus usually grows faster in media provided with nutrients under agitation and ensures the production of metabolite with uniform quality and yield. The suspension culture of C. forskohlii was studied by Mersinger et al. (1988) where it required phytohormones such as, 2, 4-D and KIN for the growth and IBA instead of 2, 4-D to induce forskolin production. Carbon source, initial cell density and light or dark conditions have an impact on the transformed cell suspension culture of C. forskohlii. It is reported that maximum forskolin production is at the stationary phase of cell growth (Mukherjee et al. 2000). Suspension culture of C. forskohlii with fungal and bacterial elicitors are used to improve the growth and forskolin content of plant cells (Swaroopa et al. 2013a, 2013b).
Hairy root culture
Genetic transformation of Agrobacterium rhizogenes results in the production of hairy roots which is an attractive strategy for secondary metabolite production (Abraham and Thomas 2017; Rawat et al. 2019). It is considered as a hormone-independent approach. The hairy root was induced in C. forskohlii by infection with A. rhizogenes for the forskolin production and the highest yield was 1.6 mg 100 mL−1 flask after five weeks of incubation (Sasaki et al. 1998). The hairy roots generally grow denser and more rapidly compared to the callus culture. Hairy root culture established from the nodal stem part of C. forskohlii can also be used to produce forskolin (Rajiv 2014). Effect of an elicitor, MeJA (500 µM) and precursor L-phenylalanine (1mM) on hairy root culture enhanced the production of forskolin by 2.7 folds (2.8 mg g−1) after two weeks of culture compared to control (Reddy et al. 2012).
Role of growth regulators in the plant tissue culture
MS medium is the most frequently used medium in plant tissue culture (Murashige and Skoog 1962) and growth hormone such as, auxin, cytokinin, gibberellin, abscisic acid, ethylene, and certain plant regulators with similar metabolic effects mediates the growth of different explants on the media (Khan et al. 2019, Koo et al. 2020). In vitro culture of C. forskohlii is mainly promoted in the presence of auxin and cytokinin. Different combinations of cytokinin such as 6-BAP, 0.25 mg L−1 and KIN, 0.25 mg L−1 generated shoot proliferation in the MS medium callus culture (Sreedevi and Pullaiah 2014). 2 mg L−1 of 6-BAP activated callus induction and shoot multiplication from shoot tip explant (Vibhuti and Kumar 2019). MS medium fortified with 4.44 μM 6-BAP produced the highest number of shoots from the nodal explant in 30 days of culture (Janarthanam and Sumathi 2020) and 2 mg L−1 produced multiple shoots in nodal segments as well (Mahmoud et al. 2019). Auxin induces callus in the order 2, 4-D) > IAA > IBA in the culturing of C. forskohlii and the maximum number of shoots were developed from the callus of shoot tip explants (Praveena et al. 2021). The amalgamation of 6-BAP, 2 mg L−1 and NAA, 1 mg L−1 produced the maximum percentage of shoot regeneration from leaf explant culture whereas, IAA (1.0 mg L−1) was felicitous for rooting in in vitro culture during micropropagation (Sharma et al.1991; Sreedevi et al. 2013; Sivakumar et al. 2021).
Growth regulator favors secondary metabolite accumulation
As discussed, growth regulators have an impact on biomass accumulation and secondary metabolite synthesis. The combined or separate and balanced ratio of the growth regulator favor callus induction and secondary metabolite accumulation. Different combinations of cytokinin and auxin in the media enhance the metabolite yield. In C. forskohlii, a lower concentration of auxin triggers callogenesis and a higher concentration induces rhizogenic callus. Moreover, forskolin synthesis is differentiation dependent and exhibited in rhizogenic callus in quantifiable amounts whereas, in trace amounts in cell cultures (Balasubramanya et al. 2012).
Efficacy of Meta-Topolin in organogenesis and secondary metabolite synthesis
Meta-topolin [6-(3-hydroxy benzylamino) purine] (mT) is a recently discovered lesser-known cytokinin, isolated from the poplar leaves coming to use in in vitro propagation of plants. mT, a natural aromatic cytokinin has the ability to induce callus regeneration in combination with auxin. The recent investigation on mT was found to be optimal when compared to other cytokinins and in combination with growth regulators for shoot/root regeneration and organogenesis (Erişen et al. 2020). Hence, it is more effective in in vitro morphogenesis and can be a replacement for benzyladenine. In C. forskohlii, mT (2 mg L−1) when augmented with MS medium, induced shoot regeneration from leaf explant and was also found superior to 6-BAP (Badhepuri et al. 2023). Also, mT is a promising candidate that can manipulate the synthesis of secondary metabolite production (Turkyilmaz 2021; Chahal et al. 2022). An increase in biomass accumulation with the addition of mT resulted in the increment of secondary metabolite after four weeks of culturing. As it is proven as the best cytokinin, the effect of mT can be investigated in forskolin production as well.
Role of elicitors to enhance forskolin synthesis
As discussed, the forskolin content in roots is generally very low and therefore, promoting forskolin biosynthesis is pivotal in increasing the yield and consistency in the recovery. Elicitation is the most widely used effective technique to induce secondary metabolite synthesis in plants (Thakur et al. 2019). An elicitor binds with a specific elicitor receptor in the plant cell membrane and activates the signal transduction pathway resulting in the secondary metabolite production. The production of ROS under stressed conditions directly or indirectly boosts the accumulation of secondary metabolite (e.g. phenolic compounds) by triggering defence mechanism (Hunyadi 2019). Two types of elicitors are generally used namely, biotic and abiotic elicitors. Biotic elicitors are derived from living sources which include microorganisms, (cell extract of yeast, bacteria and fungi) polysaccharides originated from plant or animal cell walls like chitin, lignin, pectin and cellulose. The abiotic elicitors are derived from non-living sources which include physical (water, salinity, light, temperature, drought, UV irradiation) chemical (heavy metals) and hormonal factors (plant hormones such as SA and JA) (Chamkhi et al. 2021; Nikalje et al. 2021). The efficacy of elicitation depends upon various parameters such as concentration, duration of exposure, type of explant, stages at which elicitor is introduced, media and growth regulators used and culture conditions followed during treatment (Halder et al. 2019). The procedure involves the initial growing of cells under optimum conditions for biomass formation and transferring to the media with an elicitor that induces secondary metabolite production.
Though bacterial, fungal and yeast extracts are used as elicitors in plant tissue culture, the most promising are the fungal elicitors. Various types of endophytes present in the plants have the potential to enhance the production of forskolin (Table 1). The plant probiotic fungus Piriformospora indica influenced the growth and development of C. forskohlii under field conditions (Das et al. 2012; Tarte et al. 2022). Apart from P. indica, the fungal endophytes such as F. redolens, Phialemoniopsis cornearis, and Macrophomina pseudophaseolina are also used for the growth and enhanced production of forskolin (Pullaiah 2022c). They can have a direct role in the biosynthesis of forskolin or can promote the root biomass, thereby increasing the overall yield of forskolin.
Precursor acts as a substrate in the biosynthesis of bioactive molecules. L-phenylalanine, a precursor along with MeJA improved the production of forskolin in C. forskohlii (Reddy et al. 2012). Therefore, various biosynthetic intermediates can be investigated for the induction of forskolin synthesis. NaCl, CS, SA, JA and MeJA are broadly used for terpenoid production in the cell culture (Xiang et al. 2015; Sinha et al.2018; Assaf et al. 2022; Tilkat et al. 2023). NaCl causes cellular dehydration that induces ionic and osmotic pressure resulting in an increase in secondary metabolite production. CS is a deacetylated form of chitin that can stimulate defence-related secondary metabolite accumulation (Chakraborty et al. 2009). The production rate of terpenoids changes in response to CS treatment after callus induction in cell suspension culture (Bavi et al. 2022). SA (phenolic phytohormone), JA and MeJA are well-known plant-specific endogenous signaling phytohormone elicitors and show great potential for secondary metabolite synthesis in tissue culture. They act as internal messenger molecules and stimulate the biosynthesis of bioactive molecules. Hence NaCl, CS, SA, JA and MeJA can be used to promote forskolin synthesis by optimizing their doses and exposure time in different cell culture methods.
Nano-elicitation is an emerging technology that has been used to trigger the synthesis of bioactive compounds such as phenolic, flavonoid, terpenoid etc., (Javed et al. 2022). Nano-elicitation by metal, metal oxide and carbon-based metal nanoparticles is used for improving the secondary metabolite in medicinal plants, both in vitro and in vivo (Lala 2021; Inam et al. 2023). Metals such as gold, silver, copper or metal oxide NPs, copper oxide, zinc oxide, titanium dioxide etc., are mostly explored as elicitors in combinations or alone (Fazal et al. 2016; Ghazal et al. 2018; Fatima et al. 2020; Shoja et al. 2022). However, there are no reports on the usage of nano-elicitors for the synthesis of forskolin.
Molecular mechanism of elicitation
The multiple component responses in the signal transduction network result in secondary metabolite production. The elicitor signal transduction pathways vary with different elicitor signals as they are specific to the type of elicitor to which plant cells are exposed. Multiple elicitors have been used and a synergic effect on secondary metabolite production was achieved (Halder et al. 2019). Signal perception is the initial step in the signaling pathway. The ion channels, G-protein and protein kinase get activated after the perception of elicitor signal. The elicitor recognizes and binds to the specific receptors on the plasma membrane through the elicitor binding site. This elicitor-plant cell interaction activates the phosphorylation of mitogen activated protein kinase (MAPK), calcium signaling that leads to K+ and Cl− efflux, an influx of H+ and Ca2+, cytoplasm acidification and ROS, mainly superoxide anion and hydrogen peroxide (Ramirez-Estrada et al. 2016; Halder et al. 2019; Bajwa et al. 2021). This further activates the transcription factors that regulate the expression of the defence gene that encodes enzymes responsible for secondary metabolite accumulation (Fig. 2). Calcium signaling has a critical role in physiology response to external stimuli that target cellular processes. The calcium-binding protein recognizes the increased Ca2+ intake and activates calcium-dependent protein kinase in the cytosol. Further, phosphorylates the regulatory proteins and upregulates the genes for secondary metabolite production with response to stress (Verma et al. 2022).
General mechanism of elicitation for secondary metabolite production by elicitor signal transduction pathway. Elicitor binds to the specific elicitor binding site and activates ion channels, GTP binding proteins (G proteins) and protein kinases, resulting in reversible phosphorylation and dephosphorylation of proteins, K+ and Cl− efflux/H+ influx, Ca2+ influx, cytoplasmic acidification, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation, reactive oxygen species (ROS) production, activation of mitogen activated protein (MAPK) results in the transcriptional activation of secondary metabolite producing gene (Ramirez-Estrada et al. 2016; Halder et al. 2019; Bajwa et al. 2021)
Biosynthetic pathway engineering to produce forskolin in microbial cell factories
The limited supply of raw materials demands alternate synthesis approaches for the metabolite production. The chemical synthesis of forskolin is more difficult and expensive than extraction from plants as it involves a multistep process with many organic reagents. Moreover, the chemical synthesis produces waste harmful to the environment (Corey and Jardine 1989; Colombo et al. 1992). Thus, engineering the biosynthetic pathway is considered as a better alternative to produce large quantities using microorganisms. In microbial synthesis, the biosynthetic pathway is reconstructed in the host to produce forskolin (Liu et al. 2017). Forskolin is synthesized by the mevalonate pathway (Fig. 3) which involves six regio- and stereospecific monooxygenation and is followed by regiospecific acetylation (Pateraki et al. 2017). The Cytochrome P450 enzymes (CYPs) catalyzes the key biosynthetic steps in forskolin synthesis. 13R-manoyl oxide (13R-MO) is the simplest diterpene precursor produced in the root cork cells of C. forskohlii for forskolin biosynthesis (Paterakil et al. 2014). Geranylgeranyl pyrophosphate (GGPP) is the building block of most diterpenoids through which forskolin is produced. Terpene synthase (TPSs) is the key enzyme involved in terpene synthesis. Two diterpene synthases (diTPSs) namely Cf TPS2 and Cf TPS3 catalyse the intermediate, GGPP to form 13R-MO. After an enzymatic oxidation reaction, it is converted to 7-deacetylforskolin catalysed by cytochrome, CYP76 family. And forskolin, a highly oxygenated labdane terpene is formed after acetylation. The forskolin biosynthetic pathway was engineered in Saccharomyces cerevisiae for glucose fermentation-based production of forskolin and produced forskolin of 40 mg L−1 of yeast culture, which also paved the path for the synthesis of other diterpenoids (Ignea et al. 2016; Pateraki et al. 2017; Forman et al. 2018). Moreover, there are no reports on other microorganisms other than S. cerevisiae for forskolin synthesis. The development of the heterologous synthesis of forskolin through synthetic biology approaches has emerged as an attractive possibility for high yield and high purity (Ju et al. 2021).
Synthesis of forskolin by mevalonate pathway. A pair of diterpene synthase (CfTPS2 and CfTPS3) catalyze C20 diterpenoid precursor, geranylgeranyl pyrophosphate (GGPP) to forskolin precursor 13R manoyl oxide. The highly oxygenized structure of forskolin undergoes oxidation and acetylation by the enzyme from the family cytochrome P450s (subfamily CYP76AHs from the Lamiaceae) and acetyltransferase (Paterakil et al. 2014; Pateraki et al. 2017)
Extraction and quantification of forskolin
As the forskolin content in the root is very low the use of suitable solvent and extraction methods are critical for the recovery of this plant metabolite. Various solvents, methods of extraction, and purification processes for forskolin are evaluated to improve the extraction efficiency. Quantification of forskolin using analytical techniques such as TLC, HPTLC, GC–MS, and LC–MS and HPLC were employed for the identification and estimation of forskolin (Inamdar et al. 1984; Ahmad et al. 2008; Mohamed Saleem 2013; Shukla et al. 2017; Amezcua et al. 2022; Rana et al. 2022). Forskolin is soluble in polar solvents and recovery is higher in methanol (2.91%) and ethanol (2.59%). Hence, polar group solvents are widely used for forskolin extraction (Singh and Suryanarayana 2020). The purity of the extracted forskolin is critical after isolation which further affects its commercial value. Procedures such as Soxhlet extraction followed by concentration of solvent, either by rotary evaporator or water bath and then lyophilization of the residue are generally executed for forskolin recovery (Rana et al. 2022). However, due to the demand for cost-effective and eco-friendly extraction methods, different methods are employed for the improved purity of the product (Table 2). As the yield of forskolin is 0.1% ̴ 2% of root tuber dry weight, attempts are made to increase the metabolite production of the plant by employing different culturing and extraction methods.
Medicinal importance of forskolin and its mechanism of action
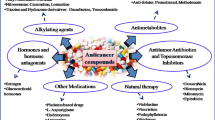
C. forskohlii has been used in traditional medicine for treating different human ailments from time immemorial as it has a broad range of medicinal activity. Administration of forskolin helps to reduce neointimal hyperplasia and atherogenesis in mice and confirms its potential to target multiple coronary artery diseases (Hao et al. 2020). In a clinical trial asthma attack was prevented with the treatment of forskolin at 10 mg/day for six months (González-Sánchez et al. 2006). Glucose metabolism was regulated and fat cell diameter was reduced in high-fat diet fed-mice after the administration of forskolin (Chen et al. 2021). Forskolin is also effective as an antidiabetic agent with antioxidant activity which improves male infertility problems caused by type 2 diabetes (Naghibi et al. 2023). The anti-stress activity of this compound can be utilized for the treatment of neurobiological disorders (Tiwari et al. 2014). In addition, the plant exerts antimicrobial activity against different standard strains of gram-positive and gram-negative bacteria such as Staphylococccus aureus, Streptococcus mutans, Salmonella typhi, and Escherichia coli (Mothana et al. 2019). Further experiments have divulged its effectiveness against pathogens causing urinary tract infections (Chakraborty et al. 2022).
In the cardiovascular system, the administration of forskolin relaxes the muscle wall of blood vessels and increases the efficiency of the myocardium by improving blood circulation (Bristow et al. 1984; Hao et al. 2020). Forskolin eye drops reduce intraocular pressure in glaucoma which regulates the changes in aqueous humor volume (Majeed et al. 2014). Forskolin is more effective in obesity as it promotes lipolysis in mature adipocytes, and decreases intracellular triglycerides (Chen et al. 2021). Curkolin®, a formulation of curcuminoids and forskolin (4:1) was found to have antimicrobial, antioxidant and cytotoxicity effects and can be used for various disorders (Sadashiva et al. 2020). Anticancer activity of forskolin is evident by sensitizing triple-negative breast cancer (TNBC) cells to doxorubicin a chemotherapy drug (Illiano et al. 2018a) and leukemia cells to GSKJ4 an antiproliferative agent (Illiano et al. 2018b). Forskolin enhances cytotoxicity in combination with paclitaxel in Non-Small-Cell Lung Cancer (NSCLC) and induces cell cycle arrest and apoptosis (Salzillo et al. 2023). Forskolin shows neuroprotective properties as demonstrated effective against Alzheimer’s disease by reducing amyloid—β peptides in the brain (Owona et al. 2016; Patole et al. 2019) and has also been proven to prevent Parkinson’s disease by activating AC/cAMP/PKA-driven CREB pathway (Alharbi et al. 2022). In tissue engineering applications biopolymer based scaffolds were developed using forskolin-modified halloysite nanotubes (HNTs) as osteoconductive materials to promote osteodifferentiation of mesenchymal stem cells (Naumenko et al. 2021). Short-term treatment of forskolin promoted bone tissue formation in the defect site without cytotoxic effect in vitro (Awale et al. 2023). Recently manufactured Celluence® with high purity forskolin is marketed and shows multiple benefits on cellulite, skin anti-aging, stretch marks, etc. (Advanced forskolin cream formulations, by Celluence®—LipoTherapeia).
The mechanism of action of forskolin is associated with the activation of cAMP in response to primary signals. The forskolin helps to study the role of cAMP in various cellular processes, and their activation and inhibition in diseased conditions. Forskolin interacts with the enzyme, adenylyl cyclase through a G Protein Coupled Receptor (GPCR) and converts ATP to cAMP in the cell (Sapio et al. 2017). Once the forskolin activates adenylyl cyclase, it increases the production of cAMP and intensifies the signals (Fig. 4). cAMP acts as a secondary messenger and regulates the different hormonal and metabolic processes such as metabolism, cell proliferation, differentiation, gene expression, apoptosis and regulation of ion channels in the human body (Yan et al. 2016; Sapio et al. 2017; Salehi et al. 2019). Based on the upstream signals, cAMP activates protein kinases that regulate cellular functions by phosphorylating target proteins through dependent or independent signaling pathways. (Liu et al. 2022). Protein kinase A (PKA) dissociates into catalytic subunit and regulatory subunit and phosphorylate serine/threonine residues of downstream target proteins and stimulates the cellular response. The phosphorylation of CREB, a transcription factor is crucial for regulating gene expression. cAMP pathways are also associated with the pathogenicity of disease and any impairment in the signal transduction pathway leads to different ailments due to decreased cAMP. The cAMP is inactivated to AMP by phosphodiesterases (PDE) through hydrolysis. The activation of cAMP levels by forskolin in cells decreases the release of histamine, increases insulin secretion, improves blood circulation and pressure, aids the breakdown of fat and increases thyroid function (Hameed et al. 2020).
Conclusions and future perspectives
C. forskohlii is an endangered species of medicinal plant and the only source of forskolin that has numerous applications in cosmetics and skin care, food and beverages and the pharmaceutical industry. The main mechanism of action of forskolin is by activating the enzyme adenylyl cyclase and elevating cAMP which regulates different cellular functions. For commercial use, the plant is propagated by different methods to ensure the availability of forskolin. The mass propagation of C. forskohlii, to improve the yield and purity of forskolin is challenging as it demands different purity for various industrial sectors. Multiple biotechnological approaches are being developed for producing a high level of forskolin without necessitating the harvest of the plant making the commercial process sustainable and environmentally friendly. Due to its high demand, there is a huge scope for improvements in forskolin production by introducing different precursors/elicitors in tissue culture approaches, which is a better alternative to the synthetic method of production. Hence the development of alternate biotechnological approaches to improve or induce the forskolin yield is the need of the hour.
A yield enhancement strategy, elicitation is currently popular in in vitro forskolin production. NaCl, CS, SA JA, and MeJA are widely used as elicitors along growth media in terpenoid production, applied alone or in combinations that can be explored for forskolin synthesis as well. Carbon-based nanomaterial such as carbon nanotubes is also an emerging approach for the induction of secondary metabolite which can be extrapolated in forskolin synthesis. Investigating the effect of elicitors or precursors on the metabolic pathway that increases the forskolin synthesis needs to be explored. This will provide a better understanding of the modification of signal transduction, transcription factors, characterization of biosynthetic genes and their encoded enzymes involved in forskolin synthesis with response to elicitation. Advanced tools such as metabolomics and transcriptomics can be used to predict the metabolic pathways and gene expression profiles in response to the elicitor.
A sustainable in vitro cultures with the effect of external stimuli for the bioactive compounds help in large-scale production. There is a need for a standardized protocol for forskolin synthesis that involves plant cell response to different conditions in a limited time without any seasonal and regional constraints. Moreover, different aspects of cell culture techniques for upscaling biomass and secondary metabolite productivity that enhance the yield can be of great significance.
Identifying the appropriate tissue culture protocol by carefully selecting elicitor an be a better choice for the production of forskolin. This method can be made more attractive if the yield concerning time, space, and scale is enhanced. The future culture technique for the production of forskolin should be designed with sustainable approaches to meet the growing demand. The exploitation of effective elicitors in the tissue culture process requires a deeper understanding of the plant cell responses to external stimuli. The effort towards reducing the time and cost while making the production goals sustainable is therefore challenging but should be prioritized. Tissue culture-based controlled production of the forskolin can reduce waste generation while maintaining product quality.
Abbreviations
- 6-BAP:
-
6-Benzylaminopurine
- KIN:
-
Kinetin
- 2,4-D:
-
2,4 Dichlorophenoxyaceticacid
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- NAA:
-
α-Naphthaleneacetic acid
- mT:
-
Meta-topolin
- ROS:
-
Reactive oxygen species
- UV:
-
Ultraviolet
- SA:
-
Salicylic acid
- JA:
-
Jasmonic acid
- MeJa:
-
Methyl jasmonate
- CS:
-
Chitosan
- NaCl:
-
Sodium Chloride
- NPs:
-
Nanoparticles
- TLC:
-
Thin-layer chromatography
- HPTLC:
-
High-performance thin-layer chromatography
- HPLC:
-
High-performance liquid chromatography
- GC–MS:
-
Gas chromatography-mass spectrometry
- LC–MS:
-
Liquid chromatography-mass spectrometry
- TPP:
-
Three phase partitioning
- SC-Co2 :
-
Supercritical carbon dioxide
- EDX:
-
Energy-dispersive X-ray spectroscopy
- cAMP:
-
Adenosine 3′, 5′-cyclic monophosphate
- ATP:
-
Adenosine triphosphate
- CREB:
-
CAMP response-element binding-protein
- AMP:
-
Adenosine monophosphate
References
Abraham J, Thomas TD (2017) Hairy root culture for the production of useful secondary metabolites. In: Malik S (ed) Biotechnology and production of anti-cancer compounds. Springer, New York, pp 201–230. https://doi.org/10.1007/978-3-319-53880-8_9
Ahmad S, Rizwan M, Parveen R, Mujeeb M, Aquil M (2008) A validated stability-indicating TLC method for determination of forskolin in crude drug and pharmaceutical dosage form. Chromatographia 67:441–447
Alharbi M, Alshammari A, Kaur G, Kalra S, Mehan S, Suri M et al (2022) Effect of natural adenylcyclase/cAMP/CREB signalling activator forskolin against intra-striatal 6-OHDA-lesioned Parkinson’s rats: preventing mitochondrial, motor and histopathological defects. Molecules 27(22):7951. https://doi.org/10.3390/molecules27227951
Amezcua IJ, Blas SR, Municio MD, Soria AC, Matute AI, Sanz ML (2022) Development of a multianalytical strategy for detection of frauds in Coleus forskohlii supplements. J Chromatogr A 1676:463198. https://doi.org/10.1016/j.chroma.2022.463198
Ammon HP, Kemper FH (1982) Ayurveda: 3000 years of Indian traditional medicine. Med Welt 33(4):148–153
Assaf M, Korkmaz A, Karaman Ş, Kulak M (2022) Effect of plant growth regulators and salt stress on secondary metabolite composition in Lamiaceae species. S Afr J B 144:480–493
Awale GM, Barajaa MA, Kan HM, Seyedsalehi A, Nam GH, Hosseini FS, Ude CC, Schmidt TA, Lo KW, Laurencin CT (2023) Regenerative engineering of long bones using the small molecule forskolin. PNAS 120(22):e2219756120. https://doi.org/10.1073/pnas.2219756120
Badhepuri MK, Manokari M, Raj MC, Jogam P, Dey A, Faisal M et al (2023) Meta-Topolin enhanced direct shoot organogenesis and regeneration from leaf explants of Coleus forskohlii (Willd) Briq. Ind Crops Prod 197:116584. https://doi.org/10.1016/j.indcrop.2023.116584
Bajwa MN, Bibi A, Idrees MZ, Zaman G, Farooq U, Bhatti TT (2021) Elicitation, a mechanistic approach to change the metabolic pathway of plants to produce pharmacological important compounds in in-vitro cell cultures. GJES 8:1–7. https://doi.org/10.33552/GJES.2021.08.000678
Balasubramanian V, Suresh J, Srinivasan R, Prabhu M, Manikandan E, Nandha VG (2020) Evaluation of soil characteristics and yield variation of coleus (Coleus forskohlii) in different agro climatic zones of Tamil Nadu. Int J Conserv Sci 8(4):2841–2845. https://doi.org/10.22271/chemi.2020.v8.i4ah.10076
Balasubramanya S, Rajanna L, Anuradha M (2012) Effect of plant growth regulators on morphogenesis and forskolin production in Plectranthus barbatus Andrews. In Vitro Cell Dev Biol Plant 48:208–215. https://doi.org/10.1007/s11627-011-9417-9
Bavi K, Khavari-Nejad RA, Najafi F, Ghanati F (2022) Phenolics and terpenoids change in response to yeast extract and chitosan elicitation in Zataria multiflora cell suspension culture. 3 Biotech 12(8):163. https://doi.org/10.1007/s13205-022-03235-x
Benjamin ED, Ishaku GA, Peingurta FA, Afolabi AS (2019) Callus culture for the production of therapeutic compounds. Am J Plant Biol 4(4):76–84
Berni R, Luyckx M, Xu X, Legay S, Sergeant K, Hausman JF, Lutts S, Cai G, Guerriero G (2019) Reactive oxygen species and heavy metal stress in plants: impact on the cell wall and secondary metabolism. Environ Exp Bot 161:98–106. https://doi.org/10.1016/j.envexpbot.2018.10.017
Bristow MR, Ginsburg R, Strosberg A, Montgomery W, Minobe W (1984) Pharmacology and inotropic potential of forskolin in the human heart. J Clin Investig 74(1):212–223. https://doi.org/10.1172/JCI111404
Chahal S, Kaur H, Lekhak MM, Shekhawat MS, Goutam U, Singh SK et al (2022) Meta-topolin-mediated regeneration and accumulation of phenolic acids in the critically endangered medicinal plant Crinum malabaricum (Amaryllidaceae): a potent source of galanthamine. S Afr J Bot 49:853–859. https://doi.org/10.1016/j.sajb.2022.01.016
Chakraborty M, KarunA MA (2009) Accumulation of phenylpropanoid derivatives in chitosan-induced cell suspension culture of Cocos nucifera. J Plant Physiol 166(1):63–71. https://doi.org/10.1016/j.jplph.2008.02.004
Chakraborty A, Haque SM, Dey D, Mukherjee S, Ghosh B (2022) Detection of UTI pathogen-killing properties of Coleus forskohlii from tissue cultured in vitro and ex vitro plants. Proc Natl Acad Sci India Sect B Biol Sci 92(1):157–169. https://doi.org/10.1007/s40011-021-01285-4
Chamkhi I, Benali T, Aanniz T, MenyiyN El, Guaouguaou FE, El Omari N et al (2021) Plant-microbial interaction: The mechanism and the application of microbial elicitor induced secondary metabolites biosynthesis in medicinal plants. Plant Physiol Biochem 167:269–295. https://doi.org/10.1016/j.plaphy.2021.08.001
Chandra AK, Rajak KK, Gururani K, Kumar H, Kumar M (2019) Influence of explants type and phytohormones on In vitro callogenesis and plantlet regeneration of patharchur (Coleus barbatus L.), an endangered ethnomedicinal plant. J Pharmacogn Phytochem 8(3):943–953
Chandran H, Meena M, Barupal T, Sharma K (2020) Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol Rep 26:e00450. https://doi.org/10.1016/j.btre.2020.e00450
Chen JY, Peng SY, Cheng YH, Lee IT, Yu YH (2021) Effect of forskolin on body weight, glucose metabolism and adipocyte size of diet-induced obesity in mice. Animals 11(3):645
Colombo MI, Zinczuk J, Rúveda EA (1992) Synthetic routes to forskolin. Tetrahedron 48:963–1037
Corey EJ, Jardine PDS (1989) A short and efficient enantioselective route to a key intermediate for the total synthesis of forskolin. Tetrahedron Lett 30:7297–7300
Das A, Kamal S, Shakil NA, Sherameti I, Oelmüller R, Dua M et al (2012) The root endophyte fungus Piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal plant, Coleus forskohlii. Plant Signal Behav 7(1):103–112. https://doi.org/10.4161/psb.7.1.18472
De Souza NJ, Shah VIRBALA (1988) Forskolin—an adenylate cyclase activating drug from an Indian herb. In: Wagner H, Hikino H, Farnsworth NR (eds) Economic and medicinal plant research, vol 2. Academic Press Ltd., New York, pp 56–61
Devendra LP, Gaikar VG (2010) Microwave-assisted extraction of forskolin from coleus roots and its purification by adsorptive separation using functionalized polymer designed by molecular simulation. Ind Eng Chem Res 49(19):271–9278
Efferth T (2019) Biotechnology applications of plant callus cultures. Engineering 5(1):50–59. https://doi.org/10.1016/j.eng.2018.11.006
Erişen S, Kurt-Gür G, Servi H (2020) In vitro propagation of Salvia sclarea L. by meta-Topolin, and assessment of genetic stability and secondary metabolite profiling of micropropagated plants. Ind Crops Prod 157:112892. https://doi.org/10.1016/j.indcrop.2020.112892
Fatima K, Abbas SR, Zia M, Sabir SM, Khan RT, Khan AA, Hassan Z, Zaman R (2020) Induction of secondary metabolites on nanoparticles stress in callus culture of Artemisia annua L. Braz J Biol 81(2):474–483. https://doi.org/10.1590/1519-6984.232937
Fazal H, Abbasi BH, Ahmad N, Ali M (2016) Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L. Appl Biochem Biotechnol 10:1076–1092. https://doi.org/10.1007/s12010-016-2153-1
Fernandes RC, Barreto RW (2003) Corynespora cassiicola causing leaf spots on Coleus barbatus. Plant Pathol 52:786. https://doi.org/10.1111/j.1365-3059.2003.00895.x
Forman V, Bjerg-Jensen N, Dyekjær JD, Møller BL, Pateraki I (2018) Engineering of CYP76AH15 can improve activity and specificity towards forskolin biosynthesis in yeast. Microb Cell Fact 17:1–17. https://doi.org/10.1186/s12934-018-1027-3
Ghazal B, Saif S, Farid K, Khan A, Rehman S, Reshma A, Fazal H, Ali M, Ahmad A, Rahman L, Ahmad N (2018) Stimulation of secondary metabolites by copper and gold nanoparticles in submerge adventitious root cultures of Stevia rebaudiana (Bert.). IET Nanobiotechnol 12(5):569–573. https://doi.org/10.1049/iet-nbt.2017.0093
Ghosh B, Chakraborty M, Chakraborty A (2021) Forskolin-a natural root extract of Coleus forskohlii—a mini review. Acta Sci Pharma Sci 5(7):113–116
González-Sánchez R, Trujillo X, Trujillo-Hernández B, Vásquez C, Huerta M, Elizalde A (2006) Forskolin versus sodium cromoglycate for prevention of asthma attacks: a single-blinded clinical trial. J Int Med Res 34(2):200–207. https://doi.org/10.1177/147323000603400210
Goswami BK, Bhattacharya C, Paul R, Khan TA (2012) Performance of pesticide and biopesticide on growth, yield and forskolin content in Coleus forskohlii infected with Meloidogyne incognita. Pak J Nematol 30(1):48–55
Grand View Research. Market analysis report. Accessed 21 Dec 2023. https://www.grandviewresearch.com/industry-analysis/forskolin-market-report
Halder M, Sarkar S, Jha S (2019) Elicitation: a biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng Life Sci 19(12):880–895. https://doi.org/10.1002/elsc.201900058
Hameed SI, Al-Shahwany AW, Salih SJ (2020) Evaluation of the activity of some plants extracts on thyroid gland regulation in female albino rats. Iraqi J Sci 61(2):254–265. https://doi.org/10.24996/ijs.2020.61.2.3
Hao H, Ma X, Chen H, Zhu L, Xu Z, Li Q, Xu C, Zhang Y, Peng Z, Wang M (2020) The cyclic adenosine monophosphate elevating medicine, forskolin, reduces neointimal formation and atherogenesis in mice. J Cell Mol Med 24(17):9638–9645. https://doi.org/10.1111/jcmm.15476
Harde SM, Singhal RS (2012) Extraction of forskolin from Coleus forskohlii roots using three phase partitioning. Sep Purif Technol 96:20–25. https://doi.org/10.1016/j.seppur.2012.05.017
Harde SM, Kagliwal LD, Singhal RS, Patravale VB (2013) Supercritical fluid extraction of forskolin from Coleus forskohlii roots. J Food Eng 117(4):443–449. https://doi.org/10.1016/j.jfoodeng.2012.12.012
Harde SM, Lonkar SL, Degani MS, Singhal RS (2014) Ionic liquid based ultrasonic-assisted extraction of forskolin from Coleus forskohlii roots. Ind Crops Prod 61:258–264. https://doi.org/10.1016/j.indcrop.2014.07.016
Hunyadi A (2019) The mechanism (s) of action of antioxidants: from scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med Res Rev 39(6):2505–2533. https://doi.org/10.1002/med.21592
Ignea C, Ioannou E, Georgantea P, Trikka FA, Athanasakoglou A, Loupassaki S et al (2016) Production of the forskolin precursor 11β-hydroxy-manoyl oxide in yeast using surrogate enzymatic activities. Microb Cell Fact 15(1):1–11. https://doi.org/10.1186/s12934-016-0440-8
Illiano M, Sapio L, Salzillo A, Capasso L, Caiafa I, Chiosi E et al (2018a) Forskolin improves sensitivity to doxorubicin of triple negative breast cancer cells via Protein Kinase A-mediated ERK1/2 inhibition. Biochem Pharmacol 152:104–113. https://doi.org/10.1016/j.bcp.2018.03.023
Illiano M, Conte M, Sapio L, Nebbioso A, Spina A, Altucci L, Naviglio S (2018b) Forskolin sensitizes human acute myeloid leukemia cells to H3K27me2/3 demethylases GSKJ4 inhibitor via protein kinase A. Front Pharmacol 9:792. https://doi.org/10.3389/fphar.2018.00792
Inam M, Attique I, Zahra M, Khan AK, Hahim M, Hano C, Anjum S (2023) Metal oxide nanoparticles and plant secondary metabolism: unraveling the game-changer nano-elicitors. Plant Cell Tissue Organ Cult. https://doi.org/10.1007/s11240-023-02587-3
Inamdar PK, Kanitkar PV, Reden J, De Souza NJ (1984) Quantitative determination of forskolin by TLC and HPLC. Planta Med 50(01):30–34
Janarthanam B, Sumathi E (2020) In vitro plant regeneration from nodal explants of Coleus forskohlii Briq.-an important medicinal plant. Plant Tissue Cult Biotechnol 30(1):143–148. https://doi.org/10.3329/ptcb.v30i1.47799
Javed R, Yucesan B, Zia M, Gurel E (2022) Nanoelicitation: a promising and emerging technology for triggering the sustainable in vitro production of secondary metabolites in medicinal plants. In: Chen JT (ed) Plant and nanoparticles. Springer, Singapore, pp 265–280. https://doi.org/10.1007/978-981-19-2503-0_10
Ju H, Zhang C, Lu W (2021) Progress in heterologous biosynthesis of forskolin. J Ind Microbiol Biotechnol 48:kuab009. https://doi.org/10.1093/jimb/kuab009
Kamalakannan A, Mohan L, Valluvaparidasan V, Mareeswari P, Karuppiah R (2006) First report of Macrophomina root rot (Macrophomina phaseolina) on medicinal coleus (Coleus forskohlii) in India. Plant Pathol 55(2):302–302. https://doi.org/10.1111/j.1365-3059.2005.01302.x
Kavitha C, Rajamani K, Vadivel E (2010) Coleus forskohlii: a comprehensive review on morphology, phytochemistry and pharmacological aspects. J Med Plants Res 4(4):278–285
Khan N, Bano A, Babar MA (2019) Metabolic and physiological changes induced by plant growth regulators and plant growth promoting rhizobacteria and their impact on drought tolerance in Cicer arietinum L. PLoS One 14(3):e0213040. https://doi.org/10.1371/journal.pone.0213040
Khatun S, Chatterjee NC, Çakılcıoğlu U (2011) The strategies for production of forskolin vis-a-vis protection against soil borne diseases of the potential herb Coleus forskohlii Briq. Eur J Med Plants 1(1):1–9
KooY M, Heo AY, Choi HW (2020) Salicylic acid as a safe plant protector and growth regulator. Plant Pathol J 36(1):1–10
Kotagiri D, Kolluru VC (2017) Effect of salinity stress on the morphology and physiology of five different Coleus species. Biomed Pharmacol J 10(4):1639–1649. https://doi.org/10.5423/PPJ.RW.12.2019.0295
Krishna G, Sairam Reddy P, Anoop Nair N, Ramteke PW, Bhattacharya P (2010) In vitro direct shoot regeneration from proximal, middle and distal segment of Coleus forskohlii leaf explants. Physiol Mol Biol Plants 16(2):195–200. https://doi.org/10.1007/s12298-010-0021-y
Lala S (2021) Nanoparticles as elicitors and harvesters of economically important secondary metabolites in higher plants: a review. IET Nanobiotechnol 15(1):28–57. https://doi.org/10.1049/nbt2.12005
Lipotherapeia. Last accessed at 21 December 2023. Available on Advanced forskolin cream formulations, by Celluence®—LipoTherapeia | London's cellulite experts
Liu L, Guan N, Li J, Shin HD, Du G, Chen J (2017) Development of GRAS strains for nutraceutical production using systems and synthetic biology approaches: advances and prospects. Crit Rev Biotechnol 37(2):139–150. https://doi.org/10.3109/07388551.2015.1121461
Liu Y, Chen J, Fontes SK, Bautista EN, Cheng Z (2022) Physiological and pathological roles of protein kinase A in the heart. Cardiovasc Res 118(2):386–398. https://doi.org/10.1093/cvr/cvab008
Mahmoud DS, Sayed LM, Diab MI, Fahmy EM (2019) In vitro propagation of Plectranthus barbatus Andrews as important medicinal plant. Arab Univ J Agric Sci 7(1):511–517. https://doi.org/10.21608/ajs.2019.43660
Majeed M, Nagabhushanam K, Natarajan S, Vaidyanathan P, Kumar SK (2014) A double-blind, randomized clinical trial to evaluate the efficacy and safety of forskolin eye drops 1% in the treatment of open angle glaucoma—a comparative study. J Clin Trials 4(5):1–6. https://doi.org/10.4172/2167-0870.1000184
Malathy S, Pai JS (1999) Monitoring of forskolin production from roots and callus by HPTLC in Coleus forskohlii Briq. J Spices Aromat Crops 8(2):153–157
Marini I, Pelzl L, Tamamushi Y, Maettler CT, Witzemann A, Althaus K, Nowak-Harnau S, Seifried E, Bakchoul T (2023) Inhibition of GPIb-α-mediated apoptosis signaling enables cold storage of platelets. Haematologica 108(11):2959–2971. https://doi.org/10.3324/haematol.2022.282572
Mastan A, Bharadwaj RKB, Kushwaha RK, Vivek Babu CS (2019) Functional fungal endophytes in Coleus forskohlii regulate labdane diterpene biosynthesis for elevated forskolin accumulation in roots. Microb Ecol 78(49):14–926. https://doi.org/10.1007/s00248-019-01376-w
Mastan A, Rane D, Dastager SG, Vivek Babu CS (2020) Plant probiotic bacterial endophyte, Alcaligenes faecalis, modulates plant growth and forskolin biosynthesis in Coleus forskohlii. Probiotics Antimicrob Proteins 12:481–493. https://doi.org/10.1007/s12602-019-09582-1
Mastan A, Rane D, Dastager SG, Babu CV (2021) Molecular insights of fungal endophyte co-inoculation with Trichoderma viride for the augmentation of forskolin biosynthesis in Coleus forskohlii. Phytochemistry 184:112654. https://doi.org/10.1016/j.phytochem.2021.112654
Mersinger R, Dornauer H, Reinhard E (1988) Formation of forskolin by suspension cultures of Coleus forskohlii1. Planta Med 54(03):200–204
Miao YH, Chen QH, Yu K, Wang YH, Liu DH (2021) First report of Fusarium wilt of Coleus forskohlii caused by Fusarium oxysporum in China. Plant Dis 105(05):1559. https://doi.org/10.1094/PDIS-11-20-2489-PDN
Mishra SP, Gaikar VG (2009) Hydrotropic extraction process for recovery of Forskolin from Coleus forskohlii roots. Ind Eng Chem Res 48(17):8083–8090. https://doi.org/10.1021/ie801728d
Mohamed Saleem A (2013) Methods of isolation and analysis of Forskolin from Coleus forskohlii. In: Ramawat K, Mérillon JM (eds) Natural products. Springer, Berlin, pp 3325–3343. https://doi.org/10.1007/978-3-642-22144-6_1773325-43
Mothana RA, Khaled JM, El-Gamal AA, Noman OM, Kumar A, Alajmi MF, Al-Said MS (2019) Comparative evaluation of cytotoxic, antimicrobial and antioxidant activities of the crude extracts of three Plectranthus species grown in Saudi Arabia. Saudi Pharm J 27(2):162–170. https://doi.org/10.1016/j.jsps.2018.09.010
Mukherjee S, Ghosh B, Jha S (2000) Establishment of forskolin yielding transformed cell suspension cultures of Coleus forskohlii as controlled by different factors. J Biotechnol 76(1):73–81. https://doi.org/10.21608/ajs.2019.43660
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Naghibi M, Nasrabadi HT, Rad JS, Garjani A, Farashah MS, Mohammadnejad D (2023) Forskolin improves male reproductive complications caused by hyperglycemia in type 2 diabetic rats. Int J Fertil Steril 17(4):268–275. https://doi.org/10.22074/IJFS.2022.544368.1235
Nagpal A, Singh B, Sharma S, Rani G, Virk GS (2008) Coleus spp.: micropropagation and in vitro production of secondary metabolites. Med Aromat Plant Sci Biotechnol 2(1):1–7
Naumenko E, Guryanov I, Zakirova E, Fakhrullin R (2021) Forskolin-loaded halloysite nanotubes as osteoconductive additive for the biopolymer tissue engineering scaffolds. Polymers 13(22):3949. https://doi.org/10.3390/polym13223949
Nikalje GC, Zimare SB, Shelke DB (2021) Effect of elicitors on plant cell suspension culture for the enhancement of secondary metabolite production. Nat J Pharm Sci 1(1):50–57
Owona BA, Zug C, Schluesener HJ, Zhang ZY (2016) Protective effects of forskolin on behavioral deficits and neuropathological changes in a mouse model of cerebral amyloidosis. J Neuropathol Exp Neurol 75(7):618–627. https://doi.org/10.1093/jnen/nlw043
Pateraki I, Andersen-Ranberg J, Jensen NB, Wubshet SG, Heskes AM, Forman V et al (2017) Total biosynthesis of the cyclic AMP booster forskolin from Coleus forskohlii. Elife 6:e23001. https://doi.org/10.7554/eLife.23001
PaterakiI A-RJ, Hamberger B, Heskes AM, Martens HJ, Zerbe P et al (2014) Manoyl oxide (13R), the biosynthetic precursor of forskolin, is synthesized in specialized root cork cells in Coleus forskohlii. Plant Physiol 64(3):1222–1236. https://doi.org/10.1104/pp.113.228429
Patole A, Lamdhade D, Dewani S, Gandhare B, Raul J (2019) Forskolin ameliorates scopolamine induced memory impairment in rats. Saudi J Med Pharm Sci 5(12):1059–1066
Paul M, Radha A, Kumar DS (2013) On the high value medicinal plant, Coleus forskohlii Briq. Hygeia J D Med 5(1):64–73
Praveena R, Vanithamani J, Devadarshini R (2021) Effect of different hormones on different explants of Coleus forskholii briq. Int J Botany Stud 6(6):285–289
Pullaiah T (2022a) Success stories and trade and commerce of Coleus forskohlii. In: Forskolin. Springer, Singapore, pp 173–177. https://doi.org/10.1007/978-981-19-6521-0_11
Pullaiah T (2022b) Micropropagation of Coleus forskohlii. In: Forskolin. Springer, Singapore. pp 107–125. https://doi.org/10.1007/978-981-19-6521-0_6
Pullaiah T (2022c) Endophytes for the enhanced growth of Coleus forskohlii and enhanced production of forskolin. In: Forskolin. Springer, Singapore, pp. 149–154. https://doi.org/10.1007/978-981-19-6521-0_9
Rajamani K, Vadivel E (2009) Marunthu Kurkan–Medicinal Coleus. Naveena mulikai sagupaddi thozhil nuttpangal, Tamil Nadu Agricultural University, Coimbatore, pp 17–22
Rajasekharan PE, Ganeshan S, Bhaskaran S (2010) In vitro regeneration and conservation of three Coleus species. Med Aromat Plant Sci Biotechnol 4(1):24–27
Rajiv P (2014) Establishment of hairy root culture and production of secondary metabolites in Coleus (Coleus forskohlii). J Med Plant Res 8(1):58–62
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21(2):182. https://doi.org/10.3390/molecules21020182
Ramprasad S (2005) Studies on collar rot complex of Coleus forskohlii (Wild.) Briq. M.Sc. thesis, UAS, Dharwad
Rana PS, Saklani P, Chandel C (2022) Analyzing the effects of altitudes on the metabolic diversity and forskolin content in Coleus forskohlii roots by HPTLC and HPLC. Plant Biosyst 156(2):323–329. https://doi.org/10.1080/11263504.2020.1857865
Rasineni GK, Siddavattam D, Reddy AR (2008) Free radical quenching activity and polyphenols in three species of Coleus. J Med Plant Res 2(10):285–291
Rawat JM, Bhandari A, Raturi M, Rawat B (2019) Agrobacterium rhizogenes mediated hairy root cultures: a promising approach for production of useful metabolites. In: Gupta VK, Pandey A (eds) New and future developments in microbial biotechnology and bioengineering. Elsevier, Amsterdam, pp 103–118. https://doi.org/10.1016/B978-0-444-63504-4.00008-6
Reddy CS, Praveena CH, Veeresham C (2012) Strategies to improve the production of Forskolin from hairy root cultures of Coleus forskohlii Briq. Int J Pharma Sci Nanotechnol 51:720–726. https://doi.org/10.37285/ijpsn.2012.5.2.7
Sadashiva CT, Hussain HM, Nanjundaiah S, Manjula AC, Kote NV, Patil R, Ranjith R, Makeswari M, Ravi S (2020) Antioxidant, antimicrobial and cytotoxic activity of Curkolin®(Curcuma longa and Coleus forskohlii formulation). IJTK 19(4):751–756
Sairam Reddy P, Rodrigues R, Rajasekharan R (2001) Shoot organogenesis and mass propagation of Coleus forskohlii from leaf derived callus. Plant Cell Tissue Organ Cult 66:183–188. https://doi.org/10.1023/A:1010697813852
Saksena AK, Green MJ, Shue HJ, Wong JK, McPhail AT, Gross PM (1985) Identity of coleonol with forskolin: structure revision of a base-catalysed rearrangement product. Tetrahedron Lett 26(5):551–554. https://doi.org/10.1016/S0040-4039(00)89145-5
Saleem AM, Dhasan PB, Rafiullah MRM (2005) Isolation of forskolin from stem of Coleus forskohlii. Pharmacogn Mag 1(3):89
Salzillo A, Ragone A, Spina A, Naviglio S, Sapio L (2023) Forskolin affects proliferation, migration and Paclitaxel-mediated cytotoxicity in non-small-cell lung cancer cell lines via adenylyl cyclase/cAMP axis. Eur J Cell Biol 102(2):151292. https://doi.org/10.1016/j.ejcb.2023.151292
Sapio L, Gallo M, Illiano M, Chiosi E, Naviglio D, Spina A, Naviglio S (2017) The natural cAMP elevating compound forskolin in cancer therapy: is it time? J Cell Physiol 232(5):922–927. https://doi.org/10.1002/jcp.25650
Sasaki K, Udagawa A, Ishimaru H, Hayashi T, Alfermann AW, Nakanishi F, Shimomura K (1998) High forskolin production in hairy roots of Coleus forskohlii. Plant Cell Rep 17:457–459. https://doi.org/10.1007/s002990050425
Schaneberg BT, Khan IA (2003) Quantitative analysis of forskolin in Coleus forskohlii (Lamiaceae) by reversed-phase liquid chromatography. J AOAC Int 86(3):467–470. https://doi.org/10.1093/jaoac/86.3.467
Seenivasan N (2010) Management of root-knot nematode, Meloidogyne incognita with organic amendments in medicinal coleus. Ann Plant Prot Sci 18(2):472–476
Sen J, Sharma AK, Sahu NP, Mahato SB (1992) Production of forskolin in in vitro cultures of Coleus forskohlii. Planta Med 58(04):324–327
Sharan AK, Singh BP, Dubey SR, Kumar R, Kishor A, Kumar G, Kumari S (2014) Effective propagation and evaluation of salt tolerance in Coleus forskohlii, an endangered herb. Int J Adv Sci Eng Technol 3(2):24–31
Sharma N, Chandel KP, Srivastava VK (1991) In vitro propagation of Coleus forskohlii Briq., a threatened medicinal plant. Plant Cell Rep 1(10):67–70. https://doi.org/10.1007/BF00236459
Shoja AA, Çirak C, Ganjeali A, Cheniany M (2022) Stimulation of phenolic compounds accumulation and antioxidant activity in in vitro culture of Salvia tebesana Bunge in response to nano-TiO2 and methyl jasmonate elicitors. PCTOC 149:423–440. https://doi.org/10.1007/s11240-022-02251-2
Shukla PK, Misra A, Kumar M, Rajan S, Agrawal PK, Rawat AKS, Srivastava S (2016) Intra-specific chemotypic variability of forskolin content in Coleus forskohlii (wild.) Briq. growing in Nilgiri hills of India. J Planar Chromatogr Mod TLC 29(5):347–355. https://doi.org/10.1556/1006.2016.29.5.4
Shukla PK, Misra A, Kumar M, Singh K, Akhtar J, Srivastava S, Rawat AKS (2017) Simultaneous quantification of forskolin and iso-forskolin in Coleus forskohlii (wild.) Briq. and identification of elite chemotype, collected from eastern ghats (India). Pharmacogn Mag 13(Suppl 4):S881–S885
Shyla M (1998) Etiology and management of root rot of Coleus forskohlii. University of Agricultural Science, Bangalore, India, M.Sc. thesis
Singh P, Suryanarayana MA (2020) Effect of solvents and extraction methods on forskolin content from Coleus forskholii roots. Indian J Pharm Sci 81(6):136–1140
Singh R, Gangwar SP, Singh SD, R, Pandey R, Kalra, A, (2011) Medicinal plant Coleus forskohlii Briq.: disease and management. Med Plants 3(1):1–7
Sinha RK, Sharma SN, Verma SS, Zha J (2018) Effects of lovastin, fosmidomycin and methyl jasmonate on andrographolide biosynthesis in the Andrographis paniculata. Acta Physiol Plant 40(9):165. https://doi.org/10.1007/s11738-018-2746-0
Sivakumar P, Bavithra VS, Ashokkumar K, Deepadharsini R, Selvaraj KV, Gopal MR (2021) Comprehensive review on phytochemistry and in vitro biotechnology of Coleus forskohlii. J Pharmacogn Phytochem 10(1):448–453. https://doi.org/10.22271/phyto.2021.v10.i1g.13346
Sreedevi E, Pullaiah T (2014) Effect of growth regulators on in vitro organogenesis and long term storage of Plectranthus barbatus Andr.(Syn.: Coleus forskohlii (Wild.) Briq.). Curr Trends Biotechnol Pharm 8(2):143–151
Sreedevi E, Anuradha M, Pullaiah T (2013) Plant regeneration from leaf-derived callus in Plectranthus barbatus Andr.[Syn.: Coleus forskohlii (Wild.) Briq.]. Afr J Biotechnol 12(18):2441–2448
Srivastava S, Misra A, Mishra P, Shukla P, Kumar M, Sundaresan V, Rawat AKS (2017) Molecular and chemotypic variability of forskolin in Coleus forskohlii Briq., a high value industrial crop collected from Western Himalayas (India). RSC Adv 7(15):8843–8851
Swaroopa G, Anuradha M, Pullaiah T (2013a) Elicitation of forskolin in suspension cultures of Coleus forskohlii (willd.) Briq. using elicitors of fungal origin. Curr Trends Biotechnol Pharm 7(3):755–762
Swaroopa G, Anuradha M, Pullaiah T (2013b) Elicitation of forskolin in suspension cultures of Coleus forskohlii (Willd.) Briq. using bacterial elicitors. J Indian Bot Soc 92(1and2):97–100
Tarte SH, Chandra K, Dev D, Khan MA (2022) Potential role and utilization of Piriformospora indica: fungal endophytes in commercial plant tissue culture. In: Gupta S, Chaturvedi P (eds) Commercial scale tissue culture for horticulture and plantation crops. Springer, Singapore, pp 85–120. https://doi.org/10.1007/978-981-19-0055-6_5
Thakur M, Bhattacharya S, Khosla PK, Puri S (2019) Improving production of plant secondary metabolites through biotic and abiotic elicitation. J Appl Res Med Aromat Plants 12:1–12. https://doi.org/10.1016/j.jarmap.2018.11.004
Thiribhuvanamala G, Meena B, Swarnakumari N, Geethalakshmi I, Muthulakshmi P, Rajamani K (2020) Current scenario on the prevalence of diseases in economically important medicinal and aromatic crops of Tamil Nadu. Ind J Pure Appl Biosci 8(2):95–103. https://doi.org/10.18782/2582-2845.8012
Tilkat EA, Hoşer A, Süzerer V, Tilkat E (2023) Influence of salinity on in vitro production of terpene: a review. IntechOpen. https://doi.org/10.5772/intechopen.111813
Tiwari N, Mishra A, Bhatt G, Chaudhary A (2014) Anti stress activity (in-vivo) of Forskolin isolated from Coleus forskohlii. Int J Pharm Phytopharmacol Res 4(3):201–204
Tripathi CKM, BasuS K, Jain S, Tandon JS (1995) Production of coleonol (forskolin) by root callus cells of plant Coleus forskohlii. Biotechnol Lett 17:423–426
Turkyilmaz Unal B (2021) The use of meta-topolin in cell and tissue cultures for increasing production of secondary metabolites. In: Ahmad N, Strnad M (eds) Meta-topolin: a growth regulator for plant biotechnology and agriculture. Springer, New York, pp 253–263. https://doi.org/10.1007/978-981-15-9046-7_18
Dinesh Valke (2018) Accessed 21 Dec 2023. https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.flickr.com%2Fphotos%2Fdinesh_valke%2F40271462130&psig=AOvVaw25jMbjKNCi8toxMyYnHjjo&ust=1703223188657000&source=images&cd=vfe&opi=89978449&ved=0CBQQjhxqFwoTCNCcwonnn4MDFQAAAAAdAAAAABAT
Verma S, Negi NP, Narwal P, Kumari P, Kisku AV, Gahlot P, Mittal N, Kumar D (2022) Calcium signaling in coordinating plant development, circadian oscillations and environmental stress responses in plants. Environ Exp Bot 201:104935. https://doi.org/10.1016/j.envexpbot.2022.104935
Vibhuti RK, Kumar D (2019) Effect of 6-BAP on callus culture and shoot multiplication of Coleus forskohlii (syn Plectranthus forskohlli wild) briq. Res J Life Sci Bioinform Chem Sci 55:74–581
Wikimedia commons. Accessed 21 Dec 2023. https://commons.wikimedia.org/wiki/File:Forskolin.svg
Xiang L, Zhu S, Zhao T, Zhang M, Liu W, Chen M et al (2015) Enhancement of artemisinin content and relative expression of genes of artemisinin biosynthesis in Artemisia annua by exogenous MeJA treatment. Plant Growth Regul 75:435–441. https://doi.org/10.1007/s10725-014-0004-z
Yan KU, Gao LN, Cui YL, Zhang YI, Zhou XI (2016) The cyclic AMP signaling pathway: exploring targets for successful drug discovery. Mol Med Rep 13(5):3715–3723. https://doi.org/10.3892/mmr.2016.5005
Acknowledgements
Roshni acknowledges Yenepoya Deemed to be University for providing the fellowship.
Funding
No funding was received to assist with the preparation of manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: PDR; Literature search, data analysis and drafted: PTR.
Corresponding author
Ethics declarations
Conflict of interest
Authors declares that they have no conflict of interest.
Ethical approval
This article does not contain the study involving humans and animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roshni, P.T., Rekha, P.D. Biotechnological interventions for the production of forskolin, an active compound from the medicinal plant, Coleus forskohlii. Physiol Mol Biol Plants 30, 213–226 (2024). https://doi.org/10.1007/s12298-024-01426-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-024-01426-9