Abstract
Trehalose being an integral part for plant growth, development and abiotic stress tolerance is accumulated in minute amounts in angiosperms with few exceptions from resurrection plants. In the current study, two rice cultivars differing in drought tolerance were used to analyse the role of trehalose in modulating photosynthesis and ROS-antioxidant balance leading to improvement in drought tolerance. Accumulation of trehalose in leaves of Vaisakh (drought-tolerant) and Aiswarya (drought-sensitive) rice cultivars was observed by spraying 50 mM trehalose and 100 µM validamycin A (trehalase inhibitor) followed by vacuum infiltration. Compared to stress sensitive Aiswarya cultivar, higher trehalose levels were observed in leaves of Vaisakh not only under control conditions but also under drought conditions corresponding with increased root length. The increase in leaf trehalose by treatment with trehalose or validamycin A corresponded well with a decrease in electrolyte leakage in sensitive and tolerant plants. Decreased ROS levels were reflected as increase in antioxidant enzyme activity and their gene expression in leaves of both the cultivars treated with trehalose or Validamycin A under control and drought conditions signifying the importance of trehalose in modulating the ROS-antioxidant balance for cellular protection. Further, higher chlorophyll, higher photosynthetic activity and modulation in other gas exchange parameters upon treatment with trehalose or validamycin A strongly suggested the beneficial role of trehalose for stress tolerance. Trehalose accumulation helped the tolerant cultivar adjust towards drought by maintaining higher water status and alleviating the ROS toxicity by effective activation and increment in antioxidant enzyme activity along with enhanced photosynthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa) is the widely cultivated crop considered as staple food of not only India but also in different parts of Asia, Latin America and Africa. Fluctuations in the environmental conditions pose a threat of stress to rice plants affecting their growth and productivity. Decades of breeding practices and research has led to development of drought tolerant rice varieties with varied stress tolerance limit (Reyes 2023). Among the varied mechanisms, accumulation of compatible solutes and sugars are considered effective as they play a crucial role in cellular protection and abiotic stress tolerance (Blum 2017; Huang et al. 2020). In many instances, some of the unusual sugars such as raffinose, stachyose, verbascose, octulose etc. were also observed in several of the plant species whose functions are not yet clearly understood (Peters et al. 2007; Dinakar and Bartels 2013; Egert et al. 2015; Sengupta et al. 2015). Drought induced accumulation of trehalose although is not common in many plants, is observed in several desiccation tolerant plants such as Selaginella lepidophylla and Myrothamnus flabellifolia (Drennan et al. 1993; Iturriaga et al. 2009). The trehalose accumulating transgenic plants, with altered trehalose biosynthesis or degradation showed tolerance towards multiple stresses suggesting its importance for abiotic stress tolerance (Li et al. 2011; Lin et al. 2019; Kosar et al. 2019; Hassan et al. 2022). Modulation in physiological, biochemical, molecular and photosynthetic functions upon exogenous application of trehalose is also observed in several plants including Arabidopsis thaliana, Triticum aestivum and Zea mays (Yang et al. 2014; Kosar et al. 2019; Hassan et al. 2022; Zhang et al. 2022).
Drought stress reduces leaf water potential, turgor pressure and affects various physiological and biochemical functions including photosynthesis, chlorophyll synthesis and carbohydrate metabolism significantly affecting the growth and cellular homeostasis ultimately leading to drastic decrease in crop productivity and causing cell death (Biswas et al. 2016; Bashir et al. 2021; Kaur et al. 2021; Challabathula et al. 2022). The declining water status is critical as most of rice cultivated areas are rain fed and the cultivars developed mostly seek good irrigation indicating the importance of water for productivity of rice plants. The prolonged drought increases the cellular ROS levels leading to oxidative damage. Although ROS production and signalling is important in eliciting the antioxidant defence responses for withstanding stress and protecting the cellular integrity, sensitive cultivars fail to employ the mechanisms that could efficiently regulate ROS accumulation thereby resulting in increased oxidative damage. The accumulation of ROS such as singlet oxygen (1O2), superoxide radical (O2−), hydrogen peroxide (H2O2) and the hydroxyl radical (HO•) constitutes unrestricted oxidation of cellular components leading to oxidative damage and cell death. Prolonged drought makes the sensitive cultivars vulnerable to ROS induced damages, predominantly the lipid peroxidation of membranes and oxidation of amino acid side chains and protein backbone (Asada 2006; Das and Roy-Choudhury 2014; Mittler 2017). Higher H2O2 accumulation was observed in the sensitive rice cultivar, Aiswarya during drought than the tolerant cultivar Vaisakh suggesting the effective ROS management in tolerant cultivar (Challabathula et al. 2022). In another study, although, the salt tolerant cultivars showed higher generation of H2O2 and higher activity of ROS generating enzyme NADPH oxidase, it was compensated by a higher activity of both enzymatic and non-enzymatic antioxidant system (Kaur et al. 2016). Compared to drought sensitive plants, the drought tolerant plants are endowed with several mechanisms for decreasing the ROS induced oxidative damage, maintenance of the cellular water status and limitation in membrane damage during stress (Sharma and Dubey 2005; Das and Roychoudhury 2014; Mishra and Panda 2017; Irato and Santovito 2021; Challabathula et al. 2022).
Plants sense the changes in water deficit and send the signals from root to leaves by accumulating abscisic acid (ABA) (Biswas et al. 2016). The ABA signalling is necessary to control and initiate the stomatal closure preventing water loss by transpiration. An ABA independent regulatory system also exists in inducing the stomatal closure which later gets the assistance from ABA accumulation (Tombesi et al. 2015). The stomatal closure, as a drawback, results in the limitation of gas exchange thereby decreasing the photosynthesis. Since the entry of CO2 is restricted due to stomatal closure, the photosynthesis majorly depends on internal CO2 availability. The inadequate availability of CO2 to chloroplasts results in decreased Ribulose-1-5-bisphosphate carboxylase oxygenase (RubisCO) activity thereby affecting photosynthesis. Drought also is known to affect the chloroplast, by damaging its ultrastructure, damaging the thylakoids and degrading chlorophyll molecules (Wang et al. 2022). Free radical generation and lipid peroxidation also are drought associated changes that occur in chloroplasts. In stress tolerant plants, photosynthesis is maintained to a certain extent by early sensing and preparing for an upcoming stress. Maintenance of higher intracellular CO2 levels, higher photosynthesis rate and enhanced antioxidant defence system are significant features of drought tolerant plants (Ullah et al. 2021). The accumulation of osmolytes is one of the important strategies adopted by tolerant plants. Stress induced expression of genes involved in osmolyte biosynthesis, water channel proteins and protective enzymes are also known to be involved in protection from drought stress induced oxidative damage (Ullah et al. 2021).
Trehalose accumulation in plants leads to stress tolerance. The accumulation of trehalose in the dehydrated tissues of resurrection plants are significant and are often discussed for the association of this sugar with to stress tolerance. The desiccation/dehydration induced changes in cells causes membrane damage and disintegration of macromolecules. Trehalose acts as an osmoprotectant molecule stabilizing the cell membranes and proteins by forming a glassy matrix around them. The modulation of oxidative stress response, induction of stress responsive genes, improved photosynthesis is observed in plants accumulating trehalose (Luo et al. 2021; Zhang et al. 2022; Mohanan et al. 2023). Rice plants exposed to drought stress showed increased accumulation of trehalose. Further, the transgenic rice plants accumulating trehalose showed improved drought tolerance (Mostofa et al. 2015a; Kosar et al. 2019; Sadak et al. 2019; Hassan et al. 2022). The accumulation of trehalose either through exogenous application or by generation of transgenic plants harbouring genes for trehalose metabolism has led to enhanced drought tolerance with better photosynthesis (Garg et al. 2002; Karim et al. 2007; Van Houtte et al. 2013). The exogenous application of trehalose leads to the accumulation of trehalose along with induction of stress responsive dehydrin genes in Arabidopsis thaliana plants (Mohanan et al. 2023). Another approach for the accumulation of trehalose in leaves can be achieved through the application of trehalase inhibitor validamycin A. Validamycin A is an aminoglycoside antibiotic with strong potential to inhibit trehalases in plants, insects and fungi. Further due to its fungicidal and antibacterial activity, it is used to control sheath-blight disease in rice and soil borne pathogens (Ishikawa et al. 2005; Yang et al. 2023). In A. thaliana, validamycin A increases the trehalose accumulation by effectively inhibiting the trehalase enzyme activity (Müller et al. 2001; Vogel et al. 2001). Further, in Tobacco plants, 100 µM validamycin inhibited more than 99% of trehalase activity indicating its effectiveness in increasing the trehalose accumulation in plants (Goddijn et al. 1997).
In our previous study, the differential modulation of photosynthesis, ROS and antioxidants were evaluated in drought stress sensitive and tolerant rice cultivars (Aiswarya and Vaisakh) along with salt stress tolerant cultivar Vyttila upon inhibition of cytochrome oxidase (COX) and alternative oxidase (AOX) pathways of mitochondrial oxidative electron transport during drought and salinity stress (Challabathula et al. 2022). The current study is focused on exploring the importance of trehalose for drought stress tolerance using the stress tolerant Vaisakh and sensitive Aiswarya plants. The modulation in photosynthesis and ROS-antioxidant balance in drought sensitive and tolerant rice cultivars upon treatment with trehalose or validamycin during drought stress conditions was analysed to shed light on the importance of trehalose for photosynthesis and ROS-antioxidant homeostasis during drought stress.
Materials and methods
Plant growth and treatment
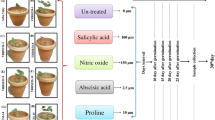
Seeds of drought sensitive rice cultivar Aiswarya were obtained from the Regional Agricultural Research Station, Pattambi, Kerala and the seeds of drought tolerant rice cultivar Vaisakh were obtained from Rice Research Station, Vyttila, Kerala, India. The seeds were surface sterilized with Sodium hypochlorite (4% v/v) followed by thoroughly washing them with sterile distilled water. After cold-stratification at 4 oC for three days, the seeds were directly sown in circular pots (7 cm height and 5 and 6 cm diameter at bottom and top respectively) containing sterile, dry, potting mixture consisting of red soil, farmyard manure and soilrite (1:1:1 ratio). The pots filled with 75 gm of the potting mixture were wetted with 100 ml of water. The pots were transferred to growth chambers maintained at 25 oC/ 22 oC the day/ night temperature, and 8/16 hrs light (110 µmol m−2 sec−1 PPFD illumination) /dark photoperiods. While control plants were regularly irrigated with 50 ml of water, drought stress was imposed by discontinuing irrigation of pots which were initially maintaining at a constant soil moisture of > 90%. The trehalose (50 mM) and validamycin A (100 µM) treatment was done by foliar spraying and vacuum infiltration (20 KPa pressure for 2 min) of 10 ml of solution prior to imposing drought stress treatment following the method described by Challabathula et al. (2022). Drought stress treatments were terminated after ten days and the leaf samples were collected for further analysis.
Measurement of relative water content (RWC) and root length
Leaf RWC was measured according to Kuroki et al. (2019). Even-sized leaves (3 replicates for each treatment) from the control and drought stress treated rice cultivars with and without trehalose and validamycin A treatment were cut from base and the fresh weight (FW) was measured. The leaves were immersed in distilled water for 24 h and the turgid weight (TW) was measured. The dry weight (DW) was measured after drying the leaves for 24 h at 80 oC in a hot air oven. The leaf RWC was calculated using the following formula:
Roots from the control and drought stress treated rice cultivars with and without trehalose and validamycin A treatment were removed from the soil and cleaned with fresh water prior to measuring the root length. Root length (in cms) of at least 10 plants from each treatment were measured using a standard measuring scale starting from the base to the tip of the longest root.
Trehalose estimation
Trehalose estimation from the leaves of control and drought stress treated rice cultivars with and without trehalose and validamycin A treatment were carried out as per the method described by Mostofa et al. (2015a). The leaves, 50 mg from each treatment were cut, ground with 1 ml of hot 80% ethanol followed by centrifugation at 10,000 rpm for 20 min at room temperature. The supernatants collected were dried at 80 °C to remove the ethanol traces and were re-suspended in 5 ml of distilled water. The 300 µl of 0.2 N sulphuric acid was added to 200 µl of the suspended solution and were boiled at 100 °C for 10 min followed by chilling on ice for 5 min. To this mixture, 300 µl of 0.6 N sodium hydroxide was added and was boiled for 10 min and were cooled on ice for 5 min. To this, 4 ml of anthrone reagent was followed by boiling for 10 min and chilling for 5 min respectively. The absorbance of the solutions was recorded at 630 nm using a UV - visible spectrophotometer. The trehalose contents were calculated using a standard curve and the graphs were plotted.
Measurement of leaf electrical conductivity (EC) and histochemical staining for ROS accumulation in leaves
For the assessment of membrane damages in leaves, leaf electrical conductivity was measured following the methodology described by Challabathula et al. (2022). Even sized leaves from control and drought stress treated rice cultivars with and without trehalose and validamycin A treatment were detached and were cut into smaller pieces and dispersed and vortexed in 10 ml of ultrapure distilled water. Initial conductivity (EC1) of the solution was measured after 2 h of incubation using a conductivity meter 304, conductivity cell type CD-10 cell K1 ± 10% (Systronics India Ltd, India) set within a range of 2 mS at 25 oC. The final conductivity (EC2) was measured after boiling the samples at 100 oC for 10 min. The relative leaf EC was calculated as per Muchate et al. (2019) using the following formula,
The histochemical staining for ROS such as H2O2 and O2− in the leaves of control and drought stress treated rice cultivars with and without trehalose and validamycin A treatment was performed according to Taj and Challabathula (2021). Histochemical staining of leaves for H2O2 was performed with 3, 3’-diaminobenzidine (DAB) and O2− was performed using nitro blue tetrazolium (NBT). The leaves that were cut from the base were immersed in either 1 mg ml−1, NBT or 1 mg ml−1 DAB in 10 mM potassium phosphate buffer pH 7.8 followed by vacuum infiltration for 2 min at 20 KPa for three times. The samples were kept under darkness for overnight followed by illumination under continuous light (300 µmol m−2 s−1) for 8 h. The pigments of the leaves were removed by destaining the leaves with methanol, acetic acid, and glycerol in a 3:2:1 ratio and fixed using fixative solution. After complete destaining, the Images were taken at 5 x magnification using a stereo zoom microscope, Nikon SMZ800N attached with Nikon DS-Fi3 camera (Nikon, Japan).
In-gel antioxidant activity staining
In-gel antioxidant enzyme activities were performed by specific staining procedures by following the methodology described by Analin et al. (2020). The proteins were extracted from 500 mg of leaf samples from control and drought stress treated rice cultivars with and without trehalose and validamycin A treatment by grinding in liquid nitrogen and thoroughly dissolving in the extraction buffer consisting of 1mM PMSF, a protease inhibitor, in 50mM sodium phosphate buffer pH 7.0 followed by centrifugation at 12,000 rpm for 5 min at 4 °C. The supernatant was used for protein quantification using Lowry’s method (Lowry et al. 1951). Native PAGE was done on gel consisting of 10% resolving gel (10% acrylamide-bisacrylamide, 375mM Tris-HCl pH 8.8, 10% glycerol, 0.05% Ammonium Persulphate; APS and 0.05% tetramethylethylenediamine; TEMED) and stacking gel containing 4% acrylamide-bisacrylamide, 126mM Tris-HCl pH 6.8, 10% glycerol, 0.05% APS and 0.05% TEMED. From the extracted total protein, 100 µg protein was loaded onto the gels and were run in the running buffer (tris - glycine for SOD and CAT, and tris-glycine-ascorbate for APX) for overnight at 4 °C at 30 V. Prior to the protein loading, the gels were pre-run in their respective running buffers. The gels were stained for the visualization of enzyme bands. For SOD, the gel was initially soaked in 2.45 mM NBT and incubated in dark for 20 min. The solution was removed and the gel was incubated in a mixture containing 28 mM TEMED and 2.4 µM riboflavin in 50 mM potassium phosphate pH 7.8 buffer and kept under illumination. Transparent bands were seen in violet stained background after 30 min of incubation. For CAT activity, the respective gel was washed with distilled water thrice, 15 min each time. The gel was then incubated in 20 mM hydrogen peroxide for 10 min in dark. After discarding the solution, the gel was washed with distilled water thrice, 5 min each time. Then, 1% ferric chloride and 1% potassium ferricyanide were prepared separately and poured onto the gel. After 10 to 15 min, transparent bands in green stained background were observed (Woodbury et al. 1971). The gel run with tris- glycine -ascorbate was used to stain for APX. After electrophoresis, the gel was initially washed thrice with 2 mM ascorbic acid in 50 mM sodium phosphate buffer pH 7.0, 10 min each time followed by incubation in a solution containing 4 mM ascorbic acid and 20 mM hydrogen peroxide in 50 mM sodium phosphate buffer pH 7.0 for 20 min. Then, a solution of 28 mM TEMED and 2.4 mM NBT in 50 mM potassium phosphate buffer pH 7.8 was prepared and added to the gel. The gel was incubated for 20 min until transparent bands were visible in dark blue background (Lee and Lee 2000). The gels were scanned using the scanner, CanoScan LiDE120 (Canon, USA).
RNA extraction and gene expression
The RNA extraction was performed following the method described by Valenzuela-Avendaño et al. (2005). The leaves of control and drought stress treated rice cultivars with and without trehalose and validamycin A treatment were ground in liquid nitrogen using the extraction buffer (38% buffer saturated phenol, 0.8 M guanidine thiocyanate, 0.4 M ammonium thiocyanate, 0.1 M sodium acetate and 5% glycerol). The leaf extracts incubated in room temperature for 10 min were centrifuged for 10 min at 9200 rpm and 300 µl of chloroform-isoamyl alcohol was added to the supernatant, vortexed and centrifuged at 9200 rpm for 10 min at 4 °C. In a fresh tube, the aqueous phase on the upper side was taken and 375 µl each of isopropanol and 0.8 M sodium citrate/1 M sodium chloride were added and incubated at room temperature for 10 min followed by centrifugation at 10,100 rpm for 10 min at 4 °C. The pellet was washed using ice cold 70% ethanol followed by centrifugation at 10,100 rpm for 10 min at 4 °C. The RNA pellets were dissolved in RNase free water and quantified using Genova nano spectrophotometer (Jenway, UK). Using iScript cDNA synthesis kit, the cDNA was synthesized using 1 µg of RNA (Bio-rad, USA) which is used as template in RT-PCR reaction along with gene specific primers for monitoring the gene expression levels. The list of primers and the sequences are provided in the Supplementary Table 1. Actin was used as a house keeping gene. The PCR program consisted of initial denaturation and denaturation both set at 95 oC for 1 min followed by annealing for 1 min set at 60 oC. The extension was set at 72 oC for 2 min. A final extension was done at 72 oC for 2 min with 28 cycles. The amplified products along with 1 kb DNA ladder were separated on 2% agarose gel, and the bands were visualized using gel documentation system (Fusion Solo S, Vilber Lour mat, France).
Chlorophyll estimation
Chlorophyll estimation was done according to Arnon (1949). From the leaves of control and drought stress treated rice cultivars with and without trehalose and validamycin A treatment, 100 mg leaves were ground with 80% acetone at 4 oC under dark. The extracts were centrifuged and the supernatants were used for chlorophyll estimation spectrophotometrically by measuring the absorbance of chlorophyll a, chlorophyll b, at 663, and 645 nm, respectively. Arnon (1949) equations were used to calculate the chlorophyll contents.
,
\(\text{C}\text{h}\text{l}\text{o}\text{r}\text{o}\text{p}\text{h}\text{y}\text{l}\text{l} \text{ b} \left(\text{m}\text{g} {\text{ g}}^{-1}\text{F}\text{W}\right)=\left(22.9\text{ x} \left(\text{A}\text{b}645\right)-4.68 \left(\text{A}\text{b}663\right)\right)\text{x}\frac{\text{V}}{100 }\text{x}\text{W}\) and
where V is final volume of chlorophyll extracted in 80% acetone; and W, fresh weight of the leaf used.
Measurements of leaf gas exchange parameters
The leaf gas exchange parameters including CO2 assimilation rate, stomatal conductance (gs), intercellular CO2 concentration and the ratio of intercellular to ambient CO2 concentration (Ci/Ca) were measured in the leaves of control and drought stress treated rice cultivars with and without trehalose and validamycin A treatment using a portable photosynthesis system (LI-6400 XT; LI-COR Inc., Lincoln, Neb. USA) following Yao et al. (2017) and Analin et al. (2020). Four leaves of the rosette were used for covering the 2 cm2 chamber area of the gas analyser. A PPFD of 1500 µmol photons m−2 s−1, CO2 concentration at 400 µmol mol−1 and temperature 25 ± 2 oC were set for the measurements.
Statistical analysis
All the experiments were conducted in at least three replicates and data represents an average of three biological replicates. The normality of the data was performed using Shapiro–Wilk test. The statistical analysis was done using Multiple comparison by means of Two-way ANOVA, the Tukey’s method. The statistically significant different groups were represented with different alphabets over the bars showing significant differences among them. (p = < 0.001) with an overall significance level = 0.05. Error bars represent means ± SE (n = 3) obtained from three biological replicates. The calculation of statistics and plotting of graphs were done using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, California USA).
Results
Trehalose accumulation in rice cultivars alters the drought associated physiological changes
As already reported by Challabathula et al. (2022), the drought tolerant cultivar Vaisakh showed superior response than the sensitive cultivar Aiswarya which was reflected in its phenotype, leaf relative water content levels and leaf electrical conductivity (EC) during drought stress (Fig. 1). Upon drought, while the sensitive cultivar maintained RWC < 20% which resulted in drying with increased EC (82%, Fig. 1a and b), the tolerant cultivar maintained higher RWC and comparatively lower EC than the sensitive cultivar (Fig. 1c). Interestingly, the leaves of the tolerant plant showed higher trehalose (3.54 µg g−1 FW) levels in its leaves than the sensitive cultivar (1.6 µg g-1 FW) under control conditions hinting towards the protective role of this sugar (Fig. 1d). Further, the trehalose accumulation in leaves of both sensitive and tolerant plants was although found to be enhanced by treatment with either 50 mM trehalose or 100 µM validamycin A, higher trehalose accumulation was observed in stress tolerant cultivar Vaisakh (Fig. 1d). The accumulation of trehalose helped both the cultivars to maintain a higher RWC and a lower EC indicating the importance of trehalose in drought tolerance.
Trehalose accumulation and associated changes in rice cultivars Vaisakh and Aiswarya during drought. The plants were subjected to drought stress for 10 days and trehalose and validamycin A treatments were given as described in Materials and methods. The treatments are represented as; control plants, C; control + 50 mM trehalose, TC; control + 100 µM validamycin A, VC; drought stressed, D; drought + 50 mM trehalose, TD; and drought + 100 µM validamycin A, VD. “dh” indicates dehydration/drought stress treatment. Unstressed and drought stressed Vaisakh (grey bars) and Aiswarya (black bars) were represented with solid and pattern filled bars respectively. a The phenotypic changes in Vaisakh and Aiswarya. b The relative water content, c electrical conductivity and d trehalose accumulation in Vaisakh and Aiswarya cultivars is plotted. Two-way ANOVA using Tukey’s multiple comparisons method was used to assess the statistical significance. Different alphabets indicate statistically significant differences among each group. P = < 0.001 with overall significance level = 0.05. Error bars represent means ± SE (n = 3)
Trehalose accumulation alters root length in rice cultivars during drought stress
Trehalose accumulating seedlings of drought stress tolerant Vaisakh and drought stress sensitive Aiswarya cultivars showed differential root growth patterns. While significant increase in root growth was observed in Vaisakh cultivar upon trehalose accumulation, the root growth was marginally increased in Aiswarya cultivar (Fig. 2a, b and c). Although, drought stress has led to decrease in root growth in Vaisakh plants, accumulation of trehalose due to trehalose/validamycin A supplementation has led to increased root growth.
Root growth in drought sensitive and drought tolerant rice cultivars treated with trehalose and validamycin A prior to imposing drought stress conditions. The validamycin A and trehalose treatments were done as described in Materials and methods and the plants were subjected to drought stress for 10 days. Root morphology of (a) Vaisakh, (b) Aiswarya and (c) root length in rice cultivars is shown. The treatments are represented as; control plants, C; control + 50 mM trehalose, TC; control + 100 µM validamycin A, VC; drought stressed, D; drought + 50 mM trehalose, TD; and drought + 100 µM validamycin A, VD. “Dehy” indicates dehydration/drought stress treatment. Unstressed and drought stressed Vaisakh (grey bars) and Aiswarya (black bars) were represented solid and pattern filled bars respectively. Two-way ANOVA using Tukey’s multiple comparisons method was used to assess the statistical significance was represented with different alphabets representing statistically significant differences among each group. P = < 0.001 with overall significance level = 0.05. Error bars represent means ± SE (n = 10). Scale bar represents 1 cm
Trehalose accumulation differentially modulates oxidative stress responses in drought sensitive and tolerant rice cultivars
The oxidative stress responses of the trehalose accumulating drought sensitive and drought tolerant rice cultivars were assessed by analysing the ROS accumulation in leaves by histochemical staining and associated antioxidant enzyme activities of SOD, CAT and APX by activity staining. Drought stress has resulted in increased ROS, (H2O2) in leaves of sensitive cultivar which was aggravated with validamycin A and trehalose treatment (Fig. 3a). Although, mild increase in ROS levels were observed in drought tolerant cultivar, the H2O2 levels were lower than those observed in leaves of stress sensitive cultivar Aiswarya. Further, trehalose accumulation resulted in decline in H2O2 accumulation during drought stress in stress tolerant cultivar. Compared to leaves of Vaisakh, higher levels of superoxide radicals were observed during drought stress conditions in the leaves of Aiswarya cultivar. However, the changes were marginal in the presence of validamycin A and trehalose. The results with ROS were in agreement with in-gel antioxidant enzyme activity wherein SOD activity was higher in both the cultivars upon trehalose and validamycin treatment during drought stress. Although, the induction in SOD and APX activities by drought were observed in both the cultivars, the induction of antioxidant enzyme activities during drought and with the treatment of trehalose and validamycin A were comparatively higher in tolerant cultivar than in sensitive cultivar (Fig. 3b). The induction in the CAT activity was observed by the treatment with trehalose and Validamycin A in leaves of Vaisakh plants, however, the CAT activity is not observed during drought stress. Compared to Vaisakh plants, the leaves of Aiswarya plants showed higher CAT activity during drought stress. Further, while the gene expression levels of APX increased with trehalose and validamycin A treatment in both Vaisakh and Aiswarya (Fig. 4), higher expression levels were observed in the Vaisakh cultivar (Fig. 4a and b). Increased expression of APX and Cu/Zn SOD in leaves of Vaisakh plants during drought upon treatment with validamycin A was observed. The expression of CAT gene was only observed in trehalose and validamyin A treated leaves of Vaisakh plants (Fig. 4a and b). The results indicated modulation of ROS accumulation and associated antioxidant activities in trehalose accumulating plants during stressed and unstressed conditions.
Histochemical staining for ROS and analysis of antioxidant enzyme activity in rice cultivars differing in drought tolerance upon treatment with trehalose and validamycin A prior to imposing drought stress conditions. The localization of (a) H2O2 and superoxide in Vaisakh (left plate) and Aiswarya (right plate) were done by staining with diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) respectively. (b) The in-gel antioxidant enzyme activity of SOD, CAT and APX. The treatments are represented as; control plants, C; drought stressed, D; control + 50 mM trehalose, T; drought + 50 mM trehalose, TD; control + 100 µM validamycin A, V; and drought + 100 µM validamycin A, VD.
The expression analysis of genes encoding antioxidant enzymes Cu/Zn SOD, CAT, and APX in the trehalose accumulating leaves of drought-tolerant Vaisakh and drought-sensitive Aiswarya during drought stress conditions. Actin was used as a reference gene for constitutive expression. The treatments are represented as; control plants, C; drought stressed, D; control + 50 mM trehalose, T; drought + 50 mM trehalose, TD; control + 100 µM validamycin A, V, and drought + 100 µM validamycin A, VD. “ne” denotes no expression and “Dehy” indicates dehydration/drought stress treatment. A densitogram was made based on the relative expression of gene of interest with actin. (a) The gel picture is with the relative expression of (b) APX, ( Cu / Zn SOD and CAT expression in Vaisakh (grey bars) and Aiswarya (black bars). Unstressed and drought stressed plants are shown as solid and pattern filled bars respectively. Two-way ANOVA using Tukey’s multiple comparisons method was used to assess the statistical significance was represented with different alphabets representing statistically significant differences among each group. P = < 0.001 with overall significance level = 0.05. Error bars represent means ± SE (n = 3)
Chlorophyll accumulation in leaves of Vaisakh and Aiswarya cultivars upon treatment with trehalose and validamycin A under drought stress conditions.
Increased chlorophyll content was observed in the leaves of Vaisakh and Aiswarya cultivars upon treatment with trehalose and validamycin A (Fig. 5). However, the chlorophyll content was significantly higher in leaves of Vaisakh than Aiswarya. Among chlorophyll a and chlorophyll b contents, while the chlorophyll a content showed significant increase in trehalose or validamycin a treated leaves of Vaisakh plants, the chlorophyll b content was decreased (Fig. 5b). Contrary to this, the decline in chlorophyll a content and an increase in chlorophyll b content was observed after trehalose and validamycin treatment in Aiswarya cultivar (Fig. 5a and b). During drought, comparatively higher chlorophyll a and chlorophyll b levels were observed in the leaves of Vaisakh plants than Aiswarya plants upon supplementation with trehalose and validamycin A (Fig. 5a, b).
Chlorophyll content in leaves of trehalose accumulating drought-tolerant Vaisakh and drought-sensitive Aiswarya subjected to drought stress. The treatments are represented as; control plants, C; control + 50 mM trehalose, TC; control + 100 µM validamycin A, VC; drought stressed, D; drought + 50 mM trehalose, TD; drought + 100 µM validamycin A, VD. “dh” indicates dehydration/drought stress treatment. Unstressed and drought stressed plants are shown as solid and pattern filled bars respectively. (a) Chlorophyll a (b) Chlorophyll b and (c) total chlorophyll in Vaisakh (grey bars) and Aiswarya (black bars). Two-way ANOVA using Tukey’s multiple comparisons method was used to assess the statistical significance. Different alphabets above the bars represent statistically significant differences among each group P = < 0.001 with overall significance level = 0.05. Error bars represent means ± SE (n = 3)
The photosynthetic responses towards trehalose accumulation in rice cultivars during drought stress
The CO2 assimilation rates were comparatively higher (5.129 µmol CO2 m−2 s−1, Fig. 6a) in the leaves of Vaisakh than Aiswarya (2.361 µmol CO2 m−2 s−1, Fig. 6a). Further, the rates were significantly increased when the leaves of Vaisakh plants were treated with 50 mM trehalose or 100 µM validamycin A. Similar observations were noticed for other gas exchange parameters such as stomatal conductance (gs, Fig. 6b) internal CO2 (Ci, Fig. 6c) and Ci/Ca ratio (Fig. 6d). In stress sensitive cultivar Aiswarya, trehalose treatment has led to 3-fold increase in CO2 assimilation and 2-fold increase when treated with validamycin A (Fig. 6a). The other gas exchange parameters also increased with trehalose treatment, but decreased with validamycin A treatment. A drastic decrease in CO2 assimilation rates and gs were observed in both the cultivars when exposed to drought. While the leaves of Vaisakh maintained comparatively higher CO2 assimilation rates (0.516 µmol CO2 m−2 s−1), the leaves of Aiswarya showed a very low CO2 assimilation rate (0.033 µmol CO2 m−2 s−1) with higher gs (0.023 mol H2O m−2 s−1, Fig. 6a and b). Trehalose accumulation through 50 mM trehalose and 100 µM validamycin, improved the gas exchange parameters where Vaisakh showed higher photosynthetic CO2 assimilation rates.
Photosynthetic gas exchange parameters analysed in trehalose accumulating drought-tolerant Vaisakh and drought-sensitive Aiswarya plants subjected to drought stress. The treatments are represented as; control plants, C; control + 50 mM trehalose, TC; control + 100 µM validamycin A, VC; drought stressed, D; drought + 50 mM trehalose, TD; drought + 100 µM validamycin A, VD. “dh” indicates dehydration/drought stress treatment. (a) CO2 assimilation rates (b) stomatal conductance (c) Ci (d) Ci/ Ca in Vaisakh (grey bars) and Aiswarya (black bars) cultivars are plotted. Two-way ANOVA using Tukey’s multiple comparisons method was used to assess the statistical significance. Different alphabets represent statistically significant differences among each group P = < 0.001 with overall significance level = 0.05. Error bars represent means ± SE (n = 3)
Correlation of physiological parameters with trehalose accumulation in leaves of Vaisakh and Aiswarya plants
While the trehalose accumulation in leaves of Vaisakh plants under unstressed condition showed a positive correlation with physiological parameters like RWC (r = 0.95), total chlorophyll content (0.94) and the gas exchange parameters like CO2 assimilation (0.65), gs (0.98), ci (0.94) and Ci/ Ca ratio (0.93), the EL was negatively correlated (-0.42). This was consistent with the antioxidant gene expression of Cu/ Zn SOD (0.99), APX (1) and CAT (0.49). Although the trehalose accumulating leaves of drought stressed Vaisakh plants showed negative correlation with root length, EL, gs, Ci and Ci/Ca, the positive correlation with RWC (0.99), CO2 assimilation (0.86) and antioxidant gene expression was observed. In contrast to this, the drought sensitive Aiswarya cultivar with trehalose treatment showed positive correlation for photosynthesis and APX gene expression under control conditions and RWC, chlorophyll content, photosynthetic CO2 assimilation and APX gene expression under drought stress conditions. These results indicated a trehalose assisted improvement of physiological, biochemical characteristics along with improvement in antioxidant gene expression and gas exchange parameters helping the tolerant cultivar to manage dehydration.
Discussion
Drought tolerance is a complex phenomenon involving mechanisms for efficient sensing of dehydration along with recruitment of mechanisms for defending and minimizing the stress associated damage. Rice cultivars differing in the sensitivity and tolerance to drought serve as valuable resource for identifying the mechanisms of stress tolerance. Analysing the intrinsic differences in between drought sensitive and tolerant cultivars and identifying the mechanisms employed by the tolerant plants is important to unravel the trivial components for stress tolerance behaviour (Bartels and Dinakar 2013). Among the two rice cultivars used in the current study, Vaisakh is a drought tolerant and Aiswarya is drought sensitive (Fig. 1a, Challabathula et al. 2022). Previously, we used these cultivars along with salt tolerant rice cultivar Vyttila to analyse the importance of cytochrome oxidase and alternative oxidase pathways of mitochondrial oxidative electron transport chain in modulating photosynthesis, ROS and antioxidants during salinity and drought stress (Dinakar et al., 2022). In the current study, the importance of trehalose in modulating the photosynthesis and ROS-antioxidant balance during drought stress in Vaisakh and Aiswarya rice cultivars is analysed. Since the drought tolerant Vaisakh plants were able to rehydrate after 10 days of drought stress as indicated in our previous study (Challabathula et al. 2022), the drought stress was imposed for 10 days duration for both sensitive and tolerant plants. In the current and previous study with Aiswarya and Vaisakh cultivars, the Relative water content of leaves was considered as the primary indicator of stress wherein the tolerant cultivar maintained around 40% RWC which supported its tolerance and revival upon rehydration. Thus 10 days of dehydration stress was imposed.
Trehalose is known to maintain the oxidative balance and protect cells from stress mediated damage. Although trehalose accumulation was once thought to be restricted to only lower order plants and certain angiosperm resurrection plants, the identification of trehalose biosynthesis genes from many angiosperm plants suggested an active biosynthesis pathway. However, it is intriguing that irrespective of the presence of trehalose biosynthesis genes in plants, the accumulation in the tissues is very scarce or in untraceable amounts. The leaves of A. thaliana plants treated with 50 mM trehalose and 100 µM Validamycin resulted in the accumulation of trehalose in the range of 1.5- 2 µg g−1 FW and has shown improved ROS- antioxidant balance and induction of stress responsive dehydrin genes under unstressed conditions in A. thaliana plants (Mohanan et al. 2023). Hence, similar concentrations were used in the current study to study the role of trehalose during drought stress. The presence of trehalose observed in the leaves both Vaisakh and Aiswarya cultivars suggests the operation of active trehalose metabolic pathway in rice cultivars (Fig. 1d). Further, compared to the stress sensitive cultivar, the significantly higher levels of trehalose observed in leaves of Vaisakh plants signifies the existence of differences in the accumulation of trehalose in between stress tolerant and sensitive cultivar. Furthermore, the drought induced increase in trehalose levels in stress tolerant Vaisakh plants indicates the active role of trehalose under stress conditions. The trehalose content in stress sensitive Aiswarya plants is not increased during drought coinciding with its stress sensitive nature (Fig. 1d). The increase in leaf trehalose content upon treatment of the leaves with trehalose or validamycin A suggests active uptake of trehalose from external supplementation (Fig. 1d). Further, the higher accumulation of trehalose in validamycin A treated leaves than trehalose treated leaves suggests higher activity of trehalase in the plants (Goddijn et al. 1997; Müller et al. 2001). Trehalase prevents the trehalose accumulation by breaking trehalose into two glucose molecules and validamycin A, due to structural similarity with trehalose shows higher affinity towards trehalase and inhibits its activity. In Arabidopsis, the trehalose accumulation gradually increased in their tissues with decreasing trehalase activity upon treatment of leaves with validamycin A (Müller et al. 2001). The increase in trehalose accumulation post trehalose and validamycin A treatment helped the rice cultivars to show positive response during drought stress. The drought along with validamycin A and trehalose promoted higher trehalose accumulation in Vaisakh plants than Aiswarya. Like the drought tolerant Vaisakh, the lower trehalose accumulation still improved stress responses as inferred from the water status and the lower membrane damages in Aiswarya (Fig. 1b, c and d).
Trehalose biosynthesis is a two-step process where trehalose is formed from glucose-6-phosphate and UDP-glucose through an intermediate sugar, trehalose-6-phosphate. Two enzymes, trehalose-6-phosphate synthase (TPS) and trehalose-6-phosphate phosphatase (TPP) are involved in the formation of trehalose-6-phosphate from the precursors and for conversion of trehalose-6-phosphate to trehalose (Iordachescu and Imai, 2008). Heterologous expression of yeast trehalose biosynthesis genes or overexpression of plant trehalose metabolism genes provided greater protection to plants from stress mediated injuries along with improvement of photosynthesis. Further, the overexpression of rice TPP led to significant increase in yield and improvement in photosynthesis during unstressed and mild drought stressed conditions in maize plants (Nuccio et al. 2015). While the overexpression of AtTPPF in A. thaliana resulted in increased trehalose accumulation with higher survival rate, improved recovery after drought stress and reduced ROS accumulation, mutant plants lacking TPPF showed higher H2O2 accumulation in its leaves (Lin et al. 2019). In agreement with this, the trehalose accumulated leaves of tolerant rice cultivar showed lower H2O2 accumulation than that was observed during drought in untreated plants (Fig. 3a). Contrastingly, the trehalose accumulation triggered the H2O2 accumulation in stress sensitive plants. This may be due to the trehalose mediated ROS accumulation and associated signalling (Fig. 3a). Similar to our observation, treatment of tobacco leaves with exogenously supplied trehalose triggered ROS accumulation (Shi et al. 2019). Trehalose induced increase in antioxidant activities are also observed in plants during abiotic stress conditions. In A. thaliana, the SOD, APX and POD activities increased with trehalose treatment during salt stress. Several reports suggested that the increased ROS accumulation followed by an increase in antioxidant activities and other positive stress responses lead to improved stress tolerance (Garg and Manchanda, 2009). The presence of multiple SOD isoforms in leaves of rice plants and their activation under stress conditions was reported earlier. Similarly, increased APX activity along with the appearance of new isoforms in stress tolerant rice plants under stress conditions was also reported (Challabathula et al. 2022). Sugars and their metabolic enzymes could possibly interact with ROS and its signalling pathways which directly or indirectly enhances the drought stress tolerance by modulating ROS production (Couée et al. 2006; Bolouri-Moghaddam et al. 2010). Exogenous application of sucrose and trehalose resulted in increased expression of antioxidant enzymes during drought (Kaur et al. 2021). In Triticum aestivum L., supplementation of trehalose resulted in increased O2- and H2O2 scavenging by activation of SOD and CAT (Luo et al. 2008). The rice cultivars, Vaisakh and Aiswarya used in the current study showed differences in their responses towards drought when they are accumulating trehalose. While the tolerant cultivar was equipped with better antioxidant system with increased activity by trehalose and stress, this was consistent even when the plants are unstressed. The higher activity of SOD and APX, might be the reason for these plants to have lower ROS during drought (Fig. 3b). Although similar response was observed in stress sensitive cultivar Aiswarya, increased ROS accumulation was observed during drought. These variations were also supported by the antioxidant gene expression where Vaisakh showed a higher Cu/Zn SOD and APX expression during trehalose treatment (Fig. 4a and b). This confirms the existence of differential modulation in oxidative balance during drought in drought tolerant and sensitive rice cultivars. The tolerant cultivar accumulated higher levels of trehalose than sensitive cultivar under non-stress conditions indicating its capability of early sensing of stress and adjusting their defence strategies even before they experience the actual stress.
Photosynthesis is an important phenomenon that is known to be severely affected by drought stress. Differences exist in the regulation of photosynthesis during drought in sensitive and tolerant cultivars. Compared to stress sensitive Aiswarya cultivar, the stress tolerant cultivar Vaisakh maintained a higher carbon assimilation and better gas exchange parameters at 1500 µmol photons m−2 s−1 PPFD (Taj and Challabathula 2021; Challabathula et al. 2022). Upon experiencing drought, plants minimize the gas exchange and transpiration process by forcing the stomata to close. The stomatal closure decreases the photosynthesis due to unavailability of CO2 and activates ABA as a mobile signal (Meyer and Genty 1998; Flexas et al. 2004). The decline in CO2 assimilation rates observed in both the cultivars during drought corresponds to lower stomatal conductance and lower internal CO2 levels (Challabathula et al. 2022; Fig. 6a-d). However, the induction in CO2 assimilation rates upon treatment with trehalose and validamycin A in both the cultivars indicates the importance of trehalose for photosynthetic activity (Fig. 6a). The association between CO2 assimilation rates and stomatal conductance reveals that the induction of CO2 assimilation rates were higher in trehalose and validamycin A treated leaves of tolerant cultivar Vaisakh than stress sensitive Aiswarya cultivar (Fig. 6). Although the stomatal conductance was relatively higher in trehalose treated leaves of Aiswarya cultivar, the photosynthetic CO2 assimilation rates were higher in Vaisakh cultivar. Lower stomatal density along with smaller stomatal size in drought tolerant cultivars exerts influence on stomatal conductance. Direct association in between the increase in the intracellular trehalose levels to increase in photosynthetic carbon assimilation rates are observed in both the cultivars suggesting the significant role played by trehalose in increasing the photosynthesis (Fig. 6a). However, since stress tolerant cultivar accumulated more amounts of trehalose, the CO2 assimilation rates were also higher. Increased Fv/Fm ratio was observed in maize and wheat plants treated with different concentrations of trehalose during heat stress indicating increased photosynthetic performance (Zhang et al. 2022). Similar responses were also observed in leaves of Ocimum basilicum L., (Zulfiqar et al. 2021). It was also reported that the exogenous trehalose application could protect the photosystem II in winter wheat by promoting the cyclic electron flow during drought stress (Luo et al. 2021). Reports also suggested trehalose interacting with the sugar signalling pathways leading to the enhancement in photosynthesis (Oszvald et al. 2018). The drought stress increased the Ci value and Ci/Ca ratio in both cultivars which was expected as a result of the mesophyll conductance (Fig. 6c and d). The trehalose accumulation comparatively reduced these parameters in drought stressed tolerant cultivar, indicating a lower CO2 release caused due to the stress induced photorespiration. It is also noted that the trehalose mediates the increase in photosynthesis during drought in tolerant cultivar which might be due to comparatively higher chlorophyll pigments.
Trehalose accumulation significantly increased the chlorophyll content in unstressed plants and protected them from drought stress induced damages. Although both Vaisakh and Aiswarya cultivars have shown increased total chlorophyll content upon trehalose or validamycin A treatment, the content was higher in Vaisakh cultivar (Fig. 5). Trehalose help the plants to maintain their photosynthesis by protecting the chloroplast ultrastructure, regulation of stomatal closure through ABA signalling, maintenance of water status, improvement in photosynthetic pigment concentration and improving the gas exchange parameters in unstressed and stressed conditions (Van Houtte et al. 2013; Wang et al. 2020; Luo et al. 2021). Vaisakh plants being stress tolerant were able to maintain a lower water loss, better oxidative balance, chlorophyll content, and improved photosynthesis and minimized drought associated damages that helped them to extend their survival to prolonged drought conditions. Drought sensitive Aiswarya, on the other hand, is susceptible to drought, showed higher water loss leading to membrane damage, increased ROS which are not compensated by timely detection and employing of sufficient antioxidant machinery leading to an altered metabolism leaving lower photosynthetic performance (Challabathula et al. 2022). Trehalose accumulation helps these cultivars in different ways to withstand drought, where the tolerant cultivar is supported by an improved root growth and lower oxidative damages. The trehalose accumulation counteracts the drought induced ROS accumulation with timely signalling and increasing the antioxidant enzyme activity along with a superior chlorophyll stability and gas exchange parameters. In sensitive cultivar, the trehalose accumulation may have role in ROS signalling which results in antioxidant defence but up to a certain extent of water loss.
Conclusions
The active trehalose metabolic pathway is operational in both drought sensitive and drought tolerant rice cultivars with differential accumulation of trehalose. Compared to the stress sensitive cultivar, significantly higher levels of trehalose observed in leaves of drought stress tolerant Vaisakh plants emphasize the importance of trehalose for stress tolerance. Further, trehalose or validamycin A treatment led to increase in trehalose content in the leaves promoting drought tolerance. Compared to stress sensitive Aiswarya plants, drought stress and the treatment of leaves with validamycin A or trehalose has led to higher trehalose accumulation in Vaisakh plants. Trehalose accumulation helped tolerant cultivar, Vaisakh, to adjust towards drought by maintaining a higher water status and decrease the ROS by effective activation of antioxidant enzyme activity and antioxidant gene expression along with enhanced photosynthesis. Further, the multifaceted roles of trehalose in signaling, activation of stress responsive genes/proteins, growth and development of plants during stress conditions needs to be clearly deciphered.
References
Analin B, Mohanan A, Bakka K, Challabathula D (2020) Cytochrome oxidase and alternative oxidase pathways of mitochondrial electron transport chain are important for the photosynthetic performance of pea plants under salinity stress conditions. Plant Physiol Biochem 154:248–259. https://doi.org/10.1016/j.plaphy.2020.05.022
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396. https://doi.org/10.1104/pp.106.082040
Bashir SS, Hussain A, Hussain SJ, Wani OA, Zahid Nabi S, Dar NA, Baloch FS, Mansoor S (2021) Plant drought stress tolerance: understanding its physiological, biochemical and molecular mechanisms. Biotechnol Biotechnol Equip 35(1):1912–1925. https://doi.org/10.1080/13102818.2021.2020161
Biswas A, Pandey H, Mishra RK et al (2016) Drought stress and metabolomics in plants. Technol Adv Plant Sci 1:65–87
Blum A (2017) Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ 40:4–10
Bolouri-Moghaddam MR, Le Roy K, Xiang L et al (2010) Sugar signalling and antioxidant network connections in plant cells. FEBS J 277:2022–2037
Challabathula D, Analin B, Mohanan A, Bakka K (2022) Differential modulation of photosynthesis, ROS and antioxidant enzyme activities in stress-sensitive and -tolerant rice cultivars during salinity and drought upon restriction of COX and AOX pathways of mitochondrial oxidative electron transport. J Plant Physiol 268:153583. https://doi.org/10.1016/j.jplph.2021.153583
Couée I, Sulmon C, Gouesbet G, El Amrani A (2006) Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot 57:449–459. https://doi.org/10.1093/jxb/erj027
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Plant Sci 2:1–13
Dinakar C, Bartels D (2012) Light response, oxidative stress management and nucleic acid stability in closely related Linderniaceae species differing in desiccation tolerance. Planta 236:541–555. https://doi.org/10.1007/s00425-012-1628-8
Dinakar C, Bartels D (2013) Desiccation tolerance in resurrection plants: new insights from transcriptome, proteome, and metabolome analysis. Front Plant Sci 4:1–14. https://doi.org/10.1016/j.jplph.2018.05.002
Dinakar C, Djilianov D, Bartels D (2012a) Photosynthesis in desiccation-tolerant plants: energy metabolism and antioxidative stress defense. Plant Sci 182:29–41. https://doi.org/10.1016/j.plantsci.2011.01.018
Drennan PM, Smith MT, Goldsworthy D, van Staden J (1993) The occurrence of trehalose in the leaves of the desiccation-tolerant angiosperm Myrothamnus Fabellifolius Welw. J Plant Physiol 142:493–496. https://doi.org/10.1016/S0176-1617(11)81257-5
Egert A, Eicher B, Keller F, Peters S (2015) Evidence for water deficit-induced mass increases of raffinose family oligosaccharides (RFOs) in the leaves of three Craterostigma resurrection plant species. Front Physiol 6:206. https://doi.org/10.3389/fphys.2015.00206
Flexas J, Bota J, Loreto F et al (2004) Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol 6:269–279
Garg AK, Kim JK, Owens TG et al (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci U S A 99:15898–15903. https://doi.org/10.1073/pnas.252637799
Goddijn OJM, Verwoerd TC, Voogd E et al (1997) Inhibition of trehalase activity enhances trehalose accumulation in transgenic plants. Plant Physiol 113:181–190. https://doi.org/10.1104/pp.113.1.181
Hassan MU, Nawaz M, Shah AN et al (2022) Trehalose: a key player in plant growth regulation and tolerance to abiotic stresses. J Plant Growth Regul. https://doi.org/10.1007/s00344-022-10851-7
Huang S, Zuo T, Ni W (2020) Important roles of glycinebetaine in stabilizing the structure and function of the photosystem II complex under abiotic stress. Planta 251:36. https://doi.org/10.1007/s00425-019-03330-z
Irato P, Santovito G (2021) Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 10:579
Ishikawa R, Shirouzu K, Nakashita H et al (2005) Foliar spray of validamycin a or validoxylamine a controls tomato fusarium wilt. Phytopathology® 95:1209–1216. https://doi.org/10.1094/phyto-95-1209
Iturriaga G, Suárez R, Nova-Franco B (2009) Trehalose metabolism: from osmoprotection to signaling. Int J Mol Sci 10:3793–3810. https://doi.org/10.3390/ijms10093793
Karim S, Aronsson H, Ericson H et al (2007) Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol Biol 64:371–386. https://doi.org/10.1007/s11103-007-9159-6
Kaur N, Dhawan M, Sharma I, Pati PK (2016) Interdependency of reactive oxygen species generating and scavenging system in salt sensitive and salt tolerant cultivars of rice. BMC Plant Biol 16:1–13. https://doi.org/10.1186/s12870-016-0824-2
Kaur H, Manna M, Thakur T et al (2021) Imperative role of sugar signaling and transport during drought stress responses in plants. Physiol Plant 171:833–848. https://doi.org/10.1111/ppl.13364
Kosar F, Akram NA, Sadiq M et al (2019) Trehalose: a key organic osmolyte efectively involved in plant abiotic stress tolerance. J Plant Growth Regul 38:606–618. https://doi.org/10.1007/s00344-018-9876-x
Kuroki S, Tsenkova R, Moyankova D, Muncan J, Morita H, Atanassova S, Djilianov D (2019) Water molecular structure underpins extreme desiccation tolerance of the resurrection plant Haberlea rhodopensis. Sci Rep 10:3049. https://doi.org/10.1038/s41598-019-39443-4
Lee DH and Lee CB (2000) Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Sci 159:75–85
Li HW, Zang BS, Deng XW, Wang XP (2011) Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 234:1007–1018. https://doi.org/10.1007/s00425-011-1458-0
Lin Q, Yang J, Wang Q, Zhu H, Chen Z, Dao Y, Wang K (2019) Overexpression of the trehalose-6-phosphate phosphatase family gene AtTPPF improves the drought tolerance of Arabidopsis thaliana. BMC Plant Biol 19:381
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/s0021-9258(19)52451-6
Luo Y, Li WM, Wang W (2008) Trehalose: protector of antioxidant enzymes or reactive oxygen species scavenger under heat stress? Environ Exp Bot 63:378–384. https://doi.org/10.1016/j.envexpbot.2007.11.016
Luo Y, Wang W, Fan YZ et al (2018) Exogenously-supplied trehalose provides better protection for D1 protein in winter wheat under heat stress. Russ J Plant Physiol 65:115–122. https://doi.org/10.1134/S1021443718010168
Luo Y, Xie Y, He D et al (2021) Exogenous trehalose protects photosystem II by promoting cyclic electron flow under heat and drought stresses in winter wheat. Plant Biol 23:770–776. https://doi.org/10.1111/plb.13277
Meyer S, Genty B (1998) Mapping intercellular CO2 mole fraction (ci) in Rosa rubiginosa leaves fed with abscisic acid by using chlorophyll fluorescence imaging: significance of Ci estimated from leaf gas exchange. Plant Physiol 116:947–957. https://doi.org/10.1104/pp.116.3.947
Mishra SS, Panda D (2017) Leaf traits and antioxidant defense for drought tolerance during early growth stage in some popular traditional rice landraces from Koraput, India. Rice Sci 24:207–217. https://doi.org/10.1016/j.rsci.2017.04.001
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19. https://doi.org/10.1016/j. tplants.2016.08.002
Mohanan A, Gandhi S, Al Ain AN, Challabathula D (2023) Intracellular trehalose modulates oxidative responses and dehydrin gene expression in Arabidopsis thaliana (L.) Heynh during dehydration. Braz J Bot 46:255–267. https://doi.org/10.1007/s40415-023-00877-w
Mostofa MG, Hossain MA, Fujita M (2015a) Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma 252:461–475. https://doi.org/10.1007/s00709-014-0691-3
Mostofa MG, Hossain MA, Fujita M, Tran L-SP (2015b) Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci Rep 5:11433
Muchate NS, Rajurkar NS, Suprasanna P, Nikam TD (2019) NaCl induced salt adaptive changes and enhanced accumulation of 20-hydroxyecdysone in the in vitro shoot cultures of Spinacia oleracea (L). Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-48737-6
Müller J, Aeschbacher RA, Wingler A et al (2001) Trehalose and trehalase in Arabidopsis. Plant Physiol 125:1086–1093. https://doi.org/10.1104/pp.125.2.1086
Nuccio ML, Wu J, Mowers R et al (2015) Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat Biotechnol 33:1–13. https://doi.org/10.1038/nbt.3277
Oliver MJ, Guo L, Alexander DC, Ryals JA, Wone BWM, Cushman JC (2011) A sister group contrast using untargeted global metabolomic analyss delineates the biochemcal regulation underlying desiccation tolerance in Sporobolus Stapfianus. Plant Cell 23:1231–1248. htpps://
Oszvald M, Primavesi LF, Griffiths CA et al (2018) Trehalose 6-phosphate regulates photosynthesis and assimilate partitioning in reproductive tissue. Plant Physiol 176:2623–2638. https://doi.org/10.1104/pp.17.01673
Pei Z-M, Murata Y, Benning G et al (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406:731–734. https://doi.org/10.1038/35021067
Peters S, Mundree SG, Thomson JA, Farrant JM, Keller F (2007) Protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water defict. J Exp Bot 58:1947–1956. https://doi.org/10.1093/jxb/erm056
Reyes VP (2023) Fantastic genes: where and how to find them? Exploiting rice genetic resources for the improvement of yeild, tolerance, and resistance to a wide array of stresses in rice. Funct Integr Genomics 23:238
Sadak MS, El-Bassiouny HMS, Dawood MG (2019) Role of trehalose on antioxidant defense system and some osmolytes of quinoa plants under water deficit. Bull Natl Res Cent 43:5. https://doi.org/10.1186/s42269-018-0039-9
Sengupta S, Mukherjee S, Basak P, Majumder AL (2015) Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front Plant Sci 6:656. https://doi.org/10.3389/fpls.2015.00656
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46:209–221. https://doi.org/10.1007/s10725-005-0002-2
Shi Y, Sun H, Wang X et al (2019) Physiological and transcriptomic analyses reveal the molecular networks of responses induced by exogenous trehalose in plant. PLoS ONE 14:1–24. https://doi.org/10.1371/journal.pone.0217204
Taj Z, Challabathula D (2021) Protection of photosynthesis by halotolerant Staphylococcus sciuri ET101 in tomato (lycoperiscon esculentum) and rice (Oryza sativa) plants during salinity stress: possible interplay between carboxylation and oxygenation in stress mitigation. Front Microbiol 11:3232. https://doi.org/10.3389/fmicb.2020.547750
Tombesi S, Nardini A, Frioni T et al (2015) Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci Rep 5:1–12. https://doi.org/10.1038/srep12449
Ullah A, Al-Rajhi RS, Al-Sadi AM, Farooq M (2021) Wheat genotypes with higher intercellular CO2 concentration, rate of photosynthesis, and antioxidant potential can better tolerate drought stress. J Soil Sci Plant Nutr 21:2378–2391. https://doi.org/10.1007/s42729-021-00529-6
Valenzuela-Avendaño JP, Estrada Mota IA, Gabriel Lizama UC et al (2005) Use of a simple method to isolate intact RNA from partially hydrated Selaginella lepidophylla plants. Plant Mol Biol Rep 23:199–200. https://doi.org/10.1007/BF02772713
Van Houtte H, Vandesteene L, Lopez-Galvis L et al (2013) Overexpression of the trehalase gene AtTRE1 leads to increased drought stress tolerance in Arabidopsis and is involved in abscisic acid-induced stomatal closure. Plant Physiol 161:1158–1171. https://doi.org/10.1104/pp.112.211391
Vogel G, Fiehn O, Jean-Richard‐dit‐Bressel L et al (2001) Trehalose metabolism in Arabidopsis: occurrence of trehalose and molecular cloning and characterization of trehalose‐6‐phosphate synthase homologues. J Exp Bot 52:1817–1826. https://doi.org/10.1093/jexbot/52.362.1817
Wang Y, Chen X, Li X et al (2022) Exogenous application of 5-aminolevulinic acid alleviated damage to wheat chloroplast ultrastructure under drought stress by transcriptionally regulating genes correlated with photosynthesis and chlorophyll biosynthesis. Acta Physiol Plant 44:1–12. https://doi.org/10.1007/s11738-021-03347-6
Woodbury W, Spencer AK, Stahmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44:301–305. https://doi.org/10.1016/0003-2697(71)90375-7
Xia XJ, Zhou YH, Shi K et al (2015) Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot 66:2839–2856. https://doi.org/10.1093/jxb/erv089
Yang L, Zhao X, Zhu H et al (2014) Exogenous trehalose largely alleviates ionic unbalance, ROS burst, and PCD occurrence induced by high salinity in arabidopsis seedlings. Front Plant Sci 5:1–11. https://doi.org/10.3389/fpls.2014.00570
Yang Y, Yao Y, Li J et al (2022) Trehalose alleviated salt stress in Tomato by regulating ROS metabolism, photosynthesis, osmolyte synthesis, and trehalose metabolic pathways. Front Plant Sci. https://doi.org/10.3389/fpls.2022.772948
Yang X, Shu Y, Cao S et al (2023) Trehalase inhibitor validamycin may have additional mechanisms of toxicology against Rhizoctonia Cerealis. J Fungi 9:846. https://doi.org/10.3390/jof9080846
Yao X, Zhou H, Zhu Q, Li C, Zhang H, Wu JJ, Xie F (2017) Photosynthetic response of soybean leaf to wide light-fluctuation in maize-soybean intercropping system. Front Plant Sci 8:1695
Zhang Z, Sun M, Gao Y, Luo Y (2022) Exogenous trehalose differently improves photosynthetic carbon assimilation capacities in maize and wheat under heat stress. J Plant Interact 17:361–370. https://doi.org/10.1080/17429145.2022.2041119
Zhao DQ, Li TT, Hao ZJ et al (2019) Exogenous trehalose confers high temperature stress tolerance to herbaceous peony by enhancing antioxidant systems, activating photosynthesis, and protecting cell structure. Cell Stress Chaperones 24:247–257. https://doi.org/10.1007/s12192-018-00961-1
Zulfiqar F, Chen J, Finnegan PM et al (2021) Stress in sweet basil and improves plant growth. Plants 10:1–14. https://doi.org/10.3390/plants10061078
Acknowledgements
AM acknowledges the UGC fellowship, Rajiv Gandhi National Fellowship 2017-18 for supporting the research work through the Fellowship. DC acknowledges the SERB funded projects (NO/SB/EMEQ-299/2014) and (CRG/2021/005916) for facilities. The authors acknowledge the Central Instrumentation Facility of Department of Life Sciences for equipment and other common facilities.
Author information
Authors and Affiliations
Contributions
AM and DC designed research and experiments. AM, AK and DR performed the experiments, interpretation of data and manuscript writing was done by DC, BK and AM. Every author contributed to the article and approved the submitted version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12298_2023_1404_MOESM2_ESM.jpg
Supplementary Fig. 1. The normal QQ plot of data of different parameters. The data normality was checked using Shapiro-Wilk test and the Normality QQ plot was generated (JPG 285.9 kb)
12298_2023_1404_MOESM3_ESM.jpg
Supplementary Fig. 2. The correlation matrix. The Pearson correlation analysis was carried out with each data set and (a) correlation matrix and (b) correlation plot was generated. The Pearson r value vas considered for generating the heat map and the plot. The highly positive correlation is seen when r =1, highly negative correlation is when r= − 1. There is no correlation existing when r = 0 (JPG 3686.4 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohanan, A., Kodigudla, A., Raman, D.R. et al. Trehalose accumulation enhances drought tolerance by modulating photosynthesis and ROS-antioxidant balance in drought sensitive and tolerant rice cultivars. Physiol Mol Biol Plants 29, 2035–2049 (2023). https://doi.org/10.1007/s12298-023-01404-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-023-01404-7