Abstract
Cadmium (Cd) is a toxic metal and an environmental pollutant that significantly reduces plant growth and productivity. Proper management can ameliorate dysfunction and improve the plant growth and productivity exposed to Cd. Therefore, the present study was conducted to explore the protective role of the fungicides tebuconazole (TEB) and trifloxystrobin (TRI) in helping wheat (Triticum aestivum L. cv. Norin 61) seedlings to tolerate Cd. Five-day-old hydroponically grown seedlings were allowed to mild (0.25 mM CdCl2) and severe (0.5 mM CdCl2) Cd stress separately and with the fungicides (2.75 µM TEB + 1.0 µM TRI) for the next four days. Compared to control, the level of H2O2 in the seedlings exposed to mild and severe Cd stress alone increased by 81 and 112%, respectively. The accumulation of Cd also increased in the wheat seedlings along with declining mineral nutrients under Cd stress. The protective effect of TEB and TRI was observed with the enhancement of the antioxidant defense and methylglyoxalase systems and reduction in oxidative damage. Applying TEB and TRI reduced MDA (by 9 and 18%), EL (by 21 and 17%), MG (by 12 and 17%), and LOX activity (by 37 and 27%), respectively, relative to Cd stress alone. Cadmium uptake also decreased in the shoots (by 48 and 50%, respectively) and roots (by 23 and 25%, respectively) of the fungicide-treated wheat seedlings under mild and severe Cd stress, relative to stress alone. These results indicate the exogenous application of TEB and TRI is a promising approach to improve Cd tolerance in wheat plants. Further investigation is needed under field conditions and for other crop species to determine the Cd-tolerance induced by TEB and TRI application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal/metalloid(s) are considered as detrimental/obnoxious environmental pollutants due to their non-degradable and high toxic and mobile nature. The increasing human activities and industrial development are the main causes for distribution and environmental damage by metal/metalloid(s) (Zhu et al. 2018). Cadmium (Cd) is a highly noxious heavy metal that increases in soil through industrialization, mining, vehicle emissions, sewage water irrigation, and pesticide and fertilizer application (Abbas et al. 2018; Guo et al. 2018). Previous investigations have identified that Cd is very deleterious to plants and can hamper plant growth and cause plant death (Hasanuzzaman et al. 2017a; Bhuyan et al. 2020). Therefore, an effective approach is necessary to mitigate Cd toxicity.
Cadmium toxicity triggers physiological changes in plants, including seed germination inhibition, growth retardation, and photosynthesis, transpiration, and respiration reduction (Susana et al. 2012; Khanna et al. 2019). Usually, Cd is a redox inactive metal, which means it does not directly initiate the generation of reactive oxygen species (ROS) in plant cells (Rahman et al. 2016). However, it induces oxidative stress by disturbing the mineral balance, inhibiting the electron transport chain function, and reducing the antioxidant defense systems (Mahmud et al. 2018), thus leads to the higher ROS generation (Raza et al. 2020). Moreover, Cd also initiates the generation of another cytotoxic compound, methylglyoxal (MG), and causes oxidative stress by damaging ultrastructural cellular components (Rahman et al. 2016). Therefore, it is necessary to discover the effective machinery for enhancing Cd tolerance. Plant cells avoid Cd-induced growth restrictions by developing certain indigenous tolerance techniques. The most common strategies are binding of metal in the cell wall, inhibition of transport through cell membranes, efflux activation, metal chelation, and compartmentalization (Bhuyan et al. 2020). To minimize oxidative stress, plants also modulate the antioxidant defense mechanisms to reduce overproduced ROS (Nahar et al. 2016a). The antioxidant defense system incorporates enzymatic [catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), glutathione S-transferase (GST), glutathione peroxidase (GPX), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR)] and non-enzymatic [ascorbate (AsA), glutathione (GSH), α-tocopherols, phenolic compounds, non-protein amino acids, and alkaloids] antioxidants (Hasanuzzaman et al. 2017b; 2020). In the glyoxalase system, plants activate two thiol-dependent enzymes [glyoxalase I (Gly-I) and glyoxalase II (Gly-II)] to detoxify MG (Rahman et al. 2016; Mohsin et al. 2020a). In recent decades, using chemical phytoprotectants has been an effective approach to minimizing metal toxicity because of their low cost and comparatively ease of use (Hasanuzzaman et al. 2019a; Hossain et al. 2020).
A fungicide is a chemical that is widely used to control plant disease by inhibiting the growth of disease-causing microorganisms. However, at a low concentration some fungicides can also mitigate the damage of abiotic stress by activating antioxidant defense mechanisms (Mohsin et al. 2020b). Triazole and strobilurin fungicides can enhance antioxidant function and alkaloid production under stress conditions (Ruske et al. 2004; Zhang et al. 2010). Triazole fungicide minimizes fungal growth by inhibiting the synthesis of the membrane component ergosterol (Koller 1987). However, some reports from previous studies have indicated that triazole fungicides can also interfere in plant physiology by increasing photosynthetic pigment content, activating antioxidant enzymes, and improving the growth and biomass of plants (Grossmann et al. 1999; Kumar et al. 2016). Strobilurin fungicide inhibits fungal growth by disrupting the function of the electron transport chain; however, it also has a positive role in plant physiology by influencing electron transport chain activity (Sauter 2007; Diaz-Espejo et al. 2012). Using strobilurin to improve tolerance in plants under abiotic stress has been reported by Köhle et al. (2002). Several studies also found that strobilurin delays flag leaf senescence, makes the leaves darker green, improves CO2 uptake, and enhances the weight of kernel and grain yield (Wu and Von-Tiedemann 2001; Zhang et al. 2010).
Cadmium toxicity negatively affects wheat growth and yield, and thus it has become an important global issue (Ni et al. 2018). Uniconazole, a triazole fungicide, can improve Cd tolerance in wheat (Singh 1993), cucumber (Thomas and Singh 1996), and okra plants (Purohit and Singh 1999). Norková et al. (2012) investigated the role of tebuconazole (TEB) in mitigating Cd toxicity in soil and found that it reduces Cd availability in soil by binding into different complex forms such as [CdI(Teb)3]+ and [Cd(Teb)4]2+. Recently, Hameed et al. (2020) observed that seed priming by some triazole compounds can inhibit Cd toxicity in wheat plants. In our previous study, we observed that TEB (triazole) and trifloxystrobin (TRI, strobilurin) mitigate salt-induced oxidative stress in cucumber (Mohsin et al. 2019) and wheat plants (Mohsin et al. 2020b). However, there are no reports on the role of TEB and TRI in alleviating oxidative damage in wheat plants under Cd stress. Therefore, the objective of our study was to explore the Cd-induced physio-biochemical changes and the alleviating role of TEB and TRI in preventing oxidative stress and MG toxicity in the seedlings of wheat.
Materials and methods
Growth condition and stress treatments
Wheat (Triticum aestivum L. cv. Norin 61) seeds were grown hydroponically. The seeds were sterilized (by 70% ethanol) and soaked in distilled water (DH2O) for 4 h. After soaking, the seeds were placed in Petri dishes (9 cm diameter) in an incubator at 25 °C for 48 h for germination. Following germination of the seeds, the seedlings were grown in a growth chamber maintaining proper relative humidity (65–70%), temperature (25 ± 2 °C), and light (350 µmol photon m−2 s−1). Hyponex solution (3300-fold diluted) was used as a nutrient solution containing N (8%), P (6.43%), K (20.94%), Mg (3.08%), Ca (11.8%), B (0.07%), Fe (0.24%), Mn (0.03%), Zn (0.008%), Mo (0.0014%), and Cu (0.003%). Prior to the experiment, several trials were conducted using different concentrations of TEB and TRI (from 0.01 to 100 µM) on wheat seedlings, and finally 2.75 µM for TEB and 1.0 µM for TRI were selected because they showed better phenotypic responses under 0.25 and 0.5 mM Cd stress. The seedlings (five-day-old) were exposed to Cd stress (0.25 and 0.5 mM CdCl2 as mild and severe doses, respectively) alone and in combination with fungicides (2.75 µM TEB + 1.0 µM TRI) for four days. Only nutrient solution was used for untreated seedlings. Nine-day-old seedlings were used to observe physiological and biochemical attributes. The experiment was designed following a completely randomized design (CRD) with three repetitions.
Cadmium toxicity symptoms and growth parameters
Cadmium-induced toxicity symptoms were observed in the wheat seedlings. After harvest, the fresh weight (FW) of the shoots and roots were determined immediately, and the dry weight (DW) was measured after drying both shoots and roots at 70 °C for 48 h.
Determination of relative water content
Relative water content (RWC) in the leaves was determined according to Barrs and Weatherley (1962). Leaf FW, DW, and turgid weight (TW) were determined and RWC was then calculated by following formula: \({\text{RWC }}\left( {\text{\% }} \right) = \left[ {\left( {{\text{FW}} - {\text{DW}}} \right)/\left( {{\text{TW}} - {\text{DW}}} \right)} \right] \times 100.\)
Estimation of proline content
Proline (Pro) content was estimated by the method of Bates et al. (1973). Leaf was extracted by sulfo-salicylic acid (3%), and glacial acetic acid, acid ninhydrin, and toluene were then used as reaction reagents. Upper toluene chromophore was used for spectrophotometric observation at 520 nm.
Estimation of photosynthetic pigment
Chlorophyll (Chl) and carotenoid (Car) content were estimated according to Lichtenthaler (1987) by using 100% ethanol. The absorbance was measured spectrophotometrically at 664, 648, and 470 nm to determine Chl a, Chl b, and Car content, respectively.
Malondialdehyde content
Malondialdehyde (MDA) content was estimated following the method of Heath and Packer (1968). The leaves were homogenized by using trichloroacetic acid (TCA), and thiobarbituric acid (TBA) was used as a reaction reagent. Thereafter, absorbance was measured spectrophotometrically at 532 nm and corrected at 600 nm.
Estimation of hydrogen peroxide content
Hydrogen peroxide (H2O2) content was estimated following the technique described by Hossain et al. (2020). The leaves were homogenized with TCA, and potassium iodide (1 M) and K-phosphate (pH 7.0) buffer were then used as reaction reagents. To calculate H2O2, a standard curve was prepared by using H2O2, and the optical absorbance was then observed at 390 nm.
Histochemical detection of hydrogen peroxide and super oxide
To detect H2O2 and super oxide (O2•−) generation, 0.01% of acidic 3,3′-diaminobenzidine (DAB) and nitroblue tetrazolium chloride (NBT) solution were used, respectively (Chen et al. 2010). The leaves were immersed in DAB and NBT solution separately for 24 h and incubated at 25 °C. Therefore, the leaves were washed in 70% ethanol followed by DH2O to remove DAB and NBT solution. Afterward, the leaves were heated at 70 °C until clear brown and blue spots were seen, which is the indicator of H2O2 and O2•− generation, respectively.
Determination of electrolyte leakage
Electrolyte leakage (EL) was determined following Dionisio-Sese and Tobita (1998). Electrical conductivity (EC) was measured by using EC meter (Eutech Instruments, Singapore). Primary (EC1) and final conductivity (EC2) were recorded, and EL was determined by following formula: \({\text{EL }}\left( {\text{\% }} \right) = \left( {{\text{EC}}1/{\text{EC}}2} \right) \times 100.\)
Methylglyoxal content
Perchloric acid (5%) was used as an extraction buffer for determination of MG content according to the technique described by Hasanuzzaman et al. (2019a). Charcoal was used with supernatant for decolorization, and sodium carbonate was mixed to neutralize the solution. N-acetyl-L-cysteine and sodium phosphate were used with a neutralized solution in the final mixture. The optical absorbance was then recorded at 288 nm and calculated by a standard curve prepared by using different concentrations of MG.
Soluble protein and antioxidant and other enzyme activity
Leaf samples were homogenized using K-phosphate buffer (pH 7.0), AsA, β-mercaptoethanol, KCl, and glycerol as an extraction buffer. After centrifuging, leaf extracts were used to determine protein and enzyme activity. Soluble protein concentration was measured using coomassie brilliant blue dye following the technique of Bradford (1976). The optical absorbance was then recorded at 595 nm and a standard curve prepared by using bovine serum albumin was used for calculation.
Lipoxygenase (LOX; EC: 1.13.11.12) activity was measured by using linoleic acid and K-phosphate buffer (pH 6.5) according to Doderer et al. (1992) and absorbance was observed at 234 nm. Superoxide dismutase (EC: 1.15.1.1) activity was assayed by using K-phosphate buffer (pH 7), NBT, xanthine, and xanthine oxidase following El-Shabrawi et al. (2010). Optical absorbance was recorded at 560 nm and expressed as Umin−1 mg−1 protein. Catalase (EC: 1.11.1.6) activity was determined by using K-phosphate buffer (pH 7.0) and H2O2 according to the method described by Hasanuzzaman et al. (2019a) and absorbance was observed at 240 nm. Peroxidase (EC: 1.11.1.7) activity was estimated by using K-phosphate buffer (pH 7), catechol, and H2O2 following Gong et al. (2015). Absorbance was recorded at 470 nm and expressed as U min−1 mg−1 protein. Glyoxalase I (EC: 4.4.1.5) activity was measured by using K-phosphate buffer (pH 7.0), GSH, MgSO4, and MG and absorbance was measured at 240 nm (Mohsin et al. 2020a). Glyoxalase II (EC: 3.1.2.6) activity was assayed by using S-D-lactoylglutathione (SLG), 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB), and Tris–HCl buffer (pH 7.2) absorbance was and observed at 412 nm (Mohsin et al. 2020a).
Ascorbate peroxidase (EC: 1.11.1.11) activity was assayed following Nakano and Asada (1981) by using K-phosphate buffer (pH 7.0), AsA, ethylenediaminetetraacetic acid (EDTA), and H2O2 then absorbance was recorded at 290 nm. The activity of MDHAR (EC: 1.6.5.4) was measured by using ascorbate oxidase (AO), Tris–HCl buffer (pH 7.5), AsA, and nicotinamide adenine dinucleotide phosphate (NADPH) according to the method described by Parvin et al. (2020). Optical absorbance was observed at 340 nm. Dehydroascorbate reductase (EC: 1.8.5.1) activity was assayed according to Nakano and Asada (1981) by using dehydroascorbate (DHA), K-phosphate buffer (pH 7.0), GSH, and EDTA and absorbance was recorded at 265 nm. Glutathione reductase (EC: 1.6.4.2) activity was determined following the method described by Parvin et al. (2019) by using oxidized glutathione (GSSG), K-phosphate buffer (pH 7.0), EDTA, and NADPH and absorbance was observed at 340 nm. Glutathione peroxidase (EC: 1.11.1.9) activity was measured by using K-phosphate buffer (pH 7.0), GSH, EDTA, GR, NaN3, NADPH, and H2O2 according to the method described by Hasanuzzaman et al. (2019a) and absorbance was recorded at 340 nm. Glutathione S-transferase (EC: 2.5.1.18) activity was assayed by using 1-chloro-2, 4-dinitrobenzene (CDNB), Tris–HCl buffer (pH 6.5), and GSH according to the technique described by Hasanuzzaman et al. (2019a) and absorbance was measured at 340 nm.
Determination of ascorbate and glutathione content
Total ascorbate and AsA content were determined following the method described by Hossain et al. (2019). The leaves were homogenized with TCA, and the extract solution was then neutralized with K-phosphate buffer (pH 7.0) and dithiothretitol (DTT) to determine total ascorbate. To determine AsA, a neutralizing solution of DH2O was used instead of DTT. Ascorbate oxidase and K-phosphate buffer (pH 6.5) were used in a final reaction mixture with the neutralized solution and observed at 265 nm, and AsA was then calculated with the help of a standard curve prepared using AsA. Dehydroascorbate content was determined by subtracting the AsA content from total ascorbate.
Glutathione content was determined following Law et al. (1983). Leaf extract solutions were neutralized by using K-phosphate buffer (pH 7.0) and DH2O to determine total glutathione. To determine GSSG, a neutralizing solution of 2-vinylpyridine was used instead of DH2O. Nicotinamide adenine dinucleotide phosphate, DTNB, and GR were used in a final reaction mixture with the neutralized solution and observed at 412 nm, and glutathione was then calculated with the help of standard curves prepared using known concentrations of GSSG and GSH. The content of GSH was determined by subtracting GSSG from total glutathione.
Determination of Cd and mineral nutrient content, BAC, BCF, and TF
The content of Cd, K, Ca, and Mg were measured according to Zasoski and Barau (1977). Dried shoot and root samples were digested with an HNO3:HClO4 (5:1 v/v) acid mixture, and the Cd and mineral concentration was then measured by using an atomic absorption spectrophotometer (GFA-7000A; Shimadzu, Japan).The biological accumulation coefficient (BAC), biological concentration factor (BCF), and translocation factor (TF) were calculated using the following the equations (Nahar et al. 2016b):
Statistical analysis
The data were statistically analyzed by one-way analysis of variance (ANOVA) with XLSTAT v.2020 (Addinsoft 2020). Fisher’s Least Significant Difference (LSD) test were employed for mean comparisons, where P ≤ 0.05 was considered significant.
Results
Improvement of plant growth and biomass by TEB and TRI under Cd stress
Cadmium stress declined the growth and biomass of wheat, but a comparatively less effect was observed in the TEB and TRI-treated seedlings (Fig. 1A). The application of Cd decreased both shoots and roots FW and DW. Relative to control, mild and severe Cd treatment reduced the shoot FW and DW by 20 and 36% and 22 and 35%, respectively. Root FW and DW also declined by 32 and 43% and 28 and 42%, respectively, due to the mild and severe Cd treatments, compared with control. However, applying TEB and TRI to the mild and severe Cd-treated seedlings improved shoot FW (by 12 and 31%, respectively), shoot DW (by 16 and 27%, respectively), root FW (by 20 and 27%, respectively), and root DW (by 21 and 24%, respectively), over the Cd-alone-treated seedlings (Fig. 1B).
Effect of TEB (2.75 µM) and TRI (1.0 µM) on appearance A, shoot and root FW and DW B, and histochemical detection of H2O2 and O2•− C of nine-day-old hydroponically grown wheat seedlings under Cd stress (0.25 and 0.5 mM CdCl2, for four days). TT, Cd1, and Cd2 indicate 2.75 µM TEB + 1.0 µM TRI, 0.25 mM CdCl2, and 0.5 mM CdCl2, respectively. Means were calculated from three replicates for each treatment and the bars represent the standard deviation (± SD). Values with different letters are significantly different at p ≤ 0.05 applying Fisher’s LSD test
TEB and TRI induced osmotic status regulation
Relative to control seedlings, RWC decreased by 21 and 35%, while the content of Pro increased by 225 and 361% under mild and severe Cd stress, respectively. Adding of TEB and TRI improved RWC by 16 and 25% and declined Pro content by 15 and 18% in the mild and severe Cd-stressed seedlings, respectively, relative to Cd treatment alone (Table 1).
Photosynthetic pigment content improved due to TEB and TRI application under Cd stress
The result of TEB and TRI on the photosynthetic pigment content (Chl a, Chl b, and Car) is shown in Table 1. Mild and severe Cd treatment reduced Chl a (by 32 and 43%, respectively), Chl b (by 50 and 54%, respectively), Chl (a + b) (by 40 and 52%, respectively), and Car (by 28 and 48%, respectively), over the control seedlings. Maximal improvement after applying TEB and TRI was observed under mild and severe stress by 47 and 54% for Chl a, 71 and 62% for Chl b, 58 and 66% for Chl (a + b), and 35 and 62% for Car, respectively, relative to stress only.
TEB and TRI reduced the oxidative stress indicators
The seedlings exposed to mild and severe Cd stress produced higher MDA, EL, H2O2, and MG (Table 2), and improved the activity of LOX (Fig. 2A). Production of H2O2 increased under mild and severe Cd stress by 81 and 112%, respectively, because of higher production MDA (by 164 and 222%, respectively), EL (by 58 and 90%, respectively), and MG (by 68 and 109%, respectively), relative to control. LOX activity causing lipid peroxidation increased by 157 and 212% under mild and severe Cd stress, respectively, relative to control seedlings. The application of TEB and TRI to the mild and severe Cd-treated seedlings declined H2O2 generation by 21 and 112%, respectively, with reduction by 9 and 18% for MDA, 21 and 17% for EL, 12 and 17% for MG, and 37 and 27% for LOX activity, respectively, over the Cd stress seedlings. The production of H2O2 (brown spots) and O2•– (blue spots) in the leaves of the mild and severe Cd-treated wheat seedlings were revealed through histochemical detection (Fig. 1C). Fewer brown and blue spots in the TEB and TRI-treated seedlings indicated reduced production of H2O2 and O2•–, compared with the Cd alone-treated seedlings.
Effect of TEB (2.75 µM) and TRI (1.0 µM) on the activities of LOX A, SOD B, CAT C, POD D, Gly I E, and Gly II F in nine-day-old hydroponically grown wheat seedlings under Cd stress (0.25 and 0.5 mM CdCl2, for four days). Means were calculated from three replicates for each treatment and the bars represents the standard deviation (± SD). Values with different letters are significantly different at p ≤ 0.05 applying Fisher’s LSD test
Up-regulation of antioxidant enzymes by TEB and TRI
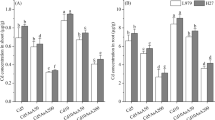
The results revealed that Cd stress, improved SOD activity, while TEB and TRI further improved SOD activity by 33 and 32% under mild and severe Cd stress, respectively, in contrast to Cd stress alone (Fig. 2B). Mild and severe Cd stress reduced the activity of CAT (by 39 and 57%, respectively), POD (by 42 and 56%, respectively), Gly I (by 41 and 64%, respectively), and Gly II (by 46 and 67%, respectively), relative to control. Maximal amelioration with TEB and TRI was observed under mild and severe stress by 29 and 46% for CAT, 25 and 59% for POD, 45 and 115% for Gly I, and 43 and 59% for Gly II, respectively, over to stress seedlings (Fig. 2C–F). Increased APX activity was observed with increasing Cd concentration, while MDHAR, DHAR, GR, GPX, and GST activity reduced, over to control seedlings. However, TEB and TRI application to the mild and severe Cd-stressed seedlings reduced APX activity by 20 and 21%, respectively, and enhanced the activity by 15 and 53% for MDHAR, 21 and 44% for DHAR, 28 and 48% for GR, 31 and 69% for GPX, and 29 and 80% for GST, respectively; relative to stress seedlings (Fig. 3A–F).
Effect of TEB (2.75 µM) and TRI (1.0 µM) on the activities of APX A, MDHAR B, DHAR C, GR D, GPX E, and GST F in nine-day-old hydroponically grown wheat seedlings under Cd stress (0.25 and 0.5 mM CdCl2, for four days). Means were calculated from three replicates for each treatment and the bars represents the standard deviation (± SD). Values with different letters are significantly different at p ≤ 0.05 applying Fisher’s LSD test
TEB and TRI enhanced the AsA-GSH pool under Cd stress
Mild and severe Cd treatment reduced the AsA content (by 38 and 48%, respectively) with increasing DHA content (by 79 and 129%, respectively); therefore, the AsA/DHA ratio reduced by 65 and 78%, respectively, over control seedlings. Applying TEB and TRI improved the AsA content by 33 and 42% and the AsA/DHA ratio by 104 and 95% with decreased DHA content by 35 and 27% in the mild and severe Cd-stressed seedlings, respectively, compared to stress seedling alone (Fig. 4A–C). Relative to control, mild and severe Cd stress decreased the GSH content by 40 and 55%, while the GSSG content increased by 96 and 148%, respectively; thus, the GSH/GSSG ratio declined by 69 and 82%, respectively. However, application of TEB and TRI to the mild and severe Cd-treated seedlings improved the GSH content by 34 and 48% and the GSH/GSSG ratio by 69 and 99% with declined GSSG content by 22 and 26%, respectively, in contrast to Cd alone-treated seedlings (Fig. 4D–F).
Effect of TEB (2.75 µM) and TRI (1.0 µM) on AsA A, DHA B, AsA/DHA ratio C, GSH D, GSSG E, and GSH/GSSG ratio F in nine-day-old hydroponically grown wheat seedlings under Cd stress (0.25 and 0.5 mM CdCl2, for four days). Means were calculated from three replicates for each treatment and the bars represent the standard deviation (± SD). Values with different letters are significantly different at p ≤ 0.05 applying Fisher’s LSD test
TEB and TRI reduced the Cd uptake and improved mineral accumulation
A higher accumulation of Cd was measured in both the shoots and roots with higher BAC, BCF, and TF under mild and severe Cd stress. After applying TEB and TRI, the concentration of Cd was reduced by 48 and 50% in the shoots and 23 and 25% in the roots under mild and severe Cd stress, respectively, over to stress alone (Fig. 5A).With TEB and TRI treatment, maximal declines were also observed by 39 and 46% for BAC, 20 and 28% for BCF, 36 and 41% for TF under mild and severe stress, respectively, over to stress seedling (Table 3). The content of K, Ca, and Mg declined in both the shoots and roots under mild and severe Cd stress, relative to control. Higher values for the mineral nutrients were observed in the TEB-and-TRI-treated seedlings under mild and severe Cd stress by 18 and 36% for K in the shoots, 19 and 41% for K in the roots, 35 and 64% for Ca in the shoots, 42 and 92% for Ca in the roots, 12 and 35% for Mg in the shoots, and 19 and 53% for Mg in the roots, respectively, compared with the Cd alone-treated seedlings. However, no significant difference was found in the shoot Mg content under mild Cd stress (Fig. 5B–D).
Effect of TEB (2.75 µM) and TRI (1.0 µM) on Cd A, K B, Ca C, and Mg D content in the shoots and roots of nine-day-old hydroponically grown wheat seedlings under Cd stress (0.25 and 0.5 mM CdCl2, for four days). Means were calculated from three replicates for each treatment and the bars represent the standard deviation (± SD). Values with different letters are significantly different at p ≤ 0.05 applying Fisher’s LSD test
Discussion
Cadmium-induced toxicity inhibits plant growth and reduces biomass (Wei et al. 2018). It alters the morphology of leaves and roots by causing leaf chlorosis and root cell wall lignification, and thus initiates the reduction of the photosynthetic rate and disturbance of the normal cellular metabolisms (Guo et al. 2019). Cadmium stress is also associated with osmotic status disturbance and inhibition of mineral nutrient uptake; thus, plants cannot maintain normal cellular mechanisms, resulting in reduced growth (Nahar et al. 2016b). Although plants possess various strategies to combat Cd-induced toxic effects, sometimes these are not enough to relieve cellular toxicity completely. Therefore, identifying a suitable technique to mitigate Cd toxicity is needed. For this reason, we investigated the Cd-tolerance mechanisms in wheat plants through the exogenous application of TEB and TRI because some other compounds of the same group have been shown to improve Cd tolerance (Singh 1993; Hameed et al. 2020).
In our study, better plant growth was observed in the TEB-and-TRI-treated seedlings under Cd stress (Fig. 1A). The lowest shoot and root biomass production was found in the plants under Cd stress without TEB and TRI. Higher Cd accumulation in plant tissues and the inhibition of mineral nutrient uptake may be one reason for the lower production of biomass (Lanier et al. 2019). Earlier studies have also shown that Cd toxicity reduces the plant biomass of wheat (Rizwan et al. 2019), rice (Bashir et al. 2018; Bhuyan et al. 2020), mung bean (Nahar et al. 2016a, b), and mustard (Hasanuzzaman et al. 2017a, b). The results of this study also indicated that the production of plant biomass improved by applying TEB and TRI to the Cd-exposed seedlings (Fig. 1B). The positive effects of uniconazole fungicide on plant biomass in different crop species have been shown previously (Singh 1993; Purohit and Singh 1999). The stunted growth of plants, may also be due to water deficiency and insufficient photosynthesis under Cd stress (Ismael et al. 2018). Our study showed that lower leaf RWC in both mild and severe Cd-treated seedlings with higher Pro content were ameliorated by applying TEB and TRI (Table 1). The accumulation of higher amounts of Cd interferes with leaf water balance and reduces RWC (Najmanova et al. 2012). To regulate water balance, the biosynthesis of osmolytes (Pro, glycine betaine, and trehalose) is a vital mechanism for plants under Cd stress (Bhuyan et al. 2020). Adding TEB and TRI reduced the Pro content in the plants under the Cd-stress condition, which indicated the Cd toxicity-mitigating role of TEB and TRI by improving leaf water balance. Cadmium stress also disrupts the photosynthetic mechanisms, particularly in the light harvesting complex II as well as photosystem (PS)I and PSII (Küpper et al. 2007), which is corroborated by the decline in the biosynthesis of Chl and Car in our present study (Table 1). The typical symptom of Cd toxicity is leaf chlorosis due to inhibited Chl biosynthesis (Xue et al. 2013). Additionally, Cd leads to modifying Chl–protein complexes by displacing Ca2+ ions of the oxygen-evolving complex and Mg2+ in Chl pigments (Küpper et al. 2007). However, improved Chl and Car content was observed in the TEB-and-TRI-treated seedlings under Cd stress. This result might be due to the influence of TEB in increasing cytokinin production, which stimulates the photosynthetic pigment biosynthesis (Fletcher et al. 2000; Mohsin et al. 2020b). Recently, Hameed et al. (2020) have also shown that seed priming by mercapto-triazole increases Chl and Car content in wheat plants under Cd toxicity.
Cadmium toxicity indirectly causes oxidative damage in plant tissues by the accumulation of higher amounts of ROS (Bashir et al. 2018). Cadmium indirectly changes the activity of NADPH oxidase in the plasma membrane, and thus initiates the production of higher amounts of O2•− and H2O2 (Guo et al. 2019). A higher amount of lipid peroxidation and membrane damage indicate the Cd-induced oxidative stress (Sytar et al. 2013). In our study, MDA and EL increased under Cd stress (Table 2). The activity of LOX, which is responsible for degrading lipid peroxides (Bhuyan et al. 2020), also markedly increased in the Cd-treated seedlings (Fig. 2A). Therefore, these responses increased the production of cellular ROS (Table 2), which was revealed by histochemical detection in the wheat leaves of our study (Fig. 1C). However, applying TEB and TRI suppressed the production of MDA, H2O2, and EL as well as reduced the activity of LOX, which indicated an improved antioxidant defense system in the Cd-stressed seedlings. Hameed et al. (2020) have also reported that mercapto-triazole alleviates Cd-induced oxidative damage in wheat plants.
Increased accumulation of the cytotoxic compound MG results in greater oxidative damage under Cd stress (Bhuyan et al. 2020). Cadmium toxicity increases the accumulation of MG by disrupting the function of the Gly-I and Gly-II enzymes (Nahar et al. 2016b). From our results, the content of MG increased in Cd-exposed seedlings in a dose-dependent fashion (Table 2) through a reduction in Gly-I and Gly-II activities (Fig. 2E, F). However, applying TEB and TRI decreased the MG content by enhancing the activities of Gly-I and Gly-II. Increased GSH is also responsible for the reduction in MG because GSH participates with Gly-I to detoxify MG into S-lactoylglutathione, thus improving the glyoxalase system (Mohsin et al. 2020b).
The antioxidant defense system is activated by enzymatic and non-enzymatic antioxidants and their mechanisms (Hasanuzzaman et al. 2020). SOD activates the frontline defense by converting O2•− into H2O2 and also reduces the possibility of hydroxyl (•OH) ion formation (Gill et al. 2015). The increased SOD activity under Cd stress in our experiment indicated the higher production of O2•−, which was reduced by TEB and TRI (Fig. 2B). In this study, CAT and POD activity declined under Cd stress, which represents the production of higher amounts of H2O2 (Bhuyan et al. 2020). However, applying TEB and TRI decreased the H2O2 content by enhancing CAT and POD activities (Fig. 2C, D), which are related to convert H2O2 into H2O (Hasanuzzaman et al. 2020). Our findings are in line with the results published by Hameed et al. (2020). In AsA-GSH cycle, there are four enzymes (APX, MDHAR, DHAR, and GR) that work jointly with AsA and GSH to mitigate ROS, and the AsA/DHA and GSH/GSSG ratios help to maintain a cellular redox state (Hasanuzzaman et al. 2019b). In the present study, Cd toxicity increased the activity of APX with decreasing MDHAR, DHAR, and GR activities (Fig. 3A–D). The non-enzymatic antioxidant, AsA, able to directly scavenge the ROS (Gill and Tuteja 2010), but Cd toxicity reduced the AsA content in our study (Fig. 4A). The mechanism for this situation might be a higher APX activity, which converts H2O2 into DHA by using AsA; therefore, a higher DHA content was observed (Fig. 4B). Lower activities of MDHAR and DHAR are also responsible for reduced AsA because these enzymes can regenerate AsA from MDHA and DHA (Hasanuzzaman et al. 2019b). TEB and TRI-mediated upregulation of AsA was accompanied by a reduced APX activity and improved MDHAR and DHAR activities, indicating that the antioxidant defense system improved. Our findings support those in a previous study, in which seed priming by mercapto-triazole improved AsA content with reduced APX activity in Cd-stressed wheat seedlings (Hameed et al. 2020). Another non-enzymatic antioxidant, GSH, also scavenges ROS and participates in AsA regeneration (Hasanuzzaman et al. 2019b). In our study, the lower GSH content (Fig. 4D) might be due to Cd toxicity inhibiting the defense system by reducing GR activity (Fig. 3D). However, TEB and TRI increased the GSH content with increased activity of GR; therefore, it regulated the cellular redox state by increasing the GSH/GSSG ratio. Cadmium toxicity also suppressed the activities of GPX and GST, but they increased with the addition of TEB and TRI (Fig. 3E, F); those enzymes can detoxify H2O2 by using GSH (Mohsin et al. 2020b).
Higher Cd accumulation was found in both shoots and roots of Cd-treated seedlings, but the shoot Cd content was lower than the root Cd content (Fig. 5A). Cadmium toxicity also increased BAC, BCF, and TF (Table 3), which indicated the higher accumulation and translocation of Cd in the wheat seedlings. Our results support those of Nahar et al. (2016b), who observed Cd transportation in mung bean plants. After adding TEB and TRI, the Cd content decreased in both the shoots and roots with reduced BAC, BCF, and TF. The possible mechanism of Cd reduction is the chelating behaviour of TEB, which tends to bind with Cd and make two probable complexes [CdI(Teb)3]+ and [Cd(Teb)4]2+ (Norková et al. 2012). Reduced Cd uptake in the roots and translocation from the roots to the upper part of a plant indicated an improvement in food hygiene through the exogenous application of TEB and TRI. Cadmium interferes with mineral nutrients (K, Ca, Mg, Cu, Zn, Mn, Fe, P, N, S, and Si) and thus decreases their concentration in plant cells (Ismael et al. 2018). The higher concentrations of K, Ca, and Mg in our study (Fig. 5B–D) showed that TEB reduced the concentration of Cd by binding various complexes, and thus improved the mineral content under Cd stress.
Conclusion
From this study, we find that Cd toxicity hampered the physiological and biochemical functions of wheat seedlings. Cadmium stress induced oxidative damage by inhibiting the normal function of the antioxidant defense and glyoxalase systems. However, this toxic effect can be alleviated by applying TEB and TRI, which promotes the detoxification of Cd (Fig. 6). The effects of alleviation are associated with improving plant biomass, promoting photosynthetic pigment content, reducing Cd accumulation, and enhancing the antioxidant defense and glyoxalase systems. TEB and TRI improved the function of the ROS-quenching antioxidants (AsA and GSH) and maintained their redox state, and thus increased the biochemical mechanisms of the plants. The activities of the antioxidant enzymes were also enhanced by TEB and TRI, and therefore, prevented ROS-induced damage in the wheat seedlings. The accumulation of Cd was reduced by applying TEB and TRI because of their binding ability. Exogenous TEB and TRI application stimulates the antioxidant defense and glyoxalase systems, and thus acts as an excellent phytoprotectant to relieve Cd toxicity. Therefore, the findings of our study indicate the protective role of TEB and TRI in regulating plant growth and improving food hygiene by detoxifying and reducing the phytoavailability of Cd in wheat plants.
References
Abbas T, Rizwan M, Ali S, Adrees M, Mahmood A, Zia-ur-Rehman M, Ibrahim M, Arshad M, Qayyum MF (2018) Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol Environ Saf 148:825–833
Addinsoft (2020) XLSTAT v. 2020.1.1: data analysis and statistics software for Microsoft Excel. Addinsoft, Paris
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428
Bashir A, Rizwan M, Ali S, Zia ur Rehman M, Ishaque W, Riaz MA, Maqbool A (2018) Effect of foliar-applied iron complexed with lysine on growth and cadmium (Cd) uptake in rice under Cd stress. Environ Sci Pollut Res 25:20691–20699
Bates LS, Waldren RP, Teari D (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bhuyan MHMB, Parvin K, Mohsin SM, Mahmud JA, Hasanuzzaman M, Fujita M (2020) Modulation of cadmium tolerance in rice: insight into vanillic acid-induced upregulation of antioxidant defense and glyoxalase systems. Plants 9:188. https://doi.org/10.3390/plants9020188
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen F, Wang F, Wu F, Mao W, Zhang G, Zhou M (2010) Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem 48:663–672
Diaz-Espejo A, Cuevas MV, Ribas-Carbo M, Flexas J, Martorell S, Fernández JE (2012) The effect of strobilurins on leaf gas exchange, water use efficiency and ABA content in grapevine under field conditions. J Plant Physiol 169:379–386
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Doderer A, Kokkelink I, Van der Veen S, Valk B, Schram A, Douma A (1992) Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta 112:97–104. https://doi.org/10.1016/0167-4838(92)90429-H
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245:85–96
Fletcher RA, Gill A, Davis TD, Sankhla N (2000) Triazoles as plant growth regulators and stress protectants. Hortic Rev 24:55–138
Gill SS, Anjum NA, Gill R, Yadav S, Hasanuzzaman M, Fujita M, Mishra P, Sabat SC, Tuteja N (2015) Superoxide dismutase—mentor of abiotic stress tolerance in crop plants. Environ Sci Pollut Res 22:10375–10394
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gong Z, Li D, Liu C, Cheng A, Wang W (2015) Partial purification and characterization of polyphenol oxidase and peroxidase from chestnut kernel. LWT-Food Sci Technol 60:1095–1099
Grossmann K, Kwiatkowski J, Retzlaff G (1999) Regulation of phytohormone levels, leaf senescence and transpiration by the strobilurin kresoxim-methyl in wheat (Triticum aestivum). J Plant Physiol 154:805–808
Guo B, Liu C, Liang Y, Li N, Fu Q (2019) Salicylic acid signals plant defence against cadmium toxicity. Int J Mol Sci 20:2960. https://doi.org/10.3390/ijms20122960
Guo J, Zhou R, Ren X, Jia H, Hua L, Xu H, Lv X, Zhao J, Wei T (2018) Effects of salicylic acid, epi-brassinolide and calcium on stress alleviation and Cd accumulation in tomato plants. Ecotoxicol Environ Saf 157:491–496
Hameed A, Hameed A, Ahmad M, Farooq T (2020) Alleviation of cadmium toxicity by mercapto-triazole priming in wheat. Arch Agron Soil Sci 66:1467–1480
Hasanuzzaman M, Alam MM, Nahar K, Mohsin SM, Bhuyan MHMB, Parvin K, Hawrylak-Nowak B, Fujita M (2019a) Silicon-induced antioxidant defense and methylglyoxal detoxification works coordinately in alleviating nickel toxicity in Oryza sativa L. Ecotoxicology 28:261–276
Hasanuzzaman M, Bhuyan MHMB, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M (2019b) Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 8:384. https://doi.org/10.3390/antiox8090384
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681. https://doi.org/10.3390/antiox9080681
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017) Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Front Plant Sci. 8:1061. https://doi.org/10.3389/fpls.2017.01061
Hasanuzzaman M, Nahar K, Gill SS, Alharby HF, Razafindrabe BH, Fujita M (2017) Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: an intrinsic study on antioxidant defense and glyoxalase systems. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00115
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hossain MS, Abdelrahman M, Tran CD, Nguyen KH, Chu HD, Watanabe Y, Hasanuzzaman M, Mohsin SM, Fujita M, Tran LSP (2020) Insights into acetate-mediated copper homeostasis and antioxidant defense in lentil under excessive copper stress. Environ Pollut 258:113544. https://doi.org/10.1016/j.envpol.2019.113544
Hossain MS, Hasanuzzaman M, Sohag MMH, Bhuyan MHMB, Fujita M (2019) Acetate-induced modulation of ascorbate: glutathione cycle and restriction of sodium accumulation in shoot confer salt tolerance in Lens culinaris Medik. Physiol Mol Biol Plants 25:443–455
Ismael MA, Elyamine AM, Moussa MG, Cai M, Zhao X, Hu C (2018) Cadmium in plants: uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 11:255–277
Khanna K, Jamwal VL, Kohli SK, Gandhi SG, Ohri P, Bhardwaj R, Abd-Allah EF, Hashem A, Ahmad P (2019) Plant growth promoting rhizobacteria induced Cd tolerance in Lycopersicon esculentum through altered antioxidative defense expression. Chemosphere 217:463–47
Köhle H, Grossmann K, Jabs T, Gerhard M, Kaiser W, Glaab J, Conrath U, Seehaus K, Herms S (2002) Physiological effects of the strobilurin fungicide F 500 on plants. In: Dehne H-W, Gisi U, Kuck KH, Russell PE, Lyr H (eds) Modern fungicides and antifungal compounds III. AgroConcept, Bonn, pp 61–74
Koller W (1987) Isomers of sterol synthesis inhibitors: fungicidal effects and plant growth regulator activities. Pesticide Sci 18:129–147
Kumar M, Chand R, Shah K (2016) Evidences for growth-promoting and fungicidal effects of low doses of tricyclazole in barley. Plant Physiol Biochem 103:176–182
Küpper H, Parameswaran A, Leitenmaier B, Trtilek M, Šetlík I (2007) Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytolo 175:655–674
Lanier C, Bernard F, Dumez S, Leclercq-Dransart J, Lemière S, Vandenbulcke F, Nesslany F, Platel A, Devred I, Hayet A, Cuny D, Deram A (2019) Combined toxic effects and DNA damage to two plant species exposed to binary metal mixtures (Cd/Pb). Ecotoxicol Environ Saf 167:278–287
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem J 210:899–903
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Packer L, Douce R (eds) Methods in enzymology. Academic Press, Cambridge, pp 350–382
Mahmud JA, Hasanuzzaman M, Nahar K, Bhuyan MHMB, Fujita M (2018) Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol Environ Saf 147:990–1001
Mohsin SM, Hasanuzzaman M, Bhuyan MHMB, Parvin K, Fujita M (2019) Exogenous tebuconazole and trifloxystrobin regulates reactive oxygen species metabolism toward mitigating salt-induced damages in cucumber seedling. Plants 8:428. https://doi.org/10.3390/plants8100428
Mohsin SM, Hasanuzzaman M, Nahar K, Hossain MS, Bhuyan MHMB, Parvin K, Fujita M (2020) Tebuconazole and trifloxystrobin regulate the physiology, antioxidant defense and methylglyoxal detoxification systems in conferring salt stress tolerance in Triticum aestivum L. Physiol Mol Biol Plants 26:1139–1154
Mohsin SM, Hasanuzzaman M, Parvin K, Fujita M (2020) Pretreatment of wheat (Triticum aestivum L.) seedlings with 2, 4-D improves tolerance to salinity-induced oxidative stress and methylglyoxal toxicity by modulating ion homeostasis, antioxidant defenses, and glyoxalase systems. Plant Physiol Biochem 152:221–231
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf 126:245–255
Nahar K, Rahman M, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ Sci Pollut Res 23:21206–21218
Najmanova J, Neumannova E, Leonhardt T, Zitka O, Kizek R, Macek T, Mackova M, Kotrba P (2012) Cadmium-induced production of phytochelatins and speciation of intracellular cadmium in organs of Linum usitatissimum seedlings. Ind Crop Prod 36:536–542
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Ni J, Wang Q, Shah FA, Liu W, Wang D, Huang S, Fu S, Wu L (2018) Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 23:799. https://doi.org/10.3390/molecules23040799
Norková R, Dytrtová JJ, Jakl M, Schröder D (2012) Formation of tebuconazole complexes with cadmium (II) investigated by electrospray ionization mass spectrometry. Water Air Soil Pollut 223:2633–2640
Parvin K, Hasanuzzaman M, Bhuyan MHMB, Mohsin SM, Fujita M (2019) Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants 8:247. https://doi.org/10.3390/plants8080247
Parvin K, Nahar K, Hasanuzzaman M, Bhuyan MHMB, Mohsin SM, Fujita M (2020) Exogenous vanillic acid enhances salt tolerance of tomato: insight into plant antioxidant defense and glyoxalase systems. Plant Physiol Biochem 150:109–120
Purohit S, Singh VP (1999) Uniconazole (S-3307) induced protection of Abelmoschus esculentus L. against cadmium stress. Photosynthetica 36:597–599
Rahman A, Nahar K, Hasanuzzaman M, Fujita M (2016) Manganese-induced cadmium stress tolerance in rice seedlings: coordinated action of antioxidant defense, glyoxalase system and nutrient homeostasis. C R Biol 339:462–474
Raza A, Habib M, Kakavand SN, Zahid Z, Zahra N, Sharif R, Hasanuzzaman M (2020) Phytoremediation of cadmium: physiological, biochemical, and molecular mechanisms. Biology 9:177. https://doi.org/10.3390/biology9070177
Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, Zia ur Rehman M, Waris AA (2019) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214:269–277
Ruske RE, Gooding MJ, Dobraszczyk BJ (2004) Effects of triazole and strobilurin fungicide programmes, with and without lateseason nitrogen fertiliser, on the baking quality of Malacca winter wheat. J Cereal Sci 40:1–8
Sauter H (2007) Fungicides acting on oxidative phosphorylation. In: Schirmer KW (ed) Modern crop protection compounds. Wiley, Weinheim, pp 457–495
Singh VP (1993) Uniconazole (S-3307) induced cadmium tolerance in wheat. J Plant Growth Regul 12:1–3
Susana MG, Liliana BP, Roberto AB, Claudia EA, María FI, Eliana PR, Myriam SZ, María DG, María PB (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Sytar O, Kumar A, Latowski D, Kuczynska P, Strzałka K, Prasad MNV (2013) Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol Plant 35:985–999
Thomas RM, Singh VP (1996) Reduction of cadmium-induced inhibition of chlorophyll and carotenoid accumulation in Cucumis sativus L. by uniconazole (S3307). Photosynthetica 32:145–148
Wei T, Lv X, Jia H, Hua L, Xu H, Zhou R, Zhao J, Ren X, Guo J (2018) Effects of salicylic acid, Fe (II) and plant growth-promoting bacteria on Cd accumulation and toxicity alleviation of Cd tolerant and sensitive tomato genotypes. J Environ Manag 214:164–171
Wu YX, Von-Tiedemann A (2001) Physiological effects of azoxystrobin and epoxiconazole on senescence and the oxidative status of wheat. Pestic Biochem Physiol 71:1–10
Xue ZC, Gao HY, Zhang LT (2013) Effects of cadmium on growth, photosynthetic rate and chlorophyll content in leaves of soybean seedlings. Biol Plant 57:587–590
Zasoski RJ, Burau RG (1977) A rapid nitric-perchloric acid digestion method for multi-element tissue analysis. Commun Soil Sci Plant Anal 8:425–436
Zhang YJ, Zhang X, Chen CJ, Zhou MG, Wang HC (2010) Effects of fungicides JS399-19, azoxystrobin, tebuconazloe, and carbendazim on the physiological and biochemical indices and grain yield of winter wheat. Pestic Biochem Physiol 98:151–157
Zhu H, Ai H, Cao L, Sui R, Ye H, Du D, Sun J, Yao J, Chen K, Chen L (2018) Transcriptome analysis providing novel insights for Cd-resistant tall fescue responses to Cd stress. Ecotoxicol Environ Saf 160:349–356
Acknowledgments
This study was funded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. Thanks to Dennis Murphy, Ehime University, Japan for English language correction of the manuscript.
Author information
Authors and Affiliations
Contributions
SMM designed, conceived, and executed the experiment and drafted the manuscript; MH designed the experiment, analyzed the data, and edited the manuscript; KP and MSH actively participated in the experiment; MF supervised the experiment, and edited and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohsin, S.M., Hasanuzzaman, M., Parvin, K. et al. Protective role of tebuconazole and trifloxystrobin in wheat (Triticum aestivum L.) under cadmium stress via enhancement of antioxidant defense and glyoxalase systems. Physiol Mol Biol Plants 27, 1043–1057 (2021). https://doi.org/10.1007/s12298-021-00983-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-00983-7