Abstract
Wheat (Triticum aestivum L.) is among the plants that are at risk from cadmium (Cd), a hazardous heavy metal that can be fatal due to its rapid absorption and high mobility. Being taken up from the soil and moving to the shoots and roots of edible plants, it enters the food chain and poses a health concern to people worldwide. A strategically important cereal crop, wheat has a demonstrated role in human health systems, particularly in poor nations. In this study, we describe the effects of nitric oxide (NO) on the growth, nutrition, and physiological functions of commercially cultivated wheat cvs. Galaxy 2013 and Akbar 2019 under Cd stress. Four-week-old plants were subjected to Cd (0.5 mM) stress, and after 2 weeks of Cd toxicity, foliar application of nitric oxide (100 and 150 μM) was carried out. As evident from excessive antioxidant production, Cd toxicity increased reactive oxygen species (ROS) level like H2O2 and significantly (p ≤ 0.001) decreased nutrient acquisition, growth, and yield attributes of plants under experiment. The severity of the effect varied between cultivars under investigation. A minimum accumulation of MDA (44%) and H2O2 (55%) was found in the cv. Akbar 2019 under Cd stress, whilst cv. Galaxy 2013 showed the highest accumulation of the oxidative stress indicators malondialdehyde content (MDA) (48%) and H2O2 (60%). Reduced and oxidized glutathione contents were also increased under Cd-induced toxicity. The application of NO resulted in a significant improvement of 22, 25, 25, and 30% in shoot fresh weight, root fresh weight, shoot dry weight, and root dry weight, respectively. Additionally, there was an increased uptake of Ca+2 (16%), K+1 (5%), chlorophyll a (46%), b (32%), a/b ratio (41%), and carotenoid (28%). When compared with Cd-stressed plants, yield parameters like 100 grain weight, number of tillers plant−1, and grain yield plant−1 improved by 14, 17, and 33%, respectively, under NO application. We concluded from the results of this study that NO treatments increased plant development by lowering oxidative stress and limiting Cd uptake. It is inferred from the results of this study that wheat production with reduced heavy metal uptake may be facilitated using NO due to its cytoprotective properties and its interaction with ROS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic stress is regarded as one of the key determinants to crop productivity and yield declines, accounting for nearly 70% of the global fall in crop yields (Godoy et al. 2021). The unbalanced relationship between crop productivity and the exponential population expansion has made this issue even worse. Therefore, it is crucial to comprehend how plants respond to such environmental constraints, especially heavy metals, and figure out how to reduce their damaging effects on plants (Singh et al. 2016).
Agricultural activities such as pesticide application, use of limestones, and inorganic fertilizers along with sewage sludge are also sources of heavy metal contamination in the environment. Different chemical processes like iron and steel smelting in industrial activities, electricity generation from coal and oil, mining, and sewage sludge all contribute to ecosystem destruction by deposition of heavy metal (Alengebawy et al. 2021). Their accumulation lowers soil quality, damages plants, and transmits pollutants to subsurface/surface water, where it enters the trophic food chain (Balali-Mood et al. 2021). Earth crust contains different types of heavy metals (Hurdebise et al. 2015). Among them, some are declared as life threatening and extremely dangerous for the environment by US Environmental Protection Agency (Terrón-Camero et al. 2019). Heavy metals such as Cd, Pb (lead), and As (arsenic) have contaminated one-fifth of agricultural land (Shi et al. 2019). These heavy metals have a variety of deleterious effects on plants, including reduced biomass accumulation, chlorosis, growth inhibition, poor photosynthesis, altered water balance, uneven nutrient absorption, and senescence. All these unfriendly processes result in plant mortality (Štofejová et al. 2021). The Cd is notoriously famous for destruction of soil-plant system due to its high solubility and mobility in agriculture systems (Anjum et al. 2016). Some previous studies marked out that Cd reduced germination, growth, dry biomass, and photosynthetic pigments in wheat (Dan-Yang et al. 2018).
Plants have different built-in mechanisms and strategies which help them to avoid and ameliorate the unfavorable and destructive effects of metal toxicity (Rather et al. 2020). Increasing nutrient uptake, triggering the antioxidant defense, and prompting biochemical and physiological processes including a cascade of signaling mediated by plant growth regulators are some of these strategies (Per et al. 2017). Soil remediation is being performed by applying different biochemical agents like charcoal, ferrous manganese, and slag to alleviate the deleterious aftereffects of Cd accumulation. There are different limitations and blockades which make soil remediation difficult by these means, including price, amount needed, and contamination due to some inorganic sources and time limitation in case of biological remediation. It is clearly evident from the results of previous study that NO has a positive outcome to reduce hazardous effects of Cd in plants (Khan et al. 2020).
In plants, the NO is a transient, multipurpose, and gaseous signaling molecule (Astier et al. 2018) which regulates different stages of plant life from germination to senescence (Corpas and Barroso 2015). Its synthesis and signaling play a vital role in alleviation and acclimation of plants in stressed environment (Kushwaha et al. 2019). The main objective of present study was based to investigate Cd uptake, its transport behavior, and its impact on cellular mechanisms in different indigenous wheat varieties of commercial interest. Understanding the mechanism underlying NO-mediated alleviation of Cd toxicity in wheat and highlighting the essential components of Cd phytotoxicity were the set goals of the study. The current study will discuss the findings of the Cd toxicity and NO cross talk in wheat in addition to making important recommendations for additional research in this field.
Materials and methods
This study aimed to examine the effects of NO foliar spray on wheat (Triticum aestivum L.) under Cd stress. We conducted pot experiments in triplicate for each treatment using a completely randomized design (CRD) to undertake this investigation. In this study, two commercial wheat cvs. Galaxy 2013 and Akbar 2019 were employed, and 12 seeds were planted in earthen pots of 30-cm diameter and 35-cm height, filled with 15-kg sandy loam soil. The soil pH was 7.8 and electrical conductivity was 2.01 dS/m. The nitrate content, available phosphorus, and exchangeable potassium were 45.5, 35.00, and 315 mg/kg, respectively.
After 3 weeks of germination, six plants were maintained in each pot and subsequently subjected to 0.5 mM of Cd stress using cadmium chloride as source material. After 2 weeks of Cd stress, we used sodium nitroprusside (SNP) as a foliar spray to give NO at 100 and 150 μM dosages. The foliar spray was supplemented with 0.1% Tween 20 as surfactant. After 3 weeks of NO application, we uprooted the plants to evaluate their growth, biochemical, and physiological characteristics. Three plants in each pot were left to grow for yield attribute study.
Growth attributes
Three samples from each replication were selected randomly. Plants were uprooted and washed with distilled water and were used for fresh and dry biomass estimation. After 1 week of shade drying, the collected shoots and roots were subjected to oven drying at 60 °C for 72 h. The dry mass of the tested subjects was determined using digital weight balance. At maturity, grain weight of 100 seeds, tillers plant−1, and grain yield plant−1 were calculated.
Chlorophyll content determination
The Arnon (1949) method was used to assay the pigment contents (chl. a and b). A leaf tissue weighing 0.5 g was pulverized using 10 mL of 80% acetone. This mixture was then stored in a refrigerator at 4 °C overnight. After 5-min centrifugation at 12,000 × g, the optical density (OD) was measured by Ultra-violet visible spectrometer (Hitachi-U2001 Tokyo Japan) at wavelengths of 645 and 663 nm.
Formula used to calculate the values of photosynthetic pigments is as follows:
V = extracted sample volume
W = fresh biomass weight (g)
Antioxidant enzymes assay
Liquid nitrogen was used to grind a leaf tissue weighing 0.5 g to halt metabolic activity and facilitate better analysis. Subsequently, a homogenized mixture was created using a phosphate buffer (PPB) with a concentration of 0.5 M and a pH of 7.5. The sample was centrifuged (12,000 × g) at 4 °C for 20 min. The resulting supernatant was carefully collected. For enzyme assays, the supernatant was preserved at − 80 °C.
As stated by Nakano and Asada (1981), the reduction in absorbance at 290 nm was observed for 120 s to verify the presence of ascorbate peroxidase (APX) activity in the mixture. Nitroblue tetrazolium chloride inoculated to the enzymes, inhibiting their photochemical activity. The absorbance at 560 nm was recorded to assess the activity of superoxide dismutase (SOD) using the method.
The Polle et al. (1994) method was used to determine the activity of peroxidase (POD). This involved adding guaiacol (20 mM) and 100 μL of enzyme extract and hydrogen peroxide (H2O2, 10 mM) to a 3-mL reaction mixture. A rise in OD confirmed the presence of H2O2, recorded at 470 nm for 3 min.
Catalase (CAT) was assessed using a technique pioneered by Chance and Maehly (1955). The composition of reaction mixture was 0.1 mL of enzyme extract, 20 mM H2O2, and a PPB with a concentration of 0.5 M with pH 7.5. Over the course of 3 min, the decrease in absorbance was measured at 240 nm.
All enzymatic antioxidant activities were quantified in enzyme units per milligram of protein (U mg −1).
MDA content
The MDA content was estimated using a modified version of technique to determine the degree of lipid peroxidation. A frozen leaf sample weighing 2 g was homogenized in 20 mL of 50 mM phosphate buffer with a pH of 7.8. Centrifuging the homogenate for 10 min at 4 °C and 12,000 × g. The recovered supernatant was combined with 1.5 mL of 0.5% TBA in a volume of 5 mL. After being heated for 20 min at 95 °C in a water bath, the resulting mixture was cooled in an ice bath. At wavelengths of 532, 600, and 440 nm, the supernatant absorbance was calculated using a spectrophotometer (UV-2550; Shimadzu, Kyoto, Japan). The MDA content was calculated using the following formulae and given as nmolg−1 FW:
H2O2 content
Leaf tissues homogenized in trichloroacetic acid (TCA) were used to assess the H2O2 content according to Velikova et al. (2000) method. Analytical grade H2O2 was used to make standard calibration curve and was used thereafter for calculation of H2O2 concentration.
Proline content
For assays of proline contents, 3% sulphosalicylic acid, volume 0.5 mL, was homogenized with fresh biomass of sample weighing 0.1 g. The homogenate material was centrifuged. Supernatant was extracted mixed and homogenized with glacial acetic acid along with ninhydrin. The resultant combination was heated for an hour before being plunged into ice water for 10 min. The OD of sample at 520 nm revealed the presence of proline content in plant biomass using a UV-Vis spectrometer (model: Hitachi-U2001, manufactured in Tokyo, Japan) (Bates et al. 1973).
GB determination
Grieve and Grattan’s (1983) method was applied to detect and analyze GB content. Fresh leaves were mixed with 2 N H2SO4 and vortexed rapidly; after that, the mixture was refrigerated. Iodine and potassium were mixed to prepare periodide solution, which then mixed with extracts and stored at 4 °C for 16 h. The supernatant was taken out after the mixture was centrifuged at 10,000 × g for 15 min at 4 °C. The 1,2-dichloroethane along with iodide crystals comprising a volume of 10 mL was vortexed and contained at 25 °C for 20 min. The OD of sample solution was then assessed at a wavelength of 365 nm revealing the presence of our desired compound.
Estimation of K+ and Ca+2 ions
The approach outlined by Allen et al. (1976) was used to detect and quantify ions. In this procedure, 0.2-mL concentrated H2SO4 was used to digest 0.1 g of dried plant material. The resultant combination underwent a 24-h incubation period. Sample with a preheating at 150 °C was subjected to 35% H2O2 treatment until it became a clear solution. Using a flame photometer of the Sherwood Model 360, the digested solution was used to measure the amounts of K+ and Ca+2 ions.
Estimation of Cd contents
Following Wolf’s (1982) method, 0.2 g of thoroughly dried, finely crushed plant material was obtained and subjected to digestion within a solution composed of H2SO4 and H2O2 in a 3:1 ratio. The concentration of Cd was determined using an atomic absorption spectrophotometer (Z-2000 240V Hitachi). By utilizing a stock solution with a 100 mg/L Cd concentration and consulting a standard curve, the levels of Cd were calculated.
Estimation of GR
Smith et al. (1988)’s method was used to extract reduced glutathione (GR) from 0.5 g of plant tissues using a 0.5-mL sample of 100 mM PPB at pH 7.5 and treated with 0.5 mM EDTA. Glutathione (GSH) reduced the DNTB, resulting in higher OD detection leading to GR activity under 412 nm. The absorbance variations in the reaction mixture were plotted against a known amount of glutathione reductase to assess GR activity.
Estimation of oxidized GSH and reduced glutathione (GSSG)
Updated Griffith’s (1980) technique to quantify GSSG and GSH concentrations in wheat leaves, a sample of 0.25 g fresh leaf mass was crushed in 0.1 M HCl and 0.01 mM EDTA (total volume 2 mL) and recovered the extract by centrifuging at 12,000 × g for 15 min at 4 °C. In the PPB, 125 mM (200 mL), 6.0 mM DTNB (100 mL), 200 mL of the extract, 0.3 mM NADPH (500 L), and 6.3 mM EDTA (pH 7.5). The absorbance at 412 nm was measured using spectrophotometer (UV-2550; Shimadzu, Kyoto, Japan).
Grain yield (g)
The total number of grains of each plant was counted and weighed using digital weight balance.
Number of tillers per plant
The number of tillers per plant of each variety was counted manually.
100-grain weight
Weight of 100 grains for each plant was measured by using digital weight balance.
Statistical analysis
Using CoStat Ver. 6.303, the significance between the mean values was assessed once the data were acquired and validated. RStudio Ver. 4.13 for Windows was used to investigate the associations between the various analyzed variables (Fig. 5) and PCA (principal component analysis) (Fig. 6).
Results
Different symptoms like stunted growth, disturbed metabolic machinery, and disturbed osmotic balance indicate that Cd accumulation in different plant tissues. In this investigation, we observed a considerable (p ≤ 0.001) downfall in shoot fresh weight (35%, 31%) and dry weight (32%, 28%) of Galaxy 2013 and Akbar 2019, respectively.
The foliar application of NO increased 22 and 19% shoot fresh weight along with 25% and 26% dry weight in Galaxy 2013 and Akbar 2019, respectively.
Both wheat cvs. Galaxy 2013 and Akbar 2019 experienced a significant decrease in root fresh (37 and 34%) and dry (36 and 22%) weight under Cd stress when compared with control. The NO foliar application under Cd stress increased fresh (25 and 22%) and dry (30 and 14%) weight of root in wheat cvs. Galaxy 2013 and Akbar 2019, respectively. The cv. Akbar 2019 performed better under control and stress condition with NO (150 μM) application (Fig. 1).
The effects of NO application on shoot fresh weight (a), shoot dry weight (b), root fresh weight (c) and root dry weight (d) of wheat (Triticum aestivum L.) cultivars under Cd stress. Treatments applied were as T0 = Control, T1 = 100 μM NO, T2 = 150 μM NO, T3 = 0.5 mM Cd, T4 = 100 μM NO + 0.5 mM Cd, T5 = 150 μM NO + 0.5 mM Cd
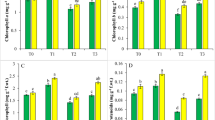
Stress causes a substantial impact on the production and working of plant pigment contents. One of the primary reactions compromised by stress in plants is photosynthesis. As compared to control, the Cd stress decreased chl a (49 and 42%), chl b (40 and 25%), chl a/b ratio (47 and 38%), and carotenoid (34 and 29%) in cvs. Galaxy 2013 and Akbar 2019. The NO application was found to be a positive stimulator of carotenoid and chlorophyll synthesis, which would have improved the photosystem I and II efficiency of wheat plants in this study. A linear relationship between NO concentration used and increment in pigments concentration was observed in the present study. At 150 μM NO application, the highest increase in chl a (46 and 39%), chl b (32 and 21%), chl a/b ratio (41 and 30%), and carotenoid (28 and 21%) was observed in both wheat cv. under study (Fig. 2).
The effects of NO application on chlorophyll a (a), chlorophyll b (b), chlorophyll a/b ratio (c), and carotenoids (d) of wheat (Triticum aestivum L.) cultivars under Cd stress. Treatments applied were as T0 = Control, T1 = 100 μM NO, T2 = 150 μM NO, T3 = 0.5 mM Cd, T4 = 100 μM NO + 0.5 mM Cd, T5 = 150 μM NO + 0.5 mM Cd
Oxidative stress in the plant cells caused by Cd toxicity resulted in significant alterations in enzyme activity. Antioxidant enzyme activities (SOD, POD, CAT, and APX) considerably enhanced in response to Cd stress. Both wheat cvs., Galaxy 2013 and Akbar 2019 when exposed to stress, had higher contents of SOD (25 and 43%), POD (52 and 24%), CAT (38 and 40%), and APX (29 and 33%) as comparison to control. While NO application improved antioxidative capacity of the cell by increasing activity of SOD (28 and 9%), POD (9 and 18%), CAT (5 and 3%), and APX (9 and 3%) in cvs. Galaxy 2013 and Akbar 2019, respectively when compared with stressed plants (Fig. 3).
The MDA and H2O2 contents were measured to examine lipid peroxidation, which is an indirect measure of the membrane damage caused by Cd stress. The MDA (48 and 44%) and H2O2 (60 and 55%) contents gradually increased with increasing Cd concentration in both wheat varieties when compared to untreated control plants. Application of NO under stressful conditions reduced MDA (36 and 31%) and H2O2 (34 and 32%) in both cvs. Galaxy 2013 and Akbar 2019, respectively (Fig. 4).
Proline and GB levels in wheat plants are normally low, but they rose when Cd stress was applied. In the present study, GB (63 and 45%) and proline (56 and 59%) accumulation in response to Cd stress in both varieties is to maintain the osmotic balance in the cells as compared to positive control plants. The NO application under stress condition further increased the GB (10 and 29%) and proline (34 and 32%) in Galaxy 2013 and Akbar 2019, respectively (Fig. 4).
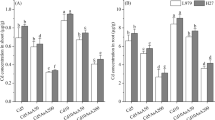
The uptake and transfer of minerals by plants are crucial for their growth, and Cd may interfere with this process and cause disruptions. In Galaxy 2013 and Akbar 2019, respectively, the results showed that calcium (23 and 22%) and potassium (9 and 10%) ions decreased more under Cd stress compared to the positive control, while NO foliar application under Cd stress significantly increased calcium (16 and 21%) and potassium (5 and 6%) contents compared to negative control plants (Table 1).
Current study demonstrated significant increase in Cd ion (96 and 61%) under Cd stress in both wheat cvs. Galaxy 2013 and Akbar 2019 as compared to controlled plants while NO application significantly decreased Cd ion (42 and 56%) in Galaxy 2013 and Akbar 2019 as compared to Cd stress condition (Table 1).
Additionally, it was discovered in this study that GR (13 and 16 %) activity considerably elevated under Cd stress. The increase in GR’s substrate, GSSG, may be the cause of this observation (33 and 30 %).GR is a flavoenzyme that catalyzes the conversion of GSSG to GSH. It increased in Galaxy 2013 (53%) and Akbar 2019 (51%) under stress conditions when compared to control plants. While NO application further increased GR (12 and 43%), GSH (40 and 5%), and GSSG (21 and 49%) level in Galaxy 2013 and Akbar 2019 cultivars as compared to negative control (Table 2).
Different yield attributes substantially decreased under Cd stress in this study. The decrease in 100-grain weight (21 and 14%), no. of tillers per plant (26 and 22%), and grain yield plant−1 (58 and 46%) was significant in cvs. Galaxy 2013 and Akbar 2019 which revealed elevated stress levels compared to the control. When no foliar application was used, however, there was a significant increase in 100-grain weight (14% and 10%), tillers per plant (17% and 5%), and grain yield per plant (33% and 20%) in Galaxy 2013 and Akbar 2019, respectively, when compared to Cd-treated plants, as shown in Table 2.
Pearson’s correlation shows the relationship between wheat plant growth, physio-biochemical traits, antioxidants, minerals, and yield attributes (Fig. 5). The SFW, RFW, SDW, RDW, Chl a, Chl b, Chl a/b ratio, Car, Ca2+, K, GY, NTTP, and 100 GW were negatively correlated with SOD, POD, CAT, APX, MDA, H2O2 GB, GR, GSH, GSSG, PR, and Cd. However, SOD, POD, PR, and GB have strong positive correlation.
Pearson’s correlation among the attributes studied. Abbreviated subjects are as follows: H2O2 (hydrogen peroxide initiation), GB (glycine betaine), Pro (proline content), POD (peroxidase activity), Ca2+ (calcium content), K+ (potassium content), GR (glutathione reductase), SOD (superoxide dismutase), GSH (oxidized glutathione content), MDA (malondialdehyde content), GSSG (reduced glutathione content), SF (shoot fresh weight), TPP (tillers plant−1), GY (grain yield), Chl a (chlorophyll a content), SD (shoot dry weight), 100 GW (100 grain weight), Chl b (chlorophyll b content), RF (root fresh weight), Chl a/b (chlorophyll a/b ratio), Car (carotenoid), APX (ascorbate peroxidase activity), CAT (catalase activity), Cd (cadmium content), and RD( root dry weight)
Results from PCAs indicated significant variations in the effects of Cd and NO treatments on various wheat growth, physio-biochemical traits, antioxidants, minerals, and yield attributes (Fig. 6). The first and second Dims explained, respectively, 72% and 14.14 % (total 86.14 %) of the variation between treatments and varieties. Among the extracted components, the major contribution Dim 1 was followed by Dim 2. In this study SOD, POD, CAT, APX, MDA, H2O2 GB, GR, GSH, GSSG, PR, and Cd are negatively correlated with SFW, RFW, SDW, RDW, Chl a, Chl b, Chl a/b ratio, Car, Ca2+, K, GY, NTTP, and 100 GW. However, H2O2 and GB are closely positively correlated with CAT and APX, while SFW also negatively correlate with SDW, RFW, RDW, Chl a, Chl b, Chl a/b ratio, Car, Ca2+, K, GY, NTTP, and 100 GW.
Principal component analysis showing association among the studied attributes. Abbreviated subjects are as follows: H2O2 (hydrogen peroxide initiation), GB (glycine betaine), Pro (proline content), POD (peroxidase activity), Ca2+ (calcium content), K+ (potassium content), GR (glutathione reductase), SOD (superoxide dismutase), GSH (oxidized glutathione content), MDA (malondialdehyde content), GSSG (reduced glutathione content), SF (shoot fresh weight), TPP (tillers plant−1), GY (grain yield), Chl a (chlorophyll a content), SD (shoot dry weight), 100 GW (100 grain weight), Chl b (chlorophyll b content), RF (root fresh weight), Chl a/b (chlorophyll a/b ratio), Car (carotenoid), APX (ascorbate peroxidase activity), CAT (catalase activity), Cd (cadmium content), and RD( root dry weight)
Discussion
Even though it is only a trace element, Cd is extremely poisonous to both plants and animals. Its buildup in plant tissues can inhibit the growth of plants in a number of ways, including by interfering with photosynthesis and other crucial chemical processes in plants (Moradi et al. 2019). The reduced biomass production in both wheat cultivars observed in this study may be the first indication that plant Cd levels are high (Menon et al. 2016). Many studies related to metal plant interaction are focused on transportation and accumulation mechanisms. Photosynthesis is one of the crucial metabolic processes disturbed by Cd toxicity along with nutrient uptake and water relations (Cuypers et al. 2009). It is well documented that all these interruptions of photosynthesis occur at structural and functional levels and ultimately reduces plant biomass. The Cd stress may hinder plant development by interfering with normal physiological and molecular processes in plants via increased ROS production (Rizwan et al. 2019).
Additionally, our findings demonstrated the beneficial effects of NO foliar application, which tends to reduce Cd toxic effect and improves plant biomass and yield. Several defensive mechanisms, including cell wall relaxation, phospholipid bilayer protection, and cell wall enlargement, minimize the Cd toxicity. The NO has been shown to have effects like those of auxin and to control plant development (Nabaei and Amooaghaie 2019) thus increasing biomass accumulation under Cd stress.
Applied Cd stress diminished chl a and chl b of both wheat varieties as compared to control in the present study. Our findings are consistent with the findings of Bahmani et al. (2020), who discovered that Cd toxicity suppressed photosynthetic pigments across different plant species. Reduced chlorophyll production may be caused by the substitution of a chlorophyll structure, which might be a result of massive uptake of Cd instead of Mg as they compete for transport channels, and therefore lead to damage or functional loss of the photosynthetic mechanism (Saleh et al. 2020). The NO application on the other hand has been shown to inhibit chlorophyll-degrading enzymes and increase internal Mg and Fe availability, promoting chlorophyll synthesis and reversing chlorotic symptoms (Nabaei and Amooaghaie 2020). Another mechanism of Cd stress alleviation by NO may be maintenance of osmotic pressure which in turn protects the chloroplast membrane and pigments.
Cd deposited into the soil and plant body induces the formation of reactive species, activating the plant’s defense mechanism. Agents of defense systems like antioxidant enzymes activate the plant action against the foreign body (Noreen et al. 2021). Rapid action by production of POD and CAT boosts the water formation by scavenging the H2O2 molecules (Gong et al. 2019), and SOD catalyzes the conversion of O2 to H2O2 (Gupta et al. 2019). Higher levels of enzymes like POD and CAT are aligned with the studies of Pandey and Dubey (2019), which elaborate the involvement of these enzymes in the endogenous defense system of plant. Previous reports have revealed that NO acts as a signal molecule which might play a role in upregulation of gene expression or antioxidant enzyme activity in the presence of abiotic stress like heavy metals. Moreover, since iron (Fe) plays a crucial structural and functional role in peroxidase (POD) and catalase (CAT), its deficiency could potentially reduce the activities of these enzymes when plants are subjected to Cd stress. However, various studies have reported that nitric oxide (NO) can potentially enhance CAT and POD activity by improving iron accessibility in Cd-exposed wheat plants, as highlighted by Tang et al. (2018).
Plant oxidative damage increases when antioxidant and oxidant levels are out of balance. Oxidative stress might lead to lipid peroxidation and destruction of plant membranes, resulting in disturbed plant osmotic levels along with cellular integrity (El-Kafafi et al. 2017). The current findings indicate that elevated amounts of ROS, such as H2O2, increased significantly and enhanced the amount of MDA in wheat cultivars. Previous studies have indicated that under Cd stress, several plant species, including Triticum aestivum, Zea mays, and Brassica napus, produced higher H2O2 and MDA contents (Nazir et al. 2020).
It has been demonstrated that NO avoided membrane damage and lowered H2O2 levels. It has already been demonstrated that the administration of NO decreases electrolyte leakage in Typha angustifolia under Cd stress. The antioxidant properties of NO may be to blame. In plants, free proline creates a non-toxic Cd-protein complex (Rady et al. 2019). The increase of proline in the cell membrane protects the membrane from oxidative damage (Szepesi and Szőllősi 2018). Exogenously applied NO increased proline levels in Cd-stressed plants by scavenging ROS, enrichment of energy source, and regulating cellular homeostasis (Aswani et al. 2019). Different studies reported under Cd-treated plants, it is well documented that the GB plays a critical role in maintaining cellular osmoregulatory activities across a wide range of plant species, as demonstrated by Zaid and Mohammad (2018)’s study. Our research has confirmed this significant fact. Furthermore, GB might have had contribution in upregulation of stress-responsive genes, along with antioxidant profile leading to the reduction of reactive oxygen species (ROS), and the conservation of protein conformations (Ahmad et al. 2021). The foliar application of nitrogen oxide (NO) brought about alterations in Golgi apparatus (GB) content through the modulation of transcriptional processes involving betaine aldehyde dehydrogenase (BADH) genes, as elucidated by. The surge in GB production triggered by NO application amidst the backdrop of Cd-induced stress appears to be correlated with heightened levels of expression of BADH genes.
Cd stress has been revealed to reduce the levels of critical nutrients such as calcium (Ca2+) and potassium (K+) in various plant species, according to the results of. The drop in concentrations of these essential elements caused by Cd-induced stress might be related to competition at adsorption sites and transporters, particularly those with comparable charge and size, as postulated by Yang et al. (2018). According to our findings, exogenous application of NO may reduce heavy metal toxicity by increasing cytosolic Ca2+ and K+ concentrations via regulating Ca2+ and K+ channels and transporters that may be implicated in the signaling cascade that governs gene expression as reported by Xu et al. (2010).
The Cd concentrations in wheat stressed plants were higher in this study, most likely due to complex formation of metal ions with sulfur ligands in root cells (Anwar et al. 2019). It was discovered that applying NO causes changes in soil properties, which cause reduction in mobility and uptake of toxic metallic ions (Fan et al. 2016). In previous investigations, it is reported that MTs (metallothioneins: basically, cysteine-rich metal-binding proteins) form chelates by binding with heavy metals through sulfhydryl groups. This leads to reduced metallic toxicity in soil. Wang et al. (2010) has reported exogenously NO application involvement in improving Cd resistance by increasing the MTs in soil exposed to Cd toxicity.
Plants have GR in their chloroplasts, cytoplasm, and mitochondria, which are involved in the ascorbate-glutathione cycle involved in H2O2 breakdown. In the present study, wheat leaves showed the maximum specific activity of GR, GSH, and GSSG in our experiment. The GR is a flavor enzyme that catalyzes the reduction of GSSG to GSH in the presence of NADPH. This reaction is necessary for the ascorbate-glutathione cycle to work properly and for cells to maintain a correct GSH/GSSG concentration ratio (Li et al. 2013). Recently, Xu et al. (2010) revealed that exogenous application of NO stimulates γ-ecs and gshs gene expression, which controls GSH production under stress, and it may be the same in our case, but variations in level are due to difference in genetic makeup of the varieties under investigation.
The Cd stress resulted in a significant reduction in different yield parameters such as grain yield, along with number of tillers (plant −1) in both wheat varieties under study. The decrease in yield is caused by a decrease in plant biomass production because of metabolic changes in biochemical and physiological processes (Hussain et al. 2015). Foliar application of NO exhibited to supplement plant growth and biomass production, resulting in increased yield characteristics as reported in previous studies (Azizi et al. 2021).
Conclusions
This research delineates the beneficial function of nitric oxide (NO) signaling ameliorating the impact of metal stress in plants. Cd stress has more damaging effects on Galaxy 2013 than Akbar 2019. The reason behind this difference in varietal response may be due to the difference in the genetic makeup of the said wheat cultivars. Our research revealed that the use of NO reduced the toxicity of Cd and improved plant development and yield as a result of an improved antioxidant profile, decreased metal accumulation, and metallic ion chelation. All of this leads to immobilization, decreased absorption from roots to shoots, and ultimately improved photosynthesis and plant development. Results revealed the reduced lipid peroxidation was due to enhanced antioxidant profile of plants under application of NO. It was found that the exogenous application of 150 μM NO is more effective than 100 μM for amelioration of Cd toxicity in commonly grown wheat cultivars under investigation. However, the need exists to execute future studies by involving different plant/crop species to understand the cross talk between NO and other molecular components. We conclude that improved plant protection, metabolism, signaling, and reduced Cd uptake in wheat are due to exogenous NO application.
Data availability
Not applicable.
References
Ahmad P, Raja V, Ashraf M, Wijaya L, Bajguz A, Alyemeni MN (2021) Jasmonic acid (JA) and gibberellic acid (GA3) mitigated Cd-toxicity in chickpea plants through restricted cd uptake and oxidative stress management. Sci Rep 11(1):1–17
Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ (2021) Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics 9(3):42
Allen S, Grimshow H, Parkinson J, Quarmby C, Roberts J (1976) Chemical analysis in methods in plant ecology by Chapman. Black Well, London
Anjum SA, Tanveer M, Hussain S, Ullah E, Wang L, Khan I et al (2016) Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. CLEAN–Soil Air Water 44(1):29–36
Anwar S, Khan S, Hussain I, Bashir R, Fahad S (2019) Chelators induced uptake of cadmium and modulation of water relation, antioxidants, and photosynthetic traits of maize. Environ Sci Pollut Res 26(17):17577–17590
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1
Astier J, Gross I, Durner J (2018) Nitric oxide production in plants: an update. J Exp Bot 69(14):3401–3411
Aswani V, Rajsheel P, Bapatla RB, Sunil B, Raghavendra AS (2019) Oxidative stress induced in chloroplasts or mitochondria promotes proline accumulation in leaves of pea (Pisum sativum): another example of chloroplast-mitochondria interactions. Protoplasma 256(2):449–457
Azizi I, Esmaielpour B, Fatemi H (2021) Exogenous nitric oxide on morphological, biochemical and antioxidant enzyme activity on savory (Satureja Hortensis L.) plants under cadmium stress. J Saudi Soc Agric Sci
Bahmani R, Modareszadeh M, reza Bihamta M (2020) Genotypic variation for cadmium tolerance in common bean (Phaseolus vulgaris L.). Ecotoxicol Environ Saf 190:110178
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Chance, B., & Maehly, A. C. (1955). [136] Assay of catalases and peroxidases
Corpas FJ, Barroso JB (2015) Nitric oxide from a “green” perspective. Nitric Oxide 45:15–19
Cuypers A, Smeets K, Vangronsveld J (2009) Heavy metal stress in plants. In: Hirt H (ed) Plant Stress Biology: From Genomics to Systems Biology. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany
Dan-Yang Q, Wan-Rong G, Li-Jie L, Jing L, Cai-Feng L, Shi W (2018) Regulation of chitosan on the ascorbate-glutathione cycle in Zea mays seedling leaves under cadmium stress. Plant Sci J 2:17
El-Kafafi S, El-Demerdash F, Taha A (2017) Toxic effect of cadmium stress on rooting, physiological and biochemical perturbations in cowpea seedling. Middle East J 6(3):646–661
Fan X, Wen X, Huang F, Cai Y, Cai K (2016) Effects of silicon on morphology, ultrastructure and exudates of rice root under heavy metal stress. Acta Physiol Plant 38(8):1–9
Godoy F, Olivos-Hernández K, Stange C, Handford M (2021) Abiotic stress in crop species: improving tolerance by applying plant metabolites. Plants 10(2):186
Gong X, Huang D, Liu Y, Zeng G, Wang R, Xu P et al (2019) Roles of multiwall carbon nanotubes in phytoremediation: cadmium uptake and oxidative burst in Boehmeria nivea (L.) Gaudich. Environ Sci Nano 6(3):851–862
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70(2):303–307
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106(1):207–212
Gupta N, Yadav KK, Kumar V, Kumar S, Chadd RP, Kumar A (2019) Trace elements in soil-vegetables interface: translocation, bioaccumulation, toxicity and amelioration-a review. Sci Total Environ 651:2927–2942
Hurdebise Q, Tarayre C, Fischer C, Colinet G, Hiligsmann S, Delvigne F (2015) Determination of zinc, cadmium and lead bioavailability in contaminated soils at the single-cell level by a combination of whole-cell biosensors and flow cytometry. Sensors 15(4):8981–8999
Hussain I, Ashraf MA, Rasheed R, Asghar A, Sajid MA, Iqbal M (2015) Exogenous application of silicon at the boot stage decreases accumulation of cadmium in wheat (Triticum aestivum L.) grains. Braz J Bot 38(2):223–234
Khan MN, Alamri S, Al-Amri AA, Alsubaie QD, Al-Munqedi B, Ali HM et al (2020) Effect of nitric oxide on seed germination and seedling development of tomato under chromium toxicity. J Plant Growth Regul:1–13
Kushwaha BK, Singh S, Tripathi DK, Sharma S, Prasad SM, Chauhan DK et al (2019) New adventitious root formation and primary root biomass accumulation are regulated by nitric oxide and reactive oxygen species in rice seedlings under arsenate stress. J Hazard Mater 361:134–140
Li FT, Qi JM, Zhang GY, Lin LH, Fang PP, Tao AF, Xu JT (2013) Effect of cadmium stress on the growth, antioxidative enzymes and lipid peroxidation in two kenaf (Hibiscus cannabinus L.) plant seedlings. J Integr Agric 12(4):610–620
Menon P, Joshi N, Joshi A (2016) Effect of heavy metals on seed germination of Trigonella foenum-graceum L. Int J Life-Sci Sci Res 2(4):488–493
Moradi R, Pourghasemian N, Naghizadeh M (2019) Effect of beeswax waste biochar on growth, physiology and cadmium uptake in saffron. J Clean Prod 229:1251–1261
Nabaei M, Amooaghaie R (2019) Nitric oxide is involved in the regulation of melatonin-induced antioxidant responses in Catharanthus roseus roots under cadmium stress. Botany 97(12):681–690
Nabaei M, Amooaghaie R (2020) Melatonin and nitric oxide enhance cadmium tolerance and phytoremediation efficiency in Catharanthus roseus (L.) G. Don. Environ Sci Pollut Res 27(7):6981–6994
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Nazir F, Fariduddin Q, Khan TA (2020) Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 252:126486
Noreen S, Sultan M, Akhter MS, Shah KH, Ummara U, Manzoor H et al (2021) Foliar fertigation of ascorbic acid and zinc improves growth, antioxidant enzyme activity and harvest index in barley (Hordeum vulgare L.) grown under salt stress. Plant Physiol Biochem 158:244–254
Pandey P, Dubey RS (2019) Metal toxicity in rice and strategies for improving stress tolerance. In: Advances in rice research for abiotic stress tolerance. Woodhead Publishing, pp 313–339
Per TS, Khan NA, Reddy PS, Masood A, Hasanuzzaman M, Khan MIR, Anjum NA (2017) Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol Biochem 115:126–140
Polle A, Otter T, Seifert F (1994) Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abies L.). Plant Physiol 106(1):53–60
Rady MM, Elrys AS, El-Maati MFA, Desoky ESM (2019) Interplaying roles of silicon and proline effectively improve salt and cadmium stress tolerance in Phaseolus vulgaris plant. Plant Physiol Biochem 139:558–568
Rather BA, Masood A, Sehar Z, Majid A, Anjum NA, Khan NA (2020) Mechanisms and role of nitric oxide in phytotoxicity-mitigation of copper. Front Plant Sci 11:675
Rizwan M, Ali S, ur Rehman MZ, Malik S, Adrees M, Qayyum MF et al (2019) Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol Plant 41(3):1–12
Saleh SR, Kandeel MM, Ghareeb D, Ghoneim TM, Talha NI, Alaoui-Sossé B et al (2020) Wheat biological responses to stress caused by cadmium, nickel and lead. Sci Total Environ 706:136013
Shi T, Ma J, Wu F, Ju T, Gong Y, Zhang Y et al (2019) Mass balance-based inventory of heavy metals inputs to and outputs from agricultural soils in Zhejiang Province, China. Sci Total Environ 649:1269–1280
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5, 5′-dithiobis (2-nitrobenzoic acid). Anal Biochem 175(2):408–413
Štofejová L, Fazekaš J, Fazekašová D (2021) Analysis of heavy metal content in soil and plants in the dumping ground of magnesite mining factory Jelšava-Lubeník (Slovakia). Sustainability 13(8):4508
Szepesi Á, Szőllősi R (2018) Mechanism of proline biosynthesis and role of proline metabolism enzymes under environmental stress in plants. In: Plant metabolites and regulation under environmental stress. Academic Press, pp 337–353
Tang Y, Lin L, Xie Y, Liu J, Sun G, Li H et al (2018) Melatonin affects the growth and cadmium accumulation of Malachium aquaticum and Galinsoga parviflora. Int J Phytoremediation 20(4):295–300
Terrón-Camero LC, Peláez-Vico MÁ, Del-Val C, Sandalio LM, Romero-Puertas MC (2019) Role of nitric oxide in plant responses to heavy metal stress: exogenous application versus endogenous production. J Exp Bot 70(17):4477–4488
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(1):59–66
Wang L, Yang L, Yang F, Li X, Song Y, Wang X, Hu X (2010) Involvements of H2O2 and metallothionein in NO-mediated tomato tolerance to copper toxicity. J Plant Physiol 167(15):1298–1306
Wolf B (1982) An improved universal extracting solution and its use for diagnosing soil fertility. Commun Soil Sci Plant Anal 13(12):1005–1033
Xu J, Wang W, Yin H, Liu X, Sun H, Mi Q (2010) Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 326(1):321–330
Yang LP, Zhu J, Wang P, Zeng J, Tan R, Yang YZ, Liu ZM (2018) Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol Environ Saf 160:10–18
Zaid A, Mohammad F (2018) Methyl jasmonate and nitrogen interact to alleviate cadmium stress in Mentha arvensis by regulating physio-biochemical damages and ROS detoxification. J Plant Growth Regul 37(4):1331–1348
Author information
Authors and Affiliations
Contributions
Conceptualization, Muhammad Nawaz and Hamzah Saleem; Data curation, Muhammad Rehan Khalid; formal analysis, Baber Ali; funding acquisition, Muhammad Nawaz and Hamzah Saleem; investigation, Muhammad Nawaz and Hamzah Saleem; methodology, Muhammad Nawaz and Hamzah Saleem Saleem; project administration, Muhammad Rehan Khalid; resources, Muhammad Rehan Khalid; software, Baber Ali; supervision, Shah Fahad; validation, Muhammad Rehan Khalid; visualization, Shah Fahad ; writing—original draft, Muhammad Hamzah Saleem and Baber Ali; writing—review and editing, Shah Fahad. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We all declare that manuscripts reporting studies do not involve any human participants, human data, or human tissue. So it is not applicable.
Consent for publication
Our manuscript does not contain data from any individual person, so it is “Not applicable.”
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nawaz, M., Saleem, M.H., Khalid, M.R. et al. Nitric oxide reduces cadmium uptake in wheat (Triticum aestivum L.) by modulating growth, mineral uptake, yield attributes, and antioxidant profile. Environ Sci Pollut Res 31, 9844–9856 (2024). https://doi.org/10.1007/s11356-024-31875-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-31875-4