Abstract
Three polymerase chain reaction (PCR) techniques were compared to analyse the genetic diversity of Clinacanthus nutans eight populations in the northern region of Peninsular Malaysia. The PCR techniques were random amplified polymorphic deoxyribonucleic acids (RAPD), inter-simple sequence repeats (ISSR) and random amplified microsatellite polymorphisms (RAMP). Leaf genomic DNA was PCR amplified using 17 RAPD, 8 ISSR and 136 RAMP primers . However, only 10 RAPD primers, 5 ISSR primers and 37 RAMP primers produced reproducible bands. The results were evaluated for polymorphic information content (PIC), marker index (MI) and resolving power (RP). The RAMP marker was the most useful marker compared to RAPD and ISSR markers because it showed the highest average value of PIC (0.25), MI (11.36) and RP (2.86). The genetic diversity showed a high percentage of polymorphism at the species level compared to the population level. Furthermore, analysis of molecular variance revealed that the genetic diversity was higher within populations, as compared to among populations of C. nutans. From the results, the RAMP technique was recommended for the analysis of genetic diversity of C. nutans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sabah Snake Grass, which is also known as Daun Belalai Gajah is a popular medicinal plant in South-East Asia. Its scientific name is Clinacanthus nutans Lindau and it belongs to the Acanthaceae family. It has been used in many local remedies and its extracts had been used to treat skin rashes, snake bites, insect stings, inflammation, cancer and as antivirals against herpes simplex virus (HSV) and varicella zoster virus (VZV) (Arullappan et al. 2014; Sakdarat et al. 2006; Wanikiat et al. 2008). C. nutans is well distributed in Malaysia, Thailand, Indonesia, Vietnam and China (Aslam et al. 2015). Local communities, and farmers have cultivated C. nutans through stem cuttings and this has resulted in informal exchanges of C. nutans. The most common method of propagation of C. nutans is via stem cutting (vegetative propagation) rather than sexual reproduction, as sexual reproduction results in poor multiplication rate and is time consuming (Fong et al. 2014). Therefore, there is a great need for wholesalers, retailers and consumers to know the genetic relationship of C. nutans in different populations. Besides that, information on genetic diversity of C. nutans can be useful in developing agricultural practices that can avoid genetic erosion and can offer conservation strategies for the long-term vigour of the species (Brake et al. 2014).
With respect to variation between species, DNA fingerprinting remains the most effective and easiest tool for genetic analyses (Ganie et al. 2015; Ntuli et al. 2015). Hence, three different kinds of markers namely RAPD, ISSR and RAMP markers were employed to determine the phylogenetic relationship in C. nutans species. RAPD has been successfully applied in genetic variation investigations of Mucuna pruriens due to its usefulness as an informative and cheap tool for plant breeding programs (Patil et al. 2016). There are also many researchers using these markers as one of their techniques to study the genetic diversity of their desired plant species (Desai et al. 2015; Kumar et al. 2009, 2010; Mei et al. 2015; Patel et al. 2015; Patil et al. 2016; Pu et al. 2009; Saleh 2015; Zhao et al. 2013; Zhao et al. 2015). RAPD and ISSR are dominant markers that function well in DNA fingerprinting studies of plant species. The combination of RAPD and ISSR can produce another type of marker, which is called RAMP. RAMP has been shown to be useful in detecting and mapping co-dominant microsatellite polymorphisms without tedious cloning and sequencing (Wu et al. 1994). Although the RAMP molecular marker has not been widely tested in other plant species, it is a useful technique because it is suitable for genetic analyses of plants whose genetic background is unclear (Zhao et al. 2013). The information on genetic diversity is useful as it serves as a guide on proper growing practice, whether by stem-cutting or sexual reproduction. Genetic diversity also offers insight as to whether a plant can survive over the long-term and its adaptability in its particular environment (Booy et al. 2000).
Currently, there is no report on the genetic variation of C. nutans using RAPD, ISSR and RAMP markers. Therefore, the objectives of this study are to provide information on the genetic diversity of C. nutans by using three different markers and, the relationship among accessions, which can be valuable for the conservation of C. nutans in herbal medicines industry.
Materials and methods
Plant collection
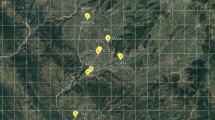
Leaf samples were collected from 80 C. nutans accessions collected from the northern region of Peninsular Malaysia (Table 1). The samples were from 8 different populations, with each population contributing 10 sample accessions. The plant identification was compared to the voucher collection (No: 11536) in the herbarium of the School of Biological Sciences, Universiti Sains Malaysia. The various sampling sites of C. nutans are shown in the map in Fig. 1.
Distribution of C. nutans populations in northern region of Peninsular Malaysia. The code names for populations above were shown in Table 1
Genomic DNA extraction
The genomic DNA of C. nutans were extracted from the leaves as described by Sunar et al. (2009). The quantity and quality of DNA were measured using a UV spectrophotometer at the wavelengths of 260/280 nm and the quality of extraction was observed using 0.8% agarose gel electrophoresis.
RAPD, ISSR and RAMP fingerprinting
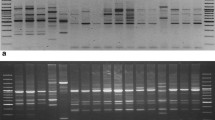
RAPD, ISSR and RAMP amplifications were performed using 17 RAPD primers, 8 ISSR primers and 136 RAMP primers (a combination of RAPD and ISSR markers). The information is in Table S1. An aliquot of 25 µL master mix was made up of 2.5 µL of 10X Taq buffer, 0.75 µL of 50 mM MgCl2, 0.5 µL of 10 mM dNTP mix, 1.0 µL of 0.4 µM primers, 0.125 µL of 5U of Taq DNA polymerase and 1.0 µL of genomic DNA and 19.125 µL of sterile deionized water. Amplification was performed in a MyCycler™ Thermal Cycler (Bio-Rad, USA) programmed for an initial 1 min denaturation at 94 °C, 45 cycles of 1 min denaturation at 94 °C, 1 min annealing (annealing temperature dependent on primers used) and 2 min extension at 72 °C followed by a final extension for 5 min at 72 °C and hold at 4 °C. Amplified PCR products were analysed using 1.5% agarose gel electrophoresis at 90 V for 30 min.
Data collection and analysis
The DNA analyses were scored by the presence (1) and absence (0) of bands for each primer genotype for RAPD, ISSR and RAMP analysis. Clear and reproducible amplified bands were chosen for the analyses. The PIC value for each locus was calculated using the formula (Roldan-Ruiz et al. 2000);
PICi—Polymorphic information content of the locus i; fi—Frequency of the amplified fragments; 1 − fi—Frequency of non-amplified fragments.
For each primer, PIC was calculated using an average PIC value from all loci of each primer. It was used to describe the linkage analysis of polymorphisms for marker locus. The MI was calculated by using the formula (Varshney et al. 2007);
EMR = n × b; n = Average number of fragments amplified by accession to a specific system marker; b = PB/(PB + MB); PB = Number of polymorphic loci; MB = Number of non-polymorphic loci.
MI was used to determine the usefulness of the system marker used in each primer. EMR analysis depends on the fraction of polymorphic fragments. The RP of each primer was calculated by using the formula (Prevost and Wilkinson 1999);
Ib = 1 − (2 × |0.5 − pi|); Ib— represents the informative fragments that can be represented on a scale of 0/1 by the following formula; pi—Proportion of accessions containing the ith band.
Data matrices of the three different types of markers were analyzed using POPGENE version 1.32 (Yeh et al. 1997) and Unweighted pair group method arithmetic average (UPGMA) cluster analysis was performed to explore the relationship among populations based on Nei’s genetic distance. Estimated gene flow (Nm) was calculated as;
where GST is the gene differentiation index.
The AMOVA and Mantel test with distance permutation (999) were performed using GenAlEx 6.5 (Peakall and Smouse 2012). The AMOVA was used to calculate the variance components and their significance levels for variation among populations and within populations.
Results and discussions
Analysis of amplified band
The uses of three different markers (RAPD, ISSR and RAMP) in this study allow us to compare the effectiveness of each genetic marker in the DNA fingerprinting of C. nutans. Based on Tables 2 and 3, only 10 primers of RAPD, 5 primers of ISSR and 37 primers of RAMP produced reproducible bands. The highest percentage of polymorphisms were from primers OPAA-16, OPAP-07 and OPAK-03 for RAPD, (CAA)5 for ISSR and (AGG)6/AGTCGGCCCA for RAMP markers. Both RAPD and ISSR markers showed 100% polymorphisms whereas RAMP markers detected 86% polymorphism. The RAMP primers on average, returned a PIC value of 0.25, which is considered as an informative marker. According to Botstein et al. (1980), primers that show a PIC value of 0.5 > PIC > 0.25 is considered informative marker. The discriminatory power and the usefulness of each marker can be evaluated by comparing its PIC, MI and RP values. Among the three different markers, the highest linkage analysis of polymorphism was from RAMP markers, which had the highest value of PIC, MI and RP compared to RAPD and ISSR (Table 4). In Table 5, the RAMP result obtained here is compared to the RAMP average value for Opuntia sp., Phoenix dactylifera L. and Arthrocnemum macrostachyum. From Table 5, C. nutans had highest MI value compared to other species, which indicated a high degree of polymorphism had been detected (Adhikari et al. 2015). Thus, RAMP markers proved to be a valuable molecular marker compared to ISSR and RAPD (Saleh 2015). This finding concurs with the report by Linh et al. (2007), who observed that the success of RAMP marker is due to the natural dominant and co-dominant characteristics of RAPD and ISSR respectively.
Analysis of genetic diversity
Table 6 shows RAPD, ISSR and RAMP results that had been analysed using POPGENE version 1.32 (Yeh et al. 1997). The highest percentage of polymorphism loci (PPB) was from the SBP population (RAPD = 80%, ISSR = 81% and RAMP = 63%) with the observed number of alleles (Na) (RAPD = 1.80 ± 0.40, ISSR = 1.81 ± 0.39 and RAMP = 1.63 ± 0.48). The effective number of alleles (Ne) of RAPD, ISSR and RAMP showed less variability than Na with an average value of 1.24 ± 0.28, 1.24 ± 0.25 and 1.38 ± 0.39 respectively among the eight populations. The Nei’s gene diversity index (H) and Shannon’s Index (I) were also highest in population SBP [RAPD: H = 0.21 ± 0.15 and I = 0.34 ± 0.21, ISSR: H = 0.20 ± 0.14 and I = 0.33 ± 0.20 and RAMP: H = 0.26 ± 0.22 and I = 0.37 ± 0.31)].
Based on Nei’s genetic diversity index, the SBP population showed the highest genetic diversity compared to other populations. SBP population is located in Taman Herba Perlis, a commercial herb garden in northern part of the west coast of Peninsular Malaysia. This finding is in line with Gao et al. (2012), who reported that populations in well-established gardens that had been managed over a long period of time showed the highest genetic diversity. This is because many gardens have maintained multiple collections from diverse wild population over the years, facilitated by the existence of a village and human society in that region. Population PBM showed low genetic diversity as it was situated in a cultivated farm that practised intensive harvesting practices preventing C. nutans to reach maturity and thus preventing production of flowers which is essential for cross-pollination. This is in contrast to other species in the Acanthaceae family, especially the herbaceous species that can be spread from seeds easily such as Ruellia nudiflora (Ramos-Zapata et al. 2010) that had higher genetic variation but cultivated in disturbed open sites and in diverse agricultural areas.
Genetic relationships of C. nutans at species level and population level
At the species level, the percentage of polymorphic loci were high compared to the average percentage at population level (RAPD: species level = 82% and population level = 63.50%, ISSR: species level = 83.78% and population level = 66.55% and RAMP: species level = 66.78% and population level = 51.79%). As shown in Table 7, AMOVA analysis from the three markers showed that variance high within populations (85–95%) than among populations (11–15%). Hence, the result of the analysis of genetic diversity by POPGENE corroborates the result of GenA1EX 6.5. Other plants that had been propagated vegetatively such as Sinopodophyllum hexandrum also have low genetic diversity at the population level (H = 0.06) but higher at the species level (H = 0.14) (Liu et al. 2014). Studies on Calanthe tsoongiana, Leersia hexandra and Jatropha curcas also showed high genetic diversity at the species level (H = 0.40, 0.26 and 0.11) than at the population level (H = 0.18, 0.15 and 0.10) respectively (Biabani et al. 2013; Song et al. 2006).
The high genetic diversity at the species levels is due to many factors such as a recent reduction of population size with inadequate time for isolation to spread and regular Nm (Maguire and Sedgley 1997). Geographical distribution is one of the factors that gives impact on genetic diversity of a species. The Acanthaceae family is consist of mainly herbs and shrubs, which have been largely distributed. In this case, C. nutans has been widely distributed in South China, Thailand, Vietnam, Malaysia and Indonesia (Chelyn et al. 2014) occurring in wild and cultivated habitat, including grasslands, hillsides, shrubs, valley, coastal regions and dense and open forest. C. nutans has a wide range of geographical distribution and is characterised by its high genetic diversity at the species level but only moderate at the population level, which will further deteriorate as commercial plantings of C. nutans practised vegetative propagation through stem-cutting. In the longer term, the moderate diversity at the population level, frequent harvesting before maturity and vegetative propagation will further reduce the genetic diversity at population level and species level due to the loss of rare alleles (Schoettle et al. (2011).
Populations relationship among C. nutans
UPGMA cluster analysis was performed to explore the relationship among populations based on Nei’s genetic distance. As shown in Fig. 2, the common nodes of similarity for RAPD, ISSR and RAMP are different, occurring at 6.4, 8.0 and 6.5% respectively. The same finding was obtained by Kumar el al. (2009), where the clusters did not show clear correlation between populations. However, the results explained a degree of commonness in the genotypes of C. nutans accessions. Among the eight populations of C. nutans, population PTG, KSP, KKK and PPS showed high similarity and low genetic distance based on the similarity matrix in Table 8. These populations are distributed in lowland areas and undergo intensive agricultural practices. On the other hand, populations PBF and PBM present low similarity and high genetic distance most probably attributed to the different growing conditions and agricultural practises. Population PBF is located on hilly coastal area in the northern coast, while PBM is situated in agricultural lowlands in the southern coast of Penang island.
The analysis on gene differentiation and Nm is summarised in Table 9. We followed the conventions proposed by Zhao et al. (2016) who defined gene differentiation (GST) values of 0 ≤ 0.05 as low, 0.05 ≤ 0.15 as medium and 0.15 < 0.25 as high level of genetic differentiation (GST). Similarly, we followed Kumar et al. (2014), who had classified Nm values <1 as low, Nm > 1 as moderate and Nm > 4 as extensive Nm. In this study, the average GST value of C. nutans is 0.17, indicating a high level of GST, while the average Nm value of 2.51 was only moderate. This result is similar to a study on a related species Justicia adhatoda, (a member of the family Acanthaceae), which reported comparable values; (GST = 0.30 for RAPD; Nm = 1.28 for RAPD and GST = 0.31 for ISSR; Nm = 1.30 for ISSR), which mirrored our findings of moderate Nm but high level of GST values. High GST values result in variations within populations, while moderate Nm indicated that one species in every generation can link the gene pools (set of genetic information) among populations (Kumar et al. 2014). Therefore, we can conclude that, C. nutans populations had been moderately dispersed among populations but showed high genetic diversity within a population.
Conclusions
We have identified 10 RAPD, 5 ISSR and 37 RAMP markers that can produced reproducible bands and can be used to study the genetic diversity of 80 accessions of C. nutans. We have shown that out of the three markers, the RAMP markers are recommended for evaluating the genetic diversity of C. nutans. Among the eight populations, SBP showed the highest genetic diversity. For future studies, we would like to compare the phytochemical profiling among populations of C. nutans. This is essential in order to understand the relationship of genetic diversity and the phytochemical profile in C. nutans, which is essential for its development for medicinal purposes.
References
Adhikari S, Saha S, Bandyopadhyay TK, Ghosh P (2015) Efficiency of ISSR marker for characterization of Cymbopogon germplasms and their suitability in molecular barcoding. Plant Syst Evol 301:439–450
Arullappan S, Rajamanickam P, Thevar N, Kodimani CC (2014) In vitro screening of cytotoxic, antimicrobial and antioxidant activities of Clinacanthus nutans (Acanthaceae) leaf extracts. Trop J Pharm Res 13:1455–1461
Aslam MS, Ahmad MS, Mamat AS (2015) A review on phytochemical constituents and pharmacological activities of Clinacanthus nutans. Int J Pharm Pharm Sci 7:30–33
Bendhifi ZM, Baraket G, Zourgui L, Souid S, Salhi HA (2014) Genetic diversity and phylogenetic relationship among Tunisian cactus species (Opuntia) as revealed by random amplified microsatellite polymorphism markers. Genet Mol Biol 14:1423–1433
Biabani A, Rafii MY, Saleh GB, Latif MA (2013) Inter-and intra-population genetic variations in Jatropha curcas populations revealed by inter-simple sequence repeat molecular markers. Maydica 58:111–118
Booy G, Hendriks RJJ, Smulders MJM, van Groenendael JM, Vosman B (2000) Genetic diversity and the survival of populations. Plant Biol 2:379–395
Botstein D, White RL, Skolnick M, Davis RW (1980) Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet 32:314–331
Brake M, Migdadi H, Al-Gharaibeh M, Ayoub S, Haddad N, El Oqlah A (2014) Characterization of Jordanian olive cultivars (Olea europaea L.) using RAPD and ISSR molecular markers. Sci Hort 176:282–289
Chelyn J, Omar M, Mohd YN, Ranggasamy R, Wasiman M, Ismail MZ (2014) Analysis of flavone C-glycosides in the leaves of Clinacanthus nutans (Burm. f.) Lindau by HPTLC and HPLC-UV/DAD. Sci World J 2014:1–6
Desai P, Gajera B, Mankad M, Shah S, Patel A, Patil G, Narayanan S, Kumar N (2015) Comparative assessment of genetic diversity among Indian bamboo genotypes using RAPD and ISSR markers. Mol Biol Rep 42:1265–1273
Fong SY, Piva T, Urban S, Huynh T (2014) Genetic homogeneity of vegetatively propagated Clinacanthus nutans (Acanthaceae). J Med Plants Res 8:903–914
Ganie SH, Ali Z, Das S, Srivastava PS, Sharma MP (2015) Genetic diversity and chemical profiling of different populations of Convolvulus pluricaulis (Convolvulaceae): an important herb of ayurvedic medicine. 3 Biotech 5:295–302
Gao J, He T, Li QM (2012) Traditional home-garden conserving genetic diversity: a case study of Acacia pennata in southwest China. Conserv Genet 13:891–898
Kumar M, Mishra GP, Singh R, Kumar J, Naik PK, Singh SB (2009) Correspondence of ISSR and RAPD markers for comparative analysis of genetic diversity among different apricot genotypes from cold arid deserts of trans-Himalayas. Physiol Mol Biol Plants 15:225–236
Kumar N, Modi AR, Singh AS, Gajera BB, Patel AR, Patel MP, Subhash N (2010) Assessment of genetic fidelity of micropropagated date palm (Phoenix dactylifera L.) plants by RAPD and ISSR markers assay. Physiol Mol Biol Plants 16:207–213
Kumar A, Mishra P, Singh SC, Sundaresan V (2014) Efficiency of ISSR and RAPD markers in genetic divergence analysis and conservation management of Justicia adhatoda L., a medicinal plant. Plant Syst Evol 300:1409–1420
Linh HTM, Son PD, Vang NT, Hien NT, Tham LX (2007) Study on application of molecular techniques (RAPD-PCR And RAMP-PCR) to detect mutation in rice breeding. Annu Rep 40:189–196
Liu W, Yin D, Liu J, Li N (2014) Genetic diversity and structure of Sinopodophyllum hexandrum (Royle) Ying in the Qinling Mountains, China. PLoS One 9:1–14
Maguire TL, Sedgley M (1997) Genetic diversity in Banksia and Dryandra (Proteaceae) with emphasis on Banksia cuneata, a rare and endangered species. Heredity 79:394–401
Mei Z, Zhang C, Khan A, Zhu Y, Tania M, Luo P, Fu J (2015) Efficiency of improved RAPD and ISSR markers in assessing genetic diversity and relationships in Angelica sinensis (Oliv.) Diels varieties of China. Electron J Biotechnol 18:96–102
Ntuli NR, Tongoona PB, Zobolo AM (2015) Genetic diversity in Cucurbita pepo landraces revealed by RAPD and SSR markers. Sci Hortic 189:192–200
Patel HK, Fougat RS, Kumar S, Mistry JG, Kumar M (2015) Detection of genetic variation in Ocimum species using RAPD and ISSR markers. 3 Biotech 5:697–707
Patil RR, Pawar KD, Rane MR, Yadav SR, Bapat VA, Jadhav JP (2016) Assessment of genetic diversity in Mucuna species of India using randomly amplified polymorphic DNA and inter simple sequence repeat markers. Physiol Mol Biol Plants 22:207–217
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in excel. Population genetic software for teaching and research-an update. Bioinformatics 28:2537–2539
Prevost A, Wilkinson M (1999) A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet 98:107–112
Pu Z, Hou Y, Xu X, Yan Z, Wei Y, Lan X, Zheng Y (2009) Genetic diversity among barley populations from west China based on RAMP and RAPD markers. Asian J Plant Sci 8:111–119
Ramos-Zapata JA, Campos-Navarrete MJ, Parra-Tabla V, Abdala-Roberts L, Navarro-Alberto J (2010) Genetic variation in the response of the weed Ruellia nudiflora (Acanthaceae) to arbuscular mycorrhizal fungi. Mycorrhiza 20:275–280
Roldan-Ruiz I, Dendauw J, van Bockstaele E, Depicker A, de Loose M (2000) AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp.). Mol Breed 6:125–134
Sakdarat S, Shuyprom A, Ayudhya TDN, Waterman PG, Karagianis G (2006) Chemical composition investigation of the Clinacanthus nutans Lindau leaves. Thai J Phytopharm 13:13–24
Saleh B (2015) Phylogenetic assessment of Arthrocnemum macrostachyum (Chenopodiaceae) genotypes using RAMP markers. Pol Bot J 60:293–299
Schoettle AW, Goodrich BA, Hipkins V, Richards C, Kray J (2011) Geographic patterns of genetic variation and population structure in Pinus aristata, Rocky Mountain bristlecone pine. Can J For Res 42:23–37
Song Z, Guan Y, Rong J, Xu X, Lu BR (2006) Inter-simple sequence repeat (ISSR) variation in populations of the cutgrass Leersia hexandra. Aquat Bot 84:359–362
Soumaya RC, Ghada B, Sonia DD, Salwa ZA, Mokhtar T (2013) Molecular research on the genetic diversity of Tunisian date palm (Phoenix dactylifera L.) using the random amplified microsatellite polymorphism (RAMPO) and amplified fragment length polymorphism (AFLP) methods. Afr J Biotechnol 10:10352–10365
Sunar S, Aksakal O, Yildirim N, Agar G, Gulluce M, Sahin F (2009) Genetic diversity and relationships detected by FAME and RAPD analysis among Thymus species growing in eastern Anatolia region of Turkey. Rom Biotechnol Lett 14:4313–4318
Varshney RK, Chabane K, Hendre PS, Aggarwal RK, Graner A (2007) Comparative assessment of EST-SSR, EST-SNP and AFLP markers for evaluation of genetic diversity and conservation of genetic resources using wild, cultivated and elite barleys. Plant Sci 173:638–649
Wanikiat P, Panthong A, Sujayanon P, Yoosook C, Rossi AG, Reutrakul V (2008) The anti-inflammatory effects and the inhibition of neutrophil responsiveness by Barleria lupulina and Clinacanthus nutans extracts. J Ethnopharmacol 116:234–244
Wu KS, Jones R, Danneberger L, Scolnik PA (1994) Detection of microsatellite polymorphisms without cloning. Nucleic Acids Res 22:3257–3258
Yeh FC, Yang RC, Boyle T (1997) POPGENE version 1.31, the user-friendly shareware for population genetic analysis. Molecular Biology and Biotechnology Center, University of Alberta, Edmonton, Canada
Zhao L, Li M, Cai G, Pan T, Shan C (2013) Assessment of the genetic diversity and genetic relationships of pomegranate (Punica granatum L.) in China using RAMP markers. Sci Hort 151:63–67
Zhao F, Nie J, Chen M, Wu G (2015) Assessment of genetic characteristics of Aconitum germplasms in Xinjiang Province (China) by RAPD and ISSR markers. Biotechnol Biotec Eq 29:309–314
Zhao H, Wang Y, Yang D, Zhao X, Li N, Zhou Y (2016) An analysis of genetic diversity in Marphysa sanguinea from different geographic populations using ISSR polymorphisms. Biochem Syst Ecol 64:65–69
Acknowledgements
Financial support for the study was provided under the Fundamental Research Grant Scheme: 203/CIPPT/6711340. The first author gratefully acknowledges Universiti Sains Malaysia for fellowship under the USM Fellowship Scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ismail, N.Z., Arsad, H., Samian, M.R. et al. Evaluation of genetic diversity of Clinacanthus nutans (Acanthaceaea) using RAPD, ISSR and RAMP markers. Physiol Mol Biol Plants 22, 523–534 (2016). https://doi.org/10.1007/s12298-016-0391-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-016-0391-x