Abstract
Objective
Lead is a toxic metal that damages neural connections (especially in children) and causes blood and brain diseases. Ellagic acid is a natural phenolic compound that has antioxidant properties. The objective of this study was to evaluate the efficacy of ellagic acid in lead-induced toxicity.
Methods
In this study, the effects of lead-induced toxicity on brain cells were investigated. The role of Ellagic acid in its antioxidant properties has also been studied. Levels of glutathione, nitric oxide, malondialdehyde, TNF-α, IL-1B, glutathione reductase, and catalase were evaluated. The amount of delay in rat fall and delay in dark box entry were also investigated.
Results
The study found that lead reduced the delay in rat fall, decreased rat entry into the dark box, decreased glutathione, increased malondialdehyde, increased nitric oxide, decreased catalase, superoxide dismutase and glutathione peroxidase, and also increased TNF-α and IL-1B. In all cases, Ellagic acid had a therapeutic role and had a significantly different function to lead in all cases (P < 0.05).
Conclusion
The results of a recent study have shown that lead is very harmful to humans and could endanger human life. It has also been extracted from this study that ellagic acid, as a natural compound, is very useful and can alleviate the damaging effects of heavy metals, especially lead, on the human body.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy metals such as mercury, lead and cadmium are not vital elements and have no beneficial effects on the life of living organisms, so their accumulation in the body of living organisms, especially mammals, can lead to dangerous diseases [1,2,3]. Routes of entry to mammals are typically through polluted air that enters the soil and groundwater in industrial areas after rainfall, as well as through the sea and oceans [3].
The results of many published studies have shown that lead is very harmful to humans and could endanger human life. It has also been extracted from this study that ellagic acid, as a natural compound, is very useful and can alleviate the damaging effects of heavy metals, especially lead, on the human body [1, 4, 5].
Lead poisoning has negative effects on the central nervous system and also affects the body's enzymes. In long-term exposure to low amounts, it has adverse effects on all functions and causes irreversible nephropathy [6], anemia, brain and nerve damage, and developmental delay in children [6, 7].
Lead builds up in bones and endangers your health in adulthood, poisoning the nervous system, affecting the heart, and increasing blood pressure [8]. Chronic poisoning can cause kidney and lung cancer because it is mutagenic and carcinogenic. It causes pain in the gastrointestinal tract and reduces in men the number and concentration of sperm in semen and interrupts the sperm production process [9]. In summary, lead, if introduced chronically, affects almost every system of the human body. Daily consumption of 5 µg of lead per kg body weight causes chronic poisoning as it affects the central and peripheral nervous system. Symptoms of toxicity include ataxia, distal skeletal muscle weakness, numbness of the hands and feet, and fatigue. In laboratory animals it produces neurological symptoms that in many ways are similar to the neurotoxicity that occurs in humans [10].
Given the importance of lead-induced neurotoxicity, several studies have been carried out to find the mechanisms involved in this toxicity, the decrease in glutathione content and increase in the peroxidation of lipids in the brain tissue, changes in the levels of proteins involved in apoptosis (Bax, Bcl-2 and Caspase3) in different parts of the brain, swelling and degeneration of the final axonal regions, including the mechanisms involved in their neurotoxicity. Since oxidative stress induction plays an important role in the neurotoxicity of lead, studies on antioxidant compounds appear necessary to inhibit the toxicity of this compound [11].
In recent years, it has been well demonstrated that consumption of fruits and vegetables containing natural antioxidants has prevented many diseases, including heart disease and even various cancers. Ellagic acid (EA) is a bioactive compound that has many pharmaceutical and industrial applications. EA is a polyphenolic acid found in fruits such as pomegranates, strawberries, raspberries and grapes. This molecule has various properties including antioxidant properties. Studies on EA in cancer cells have shown apoptosis induction and cell death and inhibition of continuous tumor growth [12,13,14].
Given the importance of lead-induced neurotoxicity, several studies have been carried out to find the mechanisms involved in this toxicity, the decrease in glutathione content and increase in the peroxidation of lipids in the brain tissue, changes in the levels of proteins involved in apoptosis (Bax, Bcl-2 and Caspase3) in different parts of the brain, swelling and degeneration of the final axonal regions, including the mechanisms involved in their neurotoxicity. Since oxidative stress induction plays an important role in the neurotoxicity of lead, studies on antioxidant compounds appear necessary to inhibit the toxicity of this compound.
Results
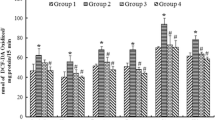
Rotarod performance in different experimental groups are presented in Fig. 1 and revealed that that in rat exposed to lead; they were falling faster than others and had significant differences to the negative control group (P < 0.01).
According to Fig. 2, the early latency was not significantly different between groups. The retention latency in lead-treated group was shorter than that of the negative group (P < 0.001). The retention latency in lead and EA-treated group was shorter than that of the negative group (P < 0.001) but was longer than lead group (P < 0.05).
Based on Fig. 3, the glutathione levels in brain tissue in rats treated with lead were significantly lower than other groups (P < 0.001). Also, the glutathione levels in rats treated with lead and EA had significant differences to the lead-exposed rats (P < 0.05).
Malondialdehyde in rats treated with lead was significantly higher than control group (P < 0.001) and in rats treated with lead and EA the amount of malondialdehyde had significant difference to the lead-exposed rats (P < 0.01) (Fig. 4).
As shown in Fig. 5, the amount of Nitric oxide in lead and EA-lead groups were significantly higher than EA and control groups with P value less than 0.001 and 0.1.
The amount of catalase enzyme in lead-exposed rats was significantly lower than control group (P < 0.001). Also in EA-lead groups the amount of catalase enzyme is significantly higher than lead-treated group (P < 0.05) and lower than control group (P < 0.001) (Fig. 6).
According to Fig. 7, the amount of Superoxide dismutase enzyme in rats treated with lead was lower than other groups (P < 0.001) and in rats treated with EA and lead was significantly lower than negative control group (P < 0.05).
The amount of Glutathione peroxidase enzyme in rats treated with lead was lower than other groups (P < 0.001) and in rats treated with EA and lead was significantly lower than negative control group (P < 0.05) (Fig. 8).
Based on Fig. 9, the amount of TNF-α in the brain tissue in lead-exposed rats was significantly higher than negative control group (P < 0.001). In rats treated with EA and lead the amount of TNF-α in the brain tissue was lower than lead-treated group and higher than negative control group (P < 0.05) (Fig. 9a).
IL-1B in lead-exposed rats was higher than negative control group and in rats treated with lead and EA was lower than lead-treated group and higher than negative control group (P < 0.001) (Fig. 9b).
Administration of lead acetate alone significantly decreased the expression level of glutathione peroxidase in brain tissue compared to normal saline group and Glutathione peroxidase enzyme gene expression in brain tissue was significantly different in lead acetate and EA groups (P < 0.05). But the level of glutathione peroxidase enzyme in brain tissue group was similar to normal saline group and there was no significant difference (P > 0.05) (Fig. 10a).
Lead acetate alone decreased the expression of SOD gene in brain tissue compared to normal saline group and expression of SOD gene expression in brain tissue was significantly different in lead acetate group and EA group in lead acetate group (P < 0.05). Expression of superoxide dismutase gene expression in brain tissue was not similar in the group receiving normal saline alone and with no significant difference (P > 0.05) (Fig. 10b).
Discussion
The results of this study showed that lead consumption caused behavioral impairments so that passive avoidance learning and pain threshold decreased significantly after lead consumption. His delay time to dark room after shock induction in the lead group showed a significant decrease compared to the control group. The results of this study also showed that administration of different doses of EA in ischemic groups increased learning, memory and pain threshold. Rotarod performance in different experimental groups revealed that that in rat exposed to lead were falling faster than others and had significant differences to the negative control group. The delaying in entry into the dark box in the early latency was not significantly different between groups but; in retention latency, in lead-treated group was shorter than that of the negative group. The results of the delay time of falling and the delay in entering the dark box of lead-exposed rats showed that lead consumption in rats accelerated the fall and increased the rate of delayed entry into the dark box. In both trials, ellagic acid has been shown to have positive therapeutic effects for treating neurological disorders.
EA is a natural agent derived from nuts and fruits [15] which is not toxic in dose of up to 50 mg/day for 45 days in rats. Anticancer, antidiabetic, anti-inflammatory, antiviral and antioxidant activities of EA are reported by Garcia-Nino et al. [14]. Also, potent protective effects of EA were reported in lung, liver and kidney organs [14]. EA exhibited anti-inflammatory effects by affection on cycloxygenase enzyme, IL-1β, TNF-α and IL-6 modulates production [16] and it will reduce inflammation and damage cells in the brain [17, 18]. Ghasemzadeh Dehkordi et al. [19] evaluated the effect of EA on memory and pain in rats with brain ischemia and revealed that EA in concentration of 10, 25 and 50 mg/kg could development the memory and decline the pain [19].
Glutathione (GSH) is a peptide composed of three essential amino acids that plays an important role in the body. Researchers believe that this substance is very important for the health of the body. Glutathione interacts with drugs to digest them, is a co-factor for some important enzymes including Glutathione peroxidase and protects the body from oxidative damage, reduces peroxide, improves cancer apoptosis, plays an important role in immune function, prevent drug resistance, protects the body against environmental toxins, and also fights cancer [20, 21]. Researchers in different fields are looking for a way to increase glutathione in the body, which has been shown in this study by EA to compensate for glutathione depletion and to significantly increase this compound [14].
Malondialdehyde and nitric oxide in rats treated with lead were significantly higher than control group and in rats treated with lead and EA the amount of them was significantly decreased. Various studies have shown that many herbs and compounds, especially ascorbic acid, have very strong antioxidant capacity, and this study has shown that EA is a potent antioxidant in reducing malondialdehyde and nitric oxide [22]. The presence of oxidant compounds in the body is very dangerous, and since oxidant compounds such as lead exist in nature and are unfortunately in contact with humans, the presence of antioxidant compounds is crucial [23].
The use of lead in the current study increases the number of inflammatory mediators such as TNF-α and IL-1B, it is clear that these inflammatory cytokines could induce apoptosis in the nervous system of neurons, and the results of this study showed that ellagic acid can reduce inflammatory cytokines. In a similar study, Goudarzi and his colleagues (2018) investigated the role of EA in reducing acrylamide-induced neurotoxicity and showed that in addition to the antioxidant effect, EA can reduce levels of TNF-α and IL-1B in rat [22].
Many natural compounds are used today to reduce the level of oxidants and reduce the toxicity of chemicals. Mehri et al. (2009) investigated the role of Silybum marianum plant in reducing acrylamide-induced neurotoxicity. As the acrylamide concentration increased, the cell viability was decreased for 5 h. S. marianum extract at all concentrations decreased acrylamide-induced toxicity after 5 h of exposure to cells. Administration of acrylamide caused significant motor abnormalities in animals. Administration of ethanolic extract of S. marianum (400 mg/kg) improved animal movements compared to acrylamide group [24].
In addition to enzymatic methods, molecular methods have been used to confirm the results of enzymatic tests. The results of molecular methods showed that SOD and GPX genes expression was decreased at the time of administration of EA and EA-Lead acetate and was significant in comparison with control groups.
Materials and methods
Study design and groups
In this experimental study, six rats were randomly assigned to different groups. The dosage and method of administration of acetate and lead acetate (LA) are selected based on reference [25]. We divided the animals into four equal groups of six, including: Group 1: as negative control; received only phosphate buffered saline (PBS), Group 2: as positive control; received lead, Group 3: received lead and EA, and Group 4: Only received EA. Passive avoidance test and rotarod test were performed 24 h after the last dose.
Passive avoidance test
In this method, the training device consists of two boxes (light and dark) separated by a guillotine blade. The bottom of the dark box is covered with stainless steel shock bars. Each mouse is placed in a light box during testing, 60 s after the separator blade is adapted, and the delay time until the mouse enters the dark box is recorded. Immediately after the mouse enters the blade dark box, the shock is given an equivalent shock of 75 V, 0.2 mA, and 50 Hz for 3 s. After 5 s, the mouse is removed from the box and returned to its cage. This test was repeated 24 h later, but not the shock. Delay memory is calculated for a maximum of 600 s [22].
Rotarod test
The Rotarod Behavioral Test is a validated test for assessing nerve damage to muscle strength, coordination of anterior and posterior organs, and balance. The measurement was based on when the animal was able to stay on the rotating machine and not fall. Once this test was done, the rotation speed was set at 5 rpm for 3 min and then gradually increased to 40 rpm over 12 min and remained constant until the end of the test. The maximum test time of each animal was 30 min. Each mouse was tested 3 times at 45-min intervals and the mean duration of tolerance on the spinning rod or the time of falling from it was recorded in seconds [22].
Sample preparations
The animals were then anesthetized with ketamine/xylazine and blood was taken directly from the animal's heart into the test tube (without heparin) and serum was separated to measure malondialdehyde and glutathione. After removal of brain tissue, it was weighed and placed in a 10% formalin solution for histological and pathological examination of the cortex. Other brain tissue was stored at − 70 °C to measure some factors such as malondialdehyde, glutathione, nitric oxide, TNF-α, IL-1B, activity of catalase, glutathione peroxidase and superoxide dismutase and glutathione peroxide gene expression.
Protein levels measurement
0.5 g of brain tissue was homogenized in 0.1 M phosphate buffer with pH = 7.4 at a 10% concentration.vol/wt using a homogenizer. Protein levels were measured using Bradford's method. The Bradford reagent was prepared with Komassi blue (10 mg), 85% phosphoric acid, 96% ethanol and distilled water (1/10). Optical absorption was then measured using a spectrophotometer at 595 nm and a calibration curve was elaborated with specified BSA concentrations of 62.5, 125, 250, 500 μg/ml BSA (as standard) and the protein concentration was measured.
Evaluation of tissue lipid peroxidation
Satho method was used to determine the amount of malondialdehyde. 1.5 ml of 10% trichloroacetic acid solution was added to 0.5 ml of homogenized tissue and then centrifuged at 4000 g for 10 min. 2 ml of 0.67% thiobarbituric acid solution was added to 1.5 ml of the supernatant, and then incubate in boiling water bath for 30 min. Then, the absorbance was read at 532 nm. Concentration of malondialdehyde was determined using tetraethoxy propane as standard.
Tissue glutathione content
Kidney tissue glutathione content was identified and measured by the reaction of glutathione (GSH) with elemental reagent (5,5′-dithiobis-(2-nitrobenzoic acid), DTNB) and the generation of TNB (yellow). To 40 μL of homogenous tissue, 2 ml of phosphate buffer was added and then 40 μL of 10 mM ellman reagent was added and optical absorption at 412 nm was read.
Tissue nitric oxide (NO) measurement
Tissue nitric oxide content was determined by a grease reagent. Briefly, brain homogenate tissue (in phosphate buffer) was deprecated using zinc sulfate and then passed through cadmium powder to convert nitrate to nitrite. Then, the nitrite was read from a grease reagent at a wavelength of 540 nm.
Catalase, glutathione peroxidase and superoxide dismutase activity in brain tissue
Measured using ZellBio commercial kits and ELISA reader according to kit instructions.
Tumor necrosis factor (TNF)-α, Interleukin (IL)-1B in brain tissue
Measured using IBL commercial kits and ELISA reader according to kit instructions.
Statistical analysis
Data were analyzed using ANOVA, t-Test and Tukey's post hoc tests and SPSS-21 and the P value less than 0.05 was considered significant.
Conclusion
Due to the increase in oxidant compounds and inflammatory cytokines from lead consumption, it has been shown to be a highly dangerous compound for human health. EA as a natural phenolic compound derived from plant sources has been shown to be a potent antioxidant and may even protect the human body from possible damage caused by inflammatory cytokines. However, the information obtained in this study should be confirmed by further testing in the in vivo and human phases.
References
Papanikolaou NC et al (2005) Lead toxicity update. A brief review. Med Sci Monit 11(10):RA329–RA336
Tchounwou PB et al (2012) Heavy metal toxicity and the environment. In: Luch A (ed) Molecular, clinical and environmental toxicology. Springer, Basel, pp 133–164
Kim J-J, Kim Y-S, Kumar V (2019) Heavy metal toxicity: an update of chelating therapeutic strategies. J Trace Elem Med Biol 54:226–231
Marsden PA (2003) Increased body lead burden-cause or consequence of chronic renal insufficiency? N Engl J Med 348(4):345–346
Balami JS et al (2011) Neurological complications of acute ischaemic stroke. Lancet Neurol 10(4):357–371
Silbergeld EK (1991) Lead in bone: implications for toxicology during pregnancy and lactation. Environ Health Perspect 91:63–70
Pentschew A, Garro F (1966) Lead encephalo-myelopathy of the suckling rat and its implications on the porphyrinopathic nervous diseases. Acta Neuropathol 6(3):266–278
Harlan WR et al (1985) Blood lead and blood pressure: relationship in the adolescent and adult US population. JAMA 253(4):530–534
Missoun F, Slimani M, Aoues A (2010) Toxic effect of lead on kidney function in rat Wistar. Afr J Biochem Res 4(2):021–027
Grice SJ et al (2015) Dominant, toxic gain-of-function mutations in gars lead to non-cell autonomous neuropathology. Hum Mol Genet 24(15):4397–4406
Carocci A et al (2016) Lead toxicity, antioxidant defense and environment. In: de Voogt P (ed) Reviews of environmental contamination and toxicology. Springer, Cham, pp 45–67
Meyer AS, Heinonen M, Frankel EN (1998) Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem 61(1–2):71–75
Losso JN et al (2004) In vitro anti-proliferative activities of ellagic acid. J nutr biochem 15(11):672–678
García-Niño WR, Zazueta C (2015) Ellagic acid: pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol Res 97:84–103
Esposito E et al (2002) A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging 23(5):719–735
El-Shitany NA, El-Bastawissy EA, El-desoky K (2014) Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism. Int Immunopharmacol 19(2):290–299
Kaur J, Kumar M, Bansal N (2016) Ellagic acid administration reverses colchicine-induced dementia in rats. J Pharm Technol Res Manag 4:31–46
Mansouri MT et al (2016) Beneficial effects of ellagic acid against animal models of scopolamine-and diazepam-induced cognitive impairments. Pharm biol 54(10):1947–1953
Ghasemzadeh DM, Rafieirad M, Rouhi L (2014) The effect of ellagic acid on memory and pain induced by brain ischemia in adult male rats. J ZanjanUniv Med Sci Health Serv 22(92):33–42
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52(1):711–760
Vina J (2017) Glutathione (1990). CRC Press, Boca Raton
Goudarzi M et al (2018) The possible neuroprotective effect of ellagic acid on sodium arsenate-induced neurotoxicity in rats. Life Sci 198:38–45
Wani AL, Ara A, Usmani JA (2015) Lead toxicity: a review. InterdiscipToxicol 8(2):55–64
Mehri S et al (2016) Evaluation of the neuroprotective effect of silymarin on acrylamide-induced neurotoxicity. Jundishapur J Nat Pharm Prod 11(4):e37644
Kaplan B et al (1991) Temporal thresholds for neocortical infarction in rats subjected to reversible focal cerebral ischemia. Stroke 22(8):1032–1039
Acknowledgements
This project did not receive any specific grant from funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ali amirahmadi, Javad babaei, Ramin Abrishami, Mehdi Goudarzi, Mojtaba kalantar declare that we have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Amirahmadi, A., Babaei, J., Abrishami, R. et al. The effect of ellagic acid on the neurotoxicity of lead exposed rats. Toxicol. Environ. Health Sci. 13, 83–89 (2021). https://doi.org/10.1007/s13530-020-00073-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-020-00073-3