Abstract
In the current study, we aimed to investigate the effect of carvacrol on the suppression of liver fibrosis progression through targeting lysyl oxidase (LOX) expression. The rats received carbon tetrachloride (CCl4) intraperitoneally and carvacrol orally for 10 weeks. Liver damage was evaluated by measuring the serum level of alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase and hepatic oxidative stress parameters including total antioxidant capacity, total thiol group and total oxidant status spectrophotometry and malondialdehyde fluorometrically. Extracellular deposition of collagen was detected using Masson’s trichrome standing. Furthermore the gene expression of lysyl oxidase homolog 2 (Loxl2) was analyzed using quantitative reverse transcription-polymerase chain reaction. And then the protein level of LOX was detected in liver tissue by western blot method. Carvacrol administration normalized serum biochemical parameters and improved oxidative stress status in liver homogenate of CCl4 treated rats. Collagen fiber bundles in interlobular spaces were decreased remarkably by carvacrol treatment. Also, carvacrol downregulated hepatic gene expression of Loxl2 and protein level of LOX. Our data clearly revealed that carvacrol suppresses progression of liver fibrosis development via attenuating of liver damage and oxidative stress status as well as via downregulation of hepatic gene expression of Loxl2 and protein level of LOX.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last years, the regression of advanced liver fibrosis has become a global concern. Over time chronic liver inflammation can be lead to liver fibrosis and subsequently develop into cirrhosis if left untreated [1]. Excessive deposition of extracellular matrix (ECM) components and collagen cross-linking limit liver fibrosis regression. Collagen cross-linking is catalyzed by lysyl oxidase (LOX) enzyme. LOX is an extracellular copper-dependent enzyme that catalyzes covalent cross-linkage formation in collagen fibers through formation of aldehydes from lysine [2]. The overexpression and increase activity of LOX has been implicated in the development of many disorders. In fact LOX provides a pathologic microenvironment in the progression of fibrotic and metastasis processes so that selective targeting of lysyl oxidase homolog 2 (Loxl2) by monoclonal antibody (AB0023) was coincided with reduction of inflammatory cytokines such as transforming growth factor-β (TGF-β) [2]. Also selective inhibiting of Loxl2 accelerated regression of hepatic fibrosis as well as prevented fibrotic process [3].
A large body of data indicate that various compounds play an important role in improvement of many disorders in the contemporary world [4,5,6]. Carvacrol is a monoterpenoid compound that mainly found in Origanum vulgare. Previous studies have indicated protective effect of carvacrol against liver injury [7, 8]. Although the molecular mechanism underlying hepatoprotective effect of carvacrol remain to be clarified. So in the present study we investigated the effect of administration of carvacrol treatment on the suppression of liver fibrosis development in CCl4-induced liver fibrosis in rats. At first we investigated the effect of carvacrol on serum biochemical markers. Then we analyzed oxidative stress parameters in liver homogenate and finally, we measured the regulatory effect of carvacrol on hepatic gene expression of lox and protein level of LOX.
Materials and Methods
Study Design
The 6 week old male Wistar rats weighing 200 ± 10 g were used for the current study. The thirty animals were obtained from Animal Care Center-Hamadan University of Medical Sciences. Animals were kept in a temperature-controlled room and received free water and food (standard chow diet) with 12-h light/dark cycles and humidity. After the acclimatization, rats were divided into 5 groups randomly.
The liver fibrosis model was induced by 10 weeks of twice weekly intraperitoneal (IP) injections of CCl4 1 ml/kg (dissolved in olive oil as the vehicle, 50% v/v). The pure carvacrol (W224502, Sigma-Aldrich, US) was mixed in distilled water and was administered daily by gavage to etch rat separately. C70 group was considered for evaluating the effect of carvacrol on normal rats. Drug dose was selected according to the previous reports [9,10,11]. All procedures were performed in accordance with guideline principles for the care and use of animals at the Faculty of Medicine at Hamadan University of Medical Sciences.
-
Group 1 Fibrotic control (FC): CCl4 + olive oil solution 1 ml/kg.
-
Group 2 Treatment 70 (T70): CCl4 + olive oil solution 1 ml/kg + carvacrol 70 mg/kg.
-
Group 3 Treatment control 70 (C70): olive oil 1 ml/kg + carvacrol 70 mg/kg.
-
Group 4 Vehicle control (VC): olive oil 1 ml/kg.
-
Group 5 Healthy control (HC): normal saline 4 ml/kg.
Following confirmation of CCl4-induced liver fibrosis, all rats were weighed 1 day after the final treatment and were sacrificed after anesthesia with ketamine. Blood samples were harvested from the vena cava and sera were collected by centrifuging at 3000 rpm for 10 min at 4 °C, then stored at − 20 °C. The liver tissues were immediately frozen in liquid nitrogen after washing with ice cold PBS and stored at − 80 °C.
Preparation of Tissue Lysate
About 40 mg piece of tissue was added to ~ 400 μL of lysis buffer containing 1 mM EDTA, 1.5 mM MgCl2, 10 mM KCl, 10 mM HEPES and 0.1% Triton X100 at pH 7.9 that supplemented with protease inhibitor cocktail (Sigma-Aldrich, US), and was shaked for 30 min at 4 °C. Then tissue homogenates were centrifuged (12000g; 20 min; 4 °C) and the supernatant was separated. Total protein of tissue lysate was quantified by Bradford method [12]. Bovine serum albumin (BSA) used as standard protein.
Serum Biochemical Analysis
The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were measured using colorimetric assay kit according to the manufacturer’s instructions (Pars Azmun, Tehran, Iran).
Masson’s Trichrome Staining
For histopathological evaluation, tissue samples were collected from the same lobule then were fixed in 10% neutral buffered formalin, embedded in paraffin and were sectioned at 5 μm thickness, and stained with Masson’s trichrome (MT) staining.
Oxidative Stress Analysis
The hepatic oxidative stress status was determined by measuring the total antioxidant capacity (TAC), total thiol group (–SH), total oxidant status (TOS) and malondialdehyde (MDA).
Total Antioxidant Capacity
TAC was determined by measuring the ability of sample to reduce ferric ion (Fe III) to ferrous (Fe II). Fe II with 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) forms a blue colored complex with maximum absorbance at 593 nm [13].
Total Oxidant Status
TOS value rely on the ability of sample to oxidize the Fe II to Fe III. Briefly, the Fe III with xylenol orange produces a colored complex in acidic conditions. The assay was calibrated with H2O2 and results were expressed in terms of micromolar hydrogen peroxide equivalents per liter [14].
Lipid Peroxidation Assay
MDA as the final product of lipid peroxidation was measured as described previously [15]. Briefly, the MDA forms a colored complex with the thiobarbituric acid. 1,1,3,3-tetraethoxypropane was used as a standard.
Total Thiol Content
The –SH level was estimated according to the previously described [16]. –SH groups react with 5,5′-Dithiobis (2-nitrobenzoic acid) (DTNB) to form a yellow colored complex that was assayed spectrophotometrically at 412 nm.
RNA Isolation and Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from frozen tissues using RNX-Plus solution (Cinnagen, Iran) according to the manufacturer’s instructions. The quantity and quality of the total RNA were measured using nanodrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., USA). The cDNA was synthesized from RNA using the cDNA synthesis kit (TaKaRa Biotechnology, Japan). The quantitative PCR was performed by SYBR Green master mix (Amplicon, Denmark) in the LightCycler®96 instruments (Roche Life Science Deutschland GmbH, Sandhofer, Germany). The specific primers were designed and evaluated with the aid of NCBI Primer-Blast tool and were synthesized (Bioneer. Korea). The forward and reverse primer sequences were listed as follow. Actb, forward: 5′CCCGCGAGTACAACCTTCT3′, and reverse: 5′CGTCATCCATGGCGAACT3′. Loxl2, forward: 5′AGTCCAGCTACCAACACAGG3′, and reverse: 5′AGACAAGCAGTGCCAAACAG3′. All reactions were performed in triplicates and the relative gene expression level was analyzed according to 2−ΔΔCt formula. The housekeeping gene (β-actin) was used as the internal control.
Western Blot Analysis
Liver tissues were homogenized in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor. The total protein content was measured by bicinchoninic acid method. Tissue lysate were resolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Then proteins were transferred to nitrocellulose membrane. After blocking, the primary antibodies, LOX (ab174316, Abcam), and β-Actin (ab119716, Abcam) were used. β-Actin protein was used as a loading control. Densitometric analysis of bands was performed with the Image J software.
Statistical Analysis
Statistical analyses were performed with SPSS 16.0 software (IBM, Armonk, NY, USA) and GraphPad Prism 5.00 software (LaJolla, CA, USA). Statistical significances were determined using one-way analysis of variance (ANOVA). All data were expressed as mean ± standard error of mean (SEM). p < 0.05 was considered significant.
Results
Carvacrol Normalized Serum Biochemical Parameters in CCl4 Induced Liver Fibrosis in Rat
It was found that liver fibrosis induction significantly increased serum level of liver markers AST, ALT and ALP compared with HC group reflecting hepatic damage. Carvacrol decreased AST and ALT dramatically near to normal level (p < 0.001). Also, ALP was decreased by carvacrol supplementation obviously (p < 0.001) (Table 1).
Carvacrol Improved Deposition of Collage Cause by CCl4 Hepatotoxicity
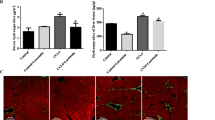
Figure 1 implies, thick collage fiber bundles were markedly increased in rats treated with CCl4. Also fibrous expansion of most portal areas with occasional portal-to-portal bridging was detected. But this increase was significantly reduced in rats treated with carvacrol whereas small amount of thin collagen fiber bundle were observed. As expected, no sign of liver fibrosis was found in the untreated CCl4 groups.
Masson’s trichrome staining (× 100 magnification). FC fibrotic control, T70 CCl4 + carvacrol 70 mg/kg, C70 olive oil + carvacrol 70 mg/kg, VC olive oil alone, HC normal saline alone. FC group; many bundles had grown into the lobules and connected the central and portal areas; T70 group, collagen deposition is markedly reduced in rats treated with carvacrol. C70, VC and HC groups, no fibrosis
Carvacrol Attenuated Oxidative Stress Caused by CCl4 in the Liver of Rats
As expected oxidant/antioxidant balance didn’t disrupt in C70 group than HC group. As shown in Fig. 2 after chronic CCl4 exposure, antioxidant indicators were decreased and oxidant indicators were enhanced as compared with HC group significantly. Carvacrol treatment increased TAC level significantly compared to the FC group in which restored TAC level to normal level completely (p < 0.001). Also, the hepatic level of –SH was restored by carvacrol treatment compared to the FC group (p < 0.001). The TOS level in T70 group was lower than FC group significantly (p < 0.01). Augmented MDA level in tissue lysate during CCl4 injection was reduced by carvacrol administration remarkably (p < 0.001).
The effect of carvacrol on oxidative stress parameters in CCl4-induced liver fibrosis. TAC total antioxidant capacity (a), –SH total thiol group (b), TOS total oxidant status (c), MDA malondialdehyde (d). FC fibrotic control, T70 CCl4 + carvacrol 70 mg/kg, C70 olive oil + carvacrol 70 mg/kg, VC olive oil alone, HC normal saline alone. ♦: compared to FC group, ϕ: restore to HC group [there was no significant difference to HC group (p > 0.05)]. Data were expressed as Mean ± SEM. +p < 0.01; #p < 0.001
Carvacrol Reduced Hepatic Protein and Gene Expression of LOX in CCl4 Induced Liver Fibrosis in Rat
On the base of previous attempt, expression of lysyl oxidase homolog 2 (Loxl2) is mainly upregulated in liver fibrosis. As shown in Fig. 3a the gene expression of Loxl2 was increased by induction of liver fibrosis. The carvacrol reduced the mRNA level of Loxl2 significantly relative to FC group (p < 0.01). Western blot analysis showed the protein level of LOX was greater in FC group than HC group through liver fibrosis induction. Also, carvacrol administration reduced protein level of LOX compared to FC group. Administration of carvacrol in untreated CCl4 rats didn’t affect on the hepatic protein level of LOX compared to HC group (Fig. 3b).
The effect of carvacrol on gene expression of Loxl2 and protein expression of LOX in CCl4-induced liver fibrosis. a the gene expression analysis of hepatic Loxl2, b Western blot analysis of hepatic LOX protein. FC fibrotic control, T70 CCl4 + carvacrol 70 mg/kg, C70 olive oil + carvacrol 70 mg/kg, VC olive oil alone, HC normal saline alone. ♦: compared to FC group. Data were expressed as Mean ± SEM. +p < 0.01
Discussion
The latest in vitro and in vivo studies highlighted collagen cross-linking plays an important role in irreversibility of liver fibrosis in late stage and could be considered as an attractive therapeutic target in the regression of liver fibrosis. In this context, we investigated the effects of carvacrol treatment on hepatic protein and gene expression of LOX in CCl4 induced liver fibrosis in rats.
The serum levels of ALT, AST and ALP as liver specific biomarkers are useful for evaluating of liver injury. Our results showed carvacrol reduced the serum level of this factors dramatically compared with FC group that was in agreement to Hussein, et al. [17] study that showed the administration of nano-encapsulated form of carvacrol normalized serum level of ALT and AST in experimental liver fibrosis model. Also Aristatile, et al. [8] have reported 3 weeks administration of carvacrol was enough for returning of liver marker enzymes and total bilirubin to normal level in d-galactosamine-hepatotoxic rats.
The MT staining indicated the carvacrol treatment markedly attenuated the liver fibrosis development compared with FC group. There was a lower fibrosis score when carvacrol was administrated and thickness of collagen fiber bundles were markedly decreased than FC group. These observation showed modulatory effect carvacrol on collagen accumulation in ECM while had no adverse effect on liver architecture in absence of CCl4.
Our observation declared administration of carvacrol 70 mg/kg attenuated oxidative stress cause by CCl4 hepatotoxicity. However our treatment approach was not able to restore oxidative stress status to normal level. These findings was in compatible to recent study. Bozkurt et al. [18] showed pretreating rats with carvacrol reduced hepatic MDA level and augmented TAC level in rat model of liver injury. Additionally, previous attempt by Balan et al. indicated carvacrol at dose of 15 mg/kg augmented the activity of antioxidant enzymes such as –SH, catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPX) in the N-nitrosodiethylamine induced liver damage in Wistar rats [19].
Collagen cross-linking in pathological conditions catalyzed mainly by LOX enzyme. Several previous studies have determined the inhibition of LOX postponed liver fibrosis development and can be lead to reverse liver fibrosis [20]. The current gene expression analysis revealed, carvacrol suppresses Loxl2 expression at the transcriptional level. Compatible to present data, Lytle et al. [21] showed mRNA level of Loxl2 was elevated more than other Loxl subtypes during liver fibrosis induction and was downregulated by feeding docosahexaenoic acid.
According to the recent study carvacrol treatment inhibited TGF-β signaling pathway as a key signaling pathway that participates in the liver fibrosis progression [22]. On the other hand previous studies showed TGF-β signaling pathway induces extracellular Lox expression during the pathogenic conditions [23, 24]. Therefore downregulation of TGF-β pathway by carvacrol treatment may be lead to reduce expression of extracellular Lox consequently decrease collagen cross-linking.
On the other hand Ikenaga et al. [25] showed specific inhibition of the activity of LOXL2 by monoclonal antibody simtuzumab facilitates liver fibrosis regression and arrests liver fibrosis progression. Although simtuzumab treatment in patients with idiopathic pulmonary fibrosis was coincided with side effects including dyspnea, cough and upper respiratory tract infection [26]. In harmony with gene expression data, carvacrol 70 mg/kg treatment reduced elevated level of hepatic LOX protein significantly. Recent attempt by Zhao et al. [27] showed in vivo inhibition of LOXL1 by LOXL1-shRNA in liver cirrhotic models suppresses liver fibrosis progression by inhibiting elastin crosslinking. As well as, in vitro silencing of LOXL1 downregulated elastin expression in LX-2 cells. Taken together, our results indicated, administration of carvacrol as a natural compound can be considered as a more effective therapeutic approach with less side effect than enzyme inhibition.
Conclusion
In conclusion, our study provided a molecular mechanism for the anti-fibrotic effect of carvacrol as a natural compound through downregulation of LOX. Further studies for identifying the effect of carvacrol on tissue stiffness and LOX activity can provide more documentation to reject or confirm our observations.
Abbreviations
- CCl4 :
-
Carbon tetrachloride
- LOX:
-
Lysyl oxidase
- ECM:
-
Extracellular matrix
- ALT:
-
Alanine aminotransferas
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- TAC:
-
Total antioxidant capacity
- –SH:
-
Total thiol group
- TOS:
-
Total oxidant status
- MDA:
-
Malondialdehyde
References
Bataller R, Brenner DA. Liver fibrosis. J Clin Investig. 2005;115(2):209–18.
Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, et al. Allosteric inhibition of lysyl oxidase–like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–17.
Ikenaga N, Peng Z-W, Vaid KA, Liu SB, Yoshida S, Sverdlov DY, et al. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017;66(9):1697–708.
Bahabadi M, Mohammadalipour A, Karimi J, Sheikh N, Solgi G, Goudarzi F, et al. Hepatoprotective effect of parthenolide in rat model of nonalcoholic fatty liver disease. Immunopharmacol Immunotoxicol. 2017;39(4):233–42.
Bhuyan B, Baishya K, Rajak P. Effects of Alternanthera sessilis on liver function in carbon tetra chloride induced hepatotoxicity in Wister rat model. Indian J Clin Biochem. 2018;33(2):190–195.
Son Y-O, Hwang J-M, Choi K-C, Lee J-C. A phenolic acid and flavonoid fraction isolated from Lolium multiflorum Lam. Prevents d-galactosamine-induced liver damages through the augmentation of Nrf2 expression. Indian J Clin Biochem. 2019;34(1):68–75.
Nafees S, Ahmad S, Arjumand W, Rashid S, Ali N, Sultana S. Carvacrol ameliorates thioacetamide-induced hepatotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in liver of Wistar rats. Hum Exp Toxicol. 2013;32(12):1292–304.
Aristatile B, Al-Assafa AH, Pugalendi KV. Carvacrol ameliorates the Ppar-Α and cytochrome P450 expression on d-galactosamine induced hepatotoxicity rats. Afr J Tradit Complement Altern Med. 2014;11(3):118–23.
Aristatile B, Al-Assaf AH, Pugalendi KV. Carvacrol suppresses the expression of inflammatory marker genes in d-galactosamine-hepatotoxic rats. Asian Pac J Trop Med. 2013;6(3):205–11.
Canbek M, Uyanoglu M, Bayramoglu G, Senturk H, Erkasap N, Koken T, et al. Effects of carvacrol on defects of ischemia-reperfusion in the rat liver. Phytomedicine. 2008;15(6–7):447–52.
da Silva M, Quintans-Júnior LJ, de Santana WA, Kaneto CM, Soares MBP, Villarreal CF. Anti-inflammatory effects of carvacrol: evidence for a key role of interleukin-10. Eur J Pharmacol. 2013;699(1–3):112–7.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–54.
Benzie IF, Strain J. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In: Packer L, editor. Methods in enzymology. Orlando FL: Academic press; 1999. p. 15–27.
Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–11.
Botsoglou NA, Fletouris DJ, Papageorgiou GE, Vassilopoulos VN, Mantis AJ, Trakatellis AG. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J Agric Food Chem. 1994;42(9):1931–7.
Hu M-L. [41] Measurement of protein thiol groups and glutathione in plasma. In: Packer L, editor. Methods in enzymology. Orlando FL: Academic press; 1994. p. 380–5.
Hussein J, El-Banna M, Mahmoud KF, Morsy S, Latif YA, Medhat D, et al. The therapeutic effect of nano-encapsulated and nano-emulsion forms of carvacrol on experimental liver fibrosis. Biomed Pharmacother. 2017;90:880–7.
Bozkurt M, Bodakci M, Turkcu G, Kuyumcu M, Akkurt M, Sula B, et al. Protective effects of carvacrol against methotrexate-induced liver toxicity in rats. Acta Chir Belg. 2014;114(6):404–9.
Rajan B, Ravikumar R, Premkumar T, Devaki T. Carvacrol attenuates N-nitrosodiethylamine induced liver injury in experimental Wistar rats. Food Sci Hum Wellness. 2015;4(2):66–74.
Liu SB, Ikenaga N, Peng Z-W, Sverdlov DY, Greenstein A, Smith V, et al. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2015;30(4):1599–609.
Lytle KA, Depner CM, Wong CP, Jump DB. Docosahexaenoic acid attenuates Western diet induced hepatic fibrosis in Ldlr-/-mice by targeting the TGFβ-Smad3 pathway. J Lipid Res. 2015;56(10):1936–46.
Mohseni R, Karimi J, Tavilani H, Khodadadi I, Hashemnia M. Carvacrol ameliorates the progression of liver fibrosis through targeting of Hippo and TGF-β signaling pathways in carbon tetrachloride (CCl4)-induced liver fibrosis in rats. Immunopharmacol Immunotoxicol. 2019;41(1):163–71.
Sethi A, Mao W, Wordinger RJ, Clark AF. Transforming growth factor–β induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Investig Ophthalmol Vis Sci. 2011;52(8):5240–50.
Taylor MA, Amin JD, Kirschmann DA, Schiemann WP. Lysyl oxidase contributes to mechanotransduction-mediated regulation of transforming growth factor-β signaling in breast cancer cells. Neoplasia (New York, NY). 2011;13(5):406.
Ikenaga N, Peng Z-W, Vaid KA, Liu SB, Yoshida S, Sverdlov DY, et al. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017;66(9):1697–708.
Raghu G, Brown KK, Collard HR, Cottin V, Gibson KF, Kaner RJ, et al. Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial. Lancet Respir Med. 2017;5(1):22–32.
Zhao W, Yang A, Chen W, Wang P, Liu T, Cong M, et al. Inhibition of lysyl oxidase-like 1 (LOXL1) expression arrests liver fibrosis progression in cirrhosis by reducing elastin crosslinking. Biochim Biophys Acta (BBA) Mol Basis Dis. 2018;1864(4):1129–37.
Acknowledgements
This study is a part of R. Mohseni Ph.D. thesis and was supported by Hamadan University of Medical Sciences (No. 960321786).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The study protocol was approved by the Hamadan University of Medical Sciences Ethics Committee (code: IR.UMSHA.REC.1396.181 approved on 13 June 2017).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohseni, R., Karimi, J., Tavilani, H. et al. Carvacrol Downregulates Lysyl Oxidase Expression and Ameliorates Oxidative Stress in the Liver of Rats with Carbon Tetrachloride‐Induced Liver Fibrosis. Ind J Clin Biochem 35, 458–464 (2020). https://doi.org/10.1007/s12291-019-00845-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-019-00845-w