Abstract
The molecular mechanism of iron transfer across placenta in response to maternal anemic status/ iron supplementation is not clear. We hypothesized that maternal iron/ anemia status during early trimesters can be utilized as a biomarker tool to get estimates of placental iron status. Early interventions can be envisaged to maintain optimum placental/ foetal iron levels for healthy pregnancy outcomes. One hundred twenty primigravida were recruited and divided into non-anemic and anemic group on the basis of hemoglobin levels. The groups were randomly allocated to receive daily and weekly iron folic acid (IFA) tablets till six weeks postpartum. Hematological and iron status markers in blood and placenta were studied along with the delivery notes. Weekly IFA supplementation in anemic primigravidas resulted in significantly reduced levels of hematological markers (p < 0.01); whereas non-anemic primigravidas showed lower ferritin and iron levels, and higher soluble transferrin receptor levels (p < 0.05). At baseline, C-reactive protein and cortisol hormone levels were also significantly lower in non-anemic primigravidas (p < 0.05). A significantly decreased placental ferritin expression (p < 0.05); and an increased placental transferrin expression was seen in anemic primigravidas supplemented with weekly IFA tablets. A significant positive correlation was observed between serum and placental ferritin expression in anemic pregnant women (r = 0.80; p < 0.007). Infant weight, gestational length and placental weight were comparable in both the supplementation groups. To conclude, mother’s serum iron / anemia status switches the modulation in placental iron transporter expression for delivering the optimum iron to the foetus for healthy pregnancy outcomes.
Trial Registration
Clinical Trial Registry-India: CTRI/2014/10/005135.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron deficiency (ID) globally impact billions of people, majority of them are pregnant women and children [1]. Mostly, ID found associated with anemia during pregnancy and prophylaxis include supplementing daily iron folic acid (IFA) tablets. Adequate supply of iron is essential during pregnancy as ID and iron excess both are harmful for mother and foetus [2]. Since, third trimester of pregnancy is the period when majority of foetal iron stores are acquired [3]; mechanism underlying placental iron transfer in response to maternal iron status is not fully understood.
Placenta is an organ that plays key role in the transportation of nutrients to the foetus. Iron transport is tightly regulated process and its transfer through placenta follows unidirectional flow against concentration gradient [4]. Foetal iron requirements are met by modulations in placental iron transporters such as transferrin receptors, divalent metal transporter 1 (DMT1), ferroportin [5,6,7]. The salutary role of transferrin receptor-1 (TfR-1) in placental iron uptake and its molecular mechanism has been elucidated [8, 9]. Furthermore, the relationship between placental TfR-1 expression with varying degree of maternal iron status/anemia has also been studied [10].
In placenta, syncytiotrophoblast functions in the storage of iron in the form of ferritin and transportation across its basolateral surface takes place through ferrportin-1 protein, a membrane protein assist in the efflux of iron [11]. There is quantum of contradictory reports on the relationship of maternal anemia/iron status/ID during pregnancy and iron/anemia status in foetus [12, 13]. Although, few studies have documented the molecular mechanism of placental iron transfer by measuring the expression of iron transporters such as TfR-1 in iron uptake, ferritin as iron storage and ferroportin-1 in iron efflux [10, 13,14,15]; still there is lack of data about the expression pattern of placental iron transport proteins in anemic and non-anemic pregnant women supplemented with daily/weekly IFA tablets. Therefore, the present study was carried out to gain better understanding about molecular mechanism of human placental iron transfer and also it will provide an insight to the comparative usefulness of both the supplementation schemes.
Methods and Materials
Subject Recruitment and Sample Size Calculation
The present study comprised a total of 120 pregnant women in their early second trimester (13–16 weeks of gestation) who are yet to start oral iron supplementation. Out of 120, 60 were non-anemic (Hb > 11 g/dl) women and 60 were anemic (Hb = 8–11 g/dl) women. The age-matched subjects were selected amongst the cohort of pregnant women those attending the Antenatal Clinic in Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India. Pregnant women fulfilling the subject selection criteria, having no chronic morbidity, 20–30 years old primigravida, and belong to middle socio-economic group willing to participate (informed consent obtained) were enrolled in the study. The socio-economic status of participants was defined as described in Kuppuswamy’s socio-economic status scale [16]; briefly, the education background, occupation and monthly family income was recorded and on the basis of scoring criteria the participants were classified as upper, middle, upper lower and lower socio-economic status. Exclusion criteria include history of any metabolic disease like diabetes mellitus, malignancy and heart disease, infectious diseases like malaria, tuberculosis, HIV, endocrine disorders or taking any iron preparations during or from past 3 months.
An estimated sample size of 20 pregnant women in each group was calculated, which would give 80% power to detect a difference in mean (at 95% confidence interval) of 1.4 g/dl with standard deviation of 1.5 and 0.2 g/dl with standard deviation of 1.0 in Hb levels during daily and weekly IFA supplementation, respectively. Further, taken into consideration of design effect, n = 20 was multiplied by factor 1.5 (20 × 1.5 = 30) to get a total of 30 pregnant women in each group was finally considered. To remove non-responsive error and loss to follow-up, we considered 10% oversampling due to which a total of 130 pregnant women were enrolled and the data of 120 pregnant women with complete follow-up visits was considered for calculation.
Randomization
After screening the eligibility and informed consent process, two groups were categorized as non-anemic and mild-anemic groups on the basis of Hb levels. The envelope containing random number and instructions for random allocation of each participant to receive either daily or weekly IFA supplementation was opened. Random numbers were generated in a ratio of 1/1 by means of a customized computer programme to ensure the equal chances for each participant to receive daily/ weekly IFA tablets.
Study Design
All the enrolled participants (non-anemic and mild-anemic pregnant women) were dewormed by giving single dose of Albendazole 400 mg to be consumed at night on the day of enrolment. The enrolled participants were asked to fasting overnight and on the next morning venous blood sample was drawn from antecubital vein in supine position followed by refreshments. Likewise, second sample (3 months after first sampling) and third sample (6 weeks postpartum) was obtained in similar manner. The compliance with IFA tablet intake and the associated side-effects were recorded through direct questioning the participants. The participants were provided pre-counted IFA tablets and were asked to bring all empty and partial empty blister packs at each visit. The number of empty blister packs was counted for observing the compliance rate [17].
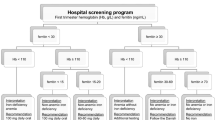
According to random allocation slip instructions, participants were given either one tablet of ferrous sulphate containing 100 mg elemental iron and 500 μg folic acid (Iron folic acid tablet/ IFA tablet) till 6 weeks postpartum daily or two IFA tablets weekly till 6 weeks postpartum. Thus, both the groups i.e. group 1 and 2 further subdivided into daily IFA supplementation group (DISG; n = 30) and weekly IFA supplementation group (WISG; n = 30). There was no placebo group in the study as ID is widely prevalent and denying iron to any participant was not possible since daily iron supplementation is included in the Indian National Programme. The flow diagram showing study design is shown in Fig. 1.
Sample Collection
6 ml venous blood was taken from each participant and serum separated by centrifuging the blood in clot activator tubes at 3000 rpm for 15 min. The different aliquots were prepared and stored in − 80 °C till further analysis.
Analytical Estimation
Measurement of Blood Index Parameters
Blood haemoglobin (Hb), haematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and red blood cell (RBC) counts were determined by using Sysmax A-380 automated cell counter.
Measurement of Iron and Inflammatory Markers
Serum was analysed for ferritin, soluble transferrin receptors (sTfR), erythropoietin, cortisol and high sensitivity C-reactive protein (HsCRP) and levels were measured using competitive ELISA kits. The results were compared with those of the standard curve obtained from the calibrators run simultaneously with the study samples for each parameter. Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES) (JY 2000 2, Jobin Horiba) technique was used for measuring the serum concentration of iron.
Placenta Tissue Collection
Placenta tissues were collected from non-anemic and anemic pregnant women allocated to DISG (n = 24 and n = 24) and WISG (n = 22 and n = 24). Four pieces of placenta tissues were immediately excised using sterile instruments from the portions nearest to the cord implantation site of placenta. The pieces were washed with ice cold saline and immediately snap freeze in liquid nitrogen before being stored at − 80 °C. Rest of the placenta was preserved in neutral formalin solution for histopathological/ immunohistochemical studies.
Western Blotting of Placenta Tissues
Placenta tissues (~ 50 mg) from each group were homogenized in 1.0 ml complete RIPA lysis buffer in the presence of protease inhibitor (10 µM). Homogenized samples were centrifuged for 20 min at 14,000 g at 4 °C and supernatant was assayed for total protein concentration using Bradford method. Supernatants were stored at − 80 °C until use. A total of 50 µg of protein lysates from placenta tissues and sample buffer were loaded on 10% SDS-PAGE. Equal amount of protein was loaded into each well. Then, the separated protein bands were transferred to nitrocellulose membrane. The membrane was blocked with the blocking agent (5% fat free milk) for 30 min followed by overnight incubation with primary antibody against ferritin (Cat. No. ab134276; Abcam, Cambridge, UK) at 1:1000 dilution followed by horseradish peroxidase-conjugated secondary antibody (goat anti-mouse antibody; Cat No. sc - 2005; Santa Cruz, USA) at 1:2000 dilution for hippocampus for 2 h. Anti-actin (mouse monoclonal to actin) antibody was used as a loading control (Cat. No. ab3280; Abcam, Cambridge, UK). The membrane was developed with Chemiluminescence reagent (Merck Millipore, USA). The optical density of bands was analysed using FluorChem M system (Model 92-15312-00; Cell Biosciences, Heidelberg, Germany).
Immunohistochemistry of Placenta Tissues
Formalin-fixed paraffin-embedded placental tissues were used for immunohistochemical staining of transferrin receptor-1 (TfR-1) protein. Immunohistochemistry was carried out on 5 μm thick tissue sections using rabbit anti-human transferrin receptor-1 antibody (Cat. No. 13113S; Cell Signaling Technology, Massachusetts, USA). Sections were deparaffinised in bioclear for 20 min then washed twice in ethanol. Slides were placed in a water bath containing 0.01 M sodium citrate at pH 6.0 for 30 min at 95 °C. Endogenous peroxidase activity was quenched with 3% H2O2 in DDW for 10 min. Slides were stained with 3, 3′—diaminobenzidine (DAB) as a chromogen. The primary antibody was applied at a concentration of 1:200 in 0.5% BSA and sodium azide and incubated 30 min at RT for TfR-1 staining. Anti-rabbit IgG, HRP conjugated antibody (Cat. No. 7074 V; Cell Signaling Technology, Massachusetts, USA) was applied at 1:1000 dilution for overnight incubation at 4 °C. Slides were visualized under a fluorescent microscope through 100 X objective (Olympus BX 60 fluorescence microscope, Japan) and digitized to a computer by a Grundig FA87 monochrome digital charge-coupled device camera. The images were captured at a resolution of 300 dots per inches.
Statistical Analysis
Data were expressed as mean ± SE. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 20.0. In order to evaluate significance level between the groups, unpaired t-test was applied and the statistical significance level (p < 0.05) was also determined using one way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests for variables such as placental ferritin expression, gestation length, infant and placental weight. The correlation between placental and serum iron status markers was evaluated using Pearson correlation assuming Gaussian distribution of population.
Results
The presented results are the part of ICMR-Anemia Task Force activity in which age-matched mild-anemic and healthy primigravid women having middle socio-economic status were longitudinally followed. The baseline anthropometric and haemodynamic characteristics of pregnant women such as mean maternal age, height, weight, BMI, blood pressure, heart rate and temperature is shown in Table 1.
The haematological indicators of anemic and non-anemic pregnant women are described in Table 2. During anemic pregnancy, third trimester Hb levels decreased significantly in WISG when compared with DISG (p ≤ 0.01). Further when compared with DISG, third trimester Hct (p ≤ 0.05) and reticulocyte count (p ≤ 0.05) was also significantly lower in WISG of anemic pregnant women despite having significantly higher second trimester Hct (p ≤ 0.05) in WISG. There was no significant difference observed in MCV and MCH levels between the supplementation groups of anemic and non-anemic pregnant women (Table 2).
Indicators of iron status and inflammation during pregnancy are shown in Table 3 which revealed that non-anemic pregnancy in DISG had elevated serum ferritin levels at six weeks postpartum (p ≤ 0.0001), higher serum iron levels (p ≤ 0.05) and significantly lower serum sTfR levels (p ≤ 0.01) at third trimester of pregnancy when compared with WISG. Inflammatory markers HsCRP (p ≤ 0.05) and stress hormone cortisol levels (p ≤ 0.01) were significantly different between the two supplementation groups during second trimester of pregnancy in non-anemic pregnant women. On the other hand, weekly IFA supplementation in anemic pregnancy resulted in significantly higher levels of sTfR (p ≤ 0.01) at six weeks postpartum, although rest of other iron makers were non-significant different in anemic pregnant women (Table 3). Despite the alterations in haematological and iron status markers, the gestational length, infant weight and placental weight were comparable between the supplementation groups in anemic and non-anemic pregnant women (Table 4).
The results obtained from protein expression studies in placental tissues were also in accordance with the serum levels of iron status markers. Placental ferritin expression was higher in DISG than WISG of both non-anemic as well as anemic pregnant women; however this difference was statistically significant in anemic pregnant women (p ≤ 0.05) when compared with WISG (Fig. 2). A positive relationship was observed between placental ferritin expressions and its serum levels at third trimester of pregnancy in all the study groups. Anemic pregnant women supplemented with weekly (r = 0.80; p ≤ 0.0001) and daily (r = 0.53; p = 0.007) IFA tablets showed significant positive correlation between placental ferritin expression and third trimester serum ferritin levels (Fig. 3).
Change in ferritin expression in human term placentas obtained from anemic and non-anemic pregnant women supplemented with daily and weekly IFA tablets (a) The representative bands of ferritin (~19 kDa) and b-actin (42 kDa) protein expression. (b) Decreased ferritin protein expression in weekly IFA supplementation group in non-anemic and anemic pregnant women compared with daily IFA supplementation group. Values are expressed as Mean ± SE. *Significant difference between WISG and DISG of anemic pregnant women (p ≤ 0.05)
Correlation between placental ferritin expression and serum ferritin during third trimester of pregnancy (a & b) Non-anemic pregnant women supplemented with weekly (n = 22) and daily (n = 24) IFA tablets respectively (c & d) Anemic pregnant women supplemented with weekly (n = 19) and daily (n = 24) IFA tablets respectively. Unit of measurement for serum ferritin expressed as ng/ml
On the other hand, placental ferritin expression was negatively associated with serum sTfR levels. As shown in Fig. 4, placental ferritin expression found negatively correlated with third trimester serum sTfR levels (r = − 0.35; p = 0.09) in anemic pregnant women supplemented with daily IFA tablets. Serum sTfR concentration during third trimester of pregnancy was lower in daily IFA supplementation groups as compared to weekly IFA supplementation groups (Table 3), which was in an inverse relation with the increased placental ferritin expression in non-anemic and anemic pregnant women (Fig. 4). A significant increased Hb concentration in DISG during third trimester of pregnancy in anemic pregnant women (Table 2) further corroborates with the significantly high ferritin expression observed in placenta tissues obtained from anemic pregnant women (Fig. 2). In addition, significantly high third trimester RBC count and haematocrit percentage in DISG of anemic pregnant women were also in agreement to the significantly increased placental expression of ferritin in anemic pregnant women.
Correlation between placental ferritin expression and soluble transferrin receptors (sTfR) during third trimester of pregnancy (a & b) Non-anemic pregnant women supplemented with weekly (n = 22) and daily (n = 24) IFA tablets respectively (c & d) Anemic pregnant women supplemented with weekly (n = 24) and daily (n = 24) IFA tablets respectively. Unit of measurement for serum sTfR expressed as μg/ml
Placental tissue localization of TfR-1 revealed immunoreactivity in the villous trophoblast cells, villous stroma, mesenchymal cells, smooth muscle cells surrounding the stem vessel and in the stem vessel endothelium. A clear demarcation in staining pattern was seen in TfR-1 protein localization among the placenta obtained from anemic and non-anemic pregnant women supplemented with daily and weekly IFA tablets (Fig. 5). In particular, stronger intensity of TfR-1 immunoreactivity was seen in placenta from anemic pregnant women supplemented with weekly IFA tablets followed by daily IFA supplementation group, followed by non-anemic placenta obtained from WISG and the placenta obtained from DISG showed least staining intensity (Fig. 5).
Immunohistochemistry on non-anemic and anemic placenta. Pictures showing transferrin receptor-1 (TfR-1) localization in the perivascular stroma (**), endothelium of stem vessel (***), villous stroma (VS), villous trophoblast (arrow head). A: Anemic pregnant women supplemented with weekly IFA tablets (n = 24), original magnification 20x; B: non-anemic pregnant women supplemented with weekly IFA tablets (n = 22), original magnification 20x; C: Anemic pregnant women supplemented with daily IFA tablets (n = 24), original magnification 20x; D: non-anemic pregnant women supplemented with daily IFA tablets (n = 24), original magnification 20x
Discussion
Main Findings
Our study has identified increased TfR-1 localization and reduced ferritin expression in placenta tissues obtained from anemic pregnant women allocated to WISG with an increased serum erythropoietin levels during third trimester. In addition, ferritin values in serum were well correlated with its placental expression during anemic pregnancy. With the presented findings, we therefore addressed a significant knowledge gap by varying supplemental dose of IFA during pregnancy and studying iron profile pattern in their blood and placenta so that the neonatal iron requirements can be envisaged right from the advancement of pregnancy.
Strengths and Limitations
Literature survey suggests limited or no data about the effect of daily and weekly IFA tablets supplementation on placenta obtained from non-anemic and anemic pregnant women. There is scanty information about the molecular mechanism underlying the iron transfer across placenta and requires careful attention in order to optimise the appropriate iron dose require to avoid bad pregnancy outcomes [18]. In the present attempt, serum levels of iron storage and transport proteins were estimated during mid and late gestation period and their levels were correlated with the placental expression of such proteins to get an estimate of iron requirements for growth and development of foetus. This study also provides an insight about differential levels of iron status markers in blood correlated with iron markers in placenta. Further, the factors such as ethnicity, age, socio-economic background, parity, gravidity, and type, dosage, make and ingredient of IFA supplement are taken into consideration. The present study not only comprised larger sample size than previous similar studies [4, 12, 19], but also a longitudinal study in which the confounders of anemia such as malaria infection, hookworm/helminth infections were also taken care of. The limitations of the study is that we could not studied placental hepcidin, ferroportin and iron response elements (IREs) expression though the reports suggest that ferroportin expression human placenta at translational and post-translational levels does not relate to maternal anemia status, and is not regulated by IREs [4].
Interpretation (in Light of Other Evidence)
According to literature, there is less controversy about the role of TfR protein expression in mechanism of placental iron uptake despite the fact that still there is much information needed about iron regulation in placenta. Localization of TfR protein in placenta was seen in previous reports which mentioned that TfR-1 was mainly expressed on the syncytiotrophoblast in villous trophoblast, although it was seen in the cytotrophoblast as well [20]. As TfR-1 protein is mainly expressed on maternal facing of placenta i.e. apical membrane also known as syncytiotrophoblast; thus it is attributed to the iron uptake in placenta. The effect of anemia on iron transport proteins involved in regulation of iron metabolism in placenta was studied, which showed upregulation of TfR-1 expression in placentas from mild anemic mothers [4]. This is in agreement with our results where we also found increased localization of TfR-1 expression in anemic pregnant women. Anemic pregnant women receiving weekly IFA had elevated serum erythropoietin levels with increased TfR-1 and reduced ferritin expression in their placenta tissues. This might be due to an increased eythropoietic activity due to ID/IDA in this group that later resulted in TfR-1 overexpression in the placenta tissues obtained from anemic mothers. The increased third trimester erythropoietin levels in this group might have contributed in increased serum sTfR levels to limit iron usage for excessive RBC production under the influence of ID/IDA. This might be the possible reason of lower RBC count and serum ferritin levels but high serum iron in anemic pregnant women received weekly IFA tablet.
Apart from cellular iron levels and anemia during pregnancy, there are other factors of maternal, placental and foetal origin, that directly or indirectly involve in the regulation of iron through placenta. It is evident from published studies that maternal ID during pregnancy results in increased mRNA and protein expression of TfR, which in turn is associated with consequently high efflux of iron through ferroportin-1 protein. This mechanism ultimately results in delivering sufficient iron supply to the growing foetus even if there is maternal ID/ iron deficiency anemia [13]. Further, it has been investigated that TfR protein expression did not get affected with foetal or placental iron status. The accumulation of ferritin protein at the end of third trimester of healthy pregnancy leads to increment in placental-foetal iron delivery via increased expression of ferroportin-1 protein [19]. Placental iron storage in the form of ferritin is influenced by iron response protein-1 (IRP-1) where IRPs attaches with IREs in 5′ untranslated region of ferritin mRNA leading to reduction in the iron storage form of ferritin. The expression of placental ferritin decreased proportionally in relation to maternal anemia [4] which is in accordance with the findings of our study. We also tried to find out the association between third trimesters maternal Hb and iron status markers with placental iron markers, and found significant associations in anemic pregnant women. Contrary to this, Langini et al. [12] reported no association between maternal serum iron markers with placental iron markers. The possible reason for this disagreement might be the different analytical technique and procedures adopted; and most importantly the quantity of iron supplement which pregnant women might have consumed during gestation; although the information related to this is not stated in the article.
Conclusions
The concluding remarks include that anemic pregnancy has an impact on placental iron distribution especially when supplemented with weekly IFA tablets, which do not seems beneficial as in case of non-anemic pregnancy [17]; though the birth outcome was not affected in either case. Placental iron transport proteins modulate their expression in response to different dosage of IFA and maternal iron/ anemia status for delivering optimum iron to the foetus. Overall, our study provide evidence that maternal serum iron status during early and mid-gestation can be exploited as a tool to figure-out neonatal iron requirements during pregnancy.
References
De Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993–2005. In: WHO Global Database on Anaemia. World Health Organization. 2008. http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf. Accessed 26 July 2013.
Milman N. Oral iron prophylaxis in pregnancy: not too little and not too much! J Pregnancy. 2012. https://doi.org/10.1155/2012/514345.
Widdowson EM, Spray CM. Chemical development in utero. Arch Dis Child. 1951;26:205–14.
Li Y-Q, Yan H, Bai B. Change in iron transporter expression in human term placenta with different maternal iron status. Eur J Obstet Gynecol Reprod Biol. 2008;140:48–54.
Orberger G, Fuchs H, Geyer R, Gessner R, Köttgen E, Tauber R. Structural and functional stability of the mature transferrin receptor from human placenta. Arch Biochem Biophys. 2001;386:79–88.
Georgieff MK, Wobken JK, Welle J, Burdo JR, Connor JR. Identification and localization of divalent metal transporter-1 (DMT-1) in term human placenta. Placenta. 2000;21:799–804.
Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–81.
Kroos MJ, Starreveld JS, Verrijt CE, van Eijk HG, van Dijk JP. Regulation of transferrin receptor synthesis by human cytotrophoblast cells in culture. Eur J Obstet Gynecol Reprod Biol. 1996;65:231–4.
Verrijt CE, Kroos MJ, Huijskes-Heins MI, Cleton-Soeteman MI, van Run PR, van Eijk HG, et al. Accumulation and release of iron in polarly and non-polarly cultured trophoblast cells isolated from human term placentas. Eur J Obstet Gynecol Reprod Biol. 1999;86:73–81.
Young MF, Pressman E, Foehr ML, McNanley T, Cooper E, Guillet R, et al. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta. 2010;31:1010–4.
Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200.
Langini SH, de Portela ML, Lázzari A, Ortega Soler CR, Lönnerdal B. Do indicators of maternal iron status reflect placental iron status at delivery? J Trace Elem Med Biol. 2006;19:243–9.
Gambling L, Danzeisen R, Gair S, Lea RG, Charania Z, Solanky N, et al. Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem J. 2001;356:883–9.
McArdle HJ, Morgan EH. Transferrin and iron movements in the rat conceptus during gestation. J Reprod Fertil. 1982;66:529–36.
Li Y, Yan H, Bai B, Zhang Q. Effect of iron status of pregnant women on ferroportin 1 expression in third-trimester placenta. Wei Sheng Yan Jiu. 2008;37:335–8.
Kumar N, Gupta N, Kishor J. Kuppuswamy’s socioeconomic scale: updating income ranges for the year 2012. Indian J Public Health. 2012;56:103–4.
Shankar H, Kumar N, Sandhir R, Mittal S, Kurra S, Dhaliwal L, et al. Weekly iron folic acid supplementation plays differential role in maintaining iron markers level in non-anaemic and anaemic primigravida: a randomized controlled study. Saudi J Biol Sci. 2016;23:724–30.
Cetin I, Berti C, Mandò C, Parisi F. Placental iron transport and maternal absorption. Ann Nutr Metab. 2011;59:55–8.
Bradley J, Leibold EA, Harris ZL, Wobken JD, Clarke S, Zumbrennen KB, et al. Influence of gestational age and fetal iron status on IRP activity and iron transporter protein expression in third-trimester human placenta. Am J Physiol Regul Integr Comp Physiol. 2004;287:R894–901.
Bastin J, Drakesmith H, Rees M, Sargent I, Townsend A. Localisation of proteins of iron metabolism in the human placenta and liver. Br J Haematol. 2006;134:532–43.
Acknowledgements
The authors are highly thankful to Mr. Santosh Kurra and Dr. Mohd. Tarique from Biochemistry department and Dr. Sandeep Mathur from Pathology department, AIIMS for their inputs in carrying out immunohistochemistry work. The authors also wish to acknowledge continuous support provided by Dr. Rohini Sehgal, Dr. Arun Kumar, Mrs. Leema Maithi and Mrs. Shobha Kandpal from HRRC, AIIMS for enrolment and follow-up of the patients.
Funding
This study was funded by Indian Council of Medical Research, New Delhi, India (grant no. 5/7/165/06-RHN and 5/7/304/08-RHN).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethics approval was obtained from the Institute Ethics Committee of All India Institute of Medical Sciences, New Delhi, India.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Shankar, H., Kumar, N., Sandhir, R. et al. Differential Iron Status and Trafficking in Blood and Placenta of Anemic and Non-anemic Primigravida Supplemented with Daily and Weekly Iron Folic Acid Tablets. Ind J Clin Biochem 35, 43–53 (2020). https://doi.org/10.1007/s12291-018-0794-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-018-0794-2