Abstract
The reference intervals (RIs) of serum aminotransferases and Gamma-glutamyl transferase (GGT) have been established many years ago. Recent RIs are not available. The prospective study was conducted to re-evaluate the RIs of liver enzymes and the effect of demographic and anthropometric variables on them in western Indian population. A total of 1059 blood donors comprised the study population. Anthropometry and serum liver enzymes levels were measured. Subjects were categorized into normal weight and overweight by using body mass index (BMI) and waist circumference (WC). For RI determination, non-parametric methodology recommended by IFCC/CLSI was adopted. Mann–Whitney test and Spearman’s rank correlation were used for statistical analysis. Upper limit of normal reference value of liver enzymes were lower in female compared to male. (ALT—23.55 F vs 36.00 M, GGT—34.58 F vs 36.20 M) When RI of liver enzymes were calculated according to body mass index, the upper limit of normal of ALT and GGT were higher in overweight group compared to normal weight group. (ALT—38.00 vs 27.00 IU/L and GGT—37.59 vs 35.26 IU/L). In both male and female, liver enzymes correlated significantly with age. WC and BMI were positively correlated with AST, ALT and GGT in both subgroups and the correlation was stronger in male. Demographic factors should be considered for making liver enzyme tests more clinically relevant. Gender based partitioning should be adopted for serum alanine aminotransferase (ALT) and GGT reference values for Western Indian population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biochemical tests for liver disease provide assistance in making a diagnosis, monitoring the disease course and documenting subclinical evidence of disease [1]. The serum enzymes that are most commonly used and readily available in evaluating liver function are alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) [2].
Studies have shown that various clinic-demographic factors, such as gender and age, body fat -its distribution pattern and serum lipid levels might interfere with the liver enzymes level in the general population [3, 4].
Serum enzyme levels can also vary with variations in diet, lifestyle, environmental factors, and race/ethnicity thus may alter the upper limit that is considered normal.
Elinav et al. studied association of serum ALT level with age in Israeli population and observed an inverted curve with peak at 40–55 years while another study by Dong et al. reported significant decrease in serum ALT levels with increasing age [5, 6].
In addition, liver enzymes levels can also be modulated by the pattern of body fat distribution. Overweight and obesity increase the risk of liver disease. The body mass index (BMI) and waist circumstance (WC) are closely linked either alone or in combination to the liver enzymes elevation in general population [7, 8].
Ethnicity differences in the liver enzymes level had been observed in the third National Health and Nutrition Examination Survey (NHANES III) where Mexican–Americans had a higher prevalence of ALT elevation (14.9 %) as compared to other ethnicities. They defined aminotransferase elevation as raised ALT or AST based on NHANES III criteria; for men this corresponded to an AST > 37 IU/L or ALT > 40 IU/L, and for women an AST or ALT > 31 IU/L [8]. Ichihara et al. described large between-city differences in some commonly measured analytes among six Asian cities [9].
Within India itself, there is an enormous regional, ethnic and racial diversity. Diverse food habits, life-style and environmental conditions are present. These factors may cause variation in reference values. That is why the values associated with a reference interval (RI) should be representative of a specific population.
In India, clinical laboratories use the reference values of commonly used analytes supplied by diagnostic reagent and equipment manufacturers in their instructions without giving details of the original source of the data [10]. Currently used RI for liver enzymes levels in India are mainly based upon western population and those provided by manufacturer of kits. The suitability of these RIs for local use is thus questionable.
Kumar et al. documented the association of serum AST and ALT levels with BMI and waist hip ratios (WHRs) and the magnitude of obesity in general healthy population in western India and urged for revision of current normal limits of serum AST and ALT levels [11].
There are few studies from India about normal limits of liver enzymes in specific areas. We therefore conducted this study to re-evaluate the RIs of liver specific enzymes and to investigate the effect of modulating factors (demographic and clinical factors) on liver specific enzymes in western Indian normal blood donors.
Methods
Study Population
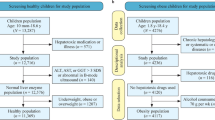
The proposed cross sectional study was conducted by the Department of Biochemistry and Department of Gastroenterology. The population for this study were individuals who attended blood donation camps held by the institute from July 2011- March 2013. The study was approved by the Institutional Ethics Committee. Total of 1132 apparently healthy individuals were selected after obtaining written informed consent. For consenting individuals, information regarding age, sex and anthropometric parameters (height, weight, waist circumference and hip circumference) were noted. Exclusion criteria were- pathophysiological states (any concurrent acute or chronic illness including acute febrile illness, thyroid disease, hypertension, diabetes, renal failure, congestive heart disease, chronic respiratory diseases, liver diseases, malabsorption syndromes, and nutritional anemia), acute viral hepatitis in last 6 months, intake of pharmacologically active agents (tobacco, oral contraceptives, replacement or supplementation therapy such as Insulin intake), family history of jaundice, H/o hepatotoxic drugs like aspirin, acetaminophen, ibuprofen etc., any other concurrent alternative medications (ayurvedic, homeopathic anticoagulant drugs etc.), blood transfusion in recent past (6 months), obese (BMI > 30 kg/m2) and modified physiological states (pregnancy, psychological and mental disorders such as severe stress and depression, exercise or physical training in previous days). The subjects whose serum AST/ALT levels were above 40.00 IU/L were referred for further evaluations of liver diseases and were excluded from analysis. After exclusion of these individuals, 1059 subjects were finally included in the study.
Anthropometry
Measurements included waist and hip circumferences, height and weight. Standing waist circumference (WC) was measured at the high point of the iliac crest. The tape was horizontally placed between the bottom of the rib cage and the top of the iliac crest (hip bones) around the smallest circumference between these two reference points. As for hip circumference, the tape horizontally placed around the hips at the biggest circumference point (maximum protrusion) between the iliac crest and either the crotch for women or the head of the femur for men. The waist-to-hip ratio was calculated as the waist measurement divided by the hip measurement. The height of subjects was determined by the use of a vertical calibrated scale, in the standing position. Heel to head-crown length was measured in centimetres. Weight was measured using a self-zeroing weight scale, with the participant wearing no shoes. The balance was pre tested with known standard weights. BMI was calculated as weight (kg) divided by height in m2 [12].
Study population was classified according to the sex (male and female) and then sub classified according to BMI (healthy weight/overweight), WC and WHR. For both male and female BMI ≤ 25 was considered healthy weight and BMI > 25 were considered overweight [13]. A WC of ≤80 cm for female and ≤85 cm for male was considered normal. WHRs of ≤0.81 in female and ≤0.88 in males were considered as normal. Values above these were considered as indication of upper body adiposity [14].
Laboratory Analysis
Venous blood samples were collected in plain evacuated tubes in aseptic condition. Serum was separated from the blood samples and stored in screw capped vials at −20 °C till processing of the sample. For each volunteer, serum samples were processed for estimation of serum ALT, AST and GGT using the IFCC approved methods [15, 16]. The kits were supplied by Mediclone Biotech Pvt. Ltd., Mumbai, India. The analysis was performed on fully automated analyzer (AU-400 by Olympus, Japan). Sample analysis was performed after standardization of instrument with the help of calibrators and controls. The quality control check was done twice in a week.
Statistical Analysis
All the enzymatic parameters revealed non-Gaussian distribution (as tested by D’Agostino–Pearso test). Data was represented as median and interquartile range (IQR). Analysis of Quantitative data between two subgroups was done using Mann–Whitney test. Analysis of Quantitative data among more than two subgroups was done using Kruskal–Wallis One Way ANOVA test. Relationship between liver enzymes and anthropometric parameters were assessed using Spearman’s rank correlation. p value of < 0.05 was considered statistically significant. All analyses were conducted using the Statistical Package for Social Sciences (SPSS-21.0, Chicago, IL). RI of liver enzymes was calculated using a “non parametric method” as described in the CLSI/IFCC Guidelines C28-A3 using MedCalc Statistical Software Version-12 [17]. Outliers were removed using Tukey’s method. The 2.5 percentile and 97.5 percentile formed the lower and upper limits of RI.
Results
Of 1059 subjects, 617 (58.26 %) were male and 442 (41.74 %) were female. The median (IQR) age of male and female subgroups was 26.00 (11.00) years and 25.00 (11.00) years respectively. The median AST and ALT of male were 26.00 (10) and 16.00 (12.50) IU/L respectively. For female, the median AST and ALT levels were 27.00 (9.00) and 13.00 (8.00) IU/L respectively. The median BMI was significantly higher in female group 23.34 (3.8) compared to male group 22.58 (4.0) (p < 0.001). The median WC and waist-hip ration of male was significantly higher in male group compared to the female group (p < 0.001). The median AST was significantly higher in female compared to male (p < 0.05) while median ALT and GGT was significantly lower in female group compared to male group (p < 0.01, 0.001 and 0.01 respectively). No significant difference in age could be observed between both the groups (Table 1).
Liver Enzymes and Age
Liver enzyme levels (median) were measured after partitioning of male and female population according to three age groups (Group1—21–30 years, Group 2—31–40 years and Group 3—41–50 years). Significant difference in serum AST and ALT levels was observed among different age groups for both genders (female—AST p < 0.001 and ALT p < 0.01, male AST p < 0.001 and ALT p < 0.001). Two female subjects had age more than 50 years, so they were also included in the group 3 (41–50 years). No significant difference could be observed in serum GGT levels with age in both genders. When pair wise comparison of AST and ALT between individual age groups was done, in male, the youngest age group (21–30 years) showed significantly lower AST and ALT activities than the age groups 2 and 3 (31–40 and 41–50 years, AST p < 0.05 and ALT p < 0.05). In women, the youngest age group (21–30 years) showed significantly lower AST and ALT activities than the age groups 2 (31–40 years, AST p < 0.001 and ALT p < 0.05) (Table 2).
BMI and Waist Hip Ratio
Table 3 reports the comparison of various variables classified by BMI into normal weight (BMI ≤ 25) and over weight (BMI > 25) for both gender. In females, out of n = 442, 126 were overweight while in male, 137 of 617 were overweight. In female, age, WC, HC, serum ALT and GGT were significantly higher in overweight group compared to the normal BMI group (p < 0.001) and AST levels was similar in the two groups. WHR of overweight females was significantly lower compared to normal weight females (p < 0.001). In males, liver enzymes (AST p < 0.05, ALT p < 0.001 and GGT p < 0.05), WC, HC and WHR were significantly higher (p < 0.001) in overweight group compared to the normal weight group; no difference in age could be observed between both the groups (Table 3).
Gender Specific Association Between Liver Enzymes and Clinical Variables
In female, AST and GGT were significantly increased with age (r = 0.24; p < 0.001 and 0.11; p < 0.05 respectively) while no significant correlation of ALT with age was observed. In male all the three liver enzymes significantly increased with age (AST—r = 0.18; p < 0.001, ALT—r = 0.20; p < 0.001 and GGT—r = 0.10; p < 0.05). Serum AST, ALT, GGT activities increased with increasing BMI for both male and female groups (Female AST—r = 0.14; p < 0.01, ALT—r = 0.27; p < 0.001, GGT—r = 0.46; p < 0.001 and Male AST—r = 0.18; p < 0.001, ALT—r = 0.39; p < 0.001, GGT—r = 0.12; p < 0.01). In male, serum AST, ALT and GGT level increased significantly with increasing WHR (AST—r = 0.20; p < 0.001, ALT—r = 0.21; p < 0.001, GGT—r = 0.15; p < 0.001). In female only ALT and GGT decreased significantly with increasing WHR (ALT—r = 0.20; p < 0.001, GGT—r = 0.25; p < 0.001) while no correlation of AST with WHR was observed.
Reference Intervals of Liver Enzymes
RIs of serum AST, ALT and GGT according to gender, BMI and WC are presented in Table 4. Figure 1 shows the graphical presentation of liver enzymes RIs in female and Fig. 2 shows the graphical presentation of liver enzymes RIs in male. The upper limit of normal of AST, ALT and GGT was significantly higher in male compared to female. The RIs were also affected by body fat and its distribution as the ULN of liver enzymes were high in overweight groups compared to healthy weight groups (Table 4).
Discussion
This prospective study was conducted to evaluate the effect of various modulating factors (age, gender, BMI and WHR) on liver enzyme levels and to re-evaluate their healthy ranges in western Indian population.
Demographic factors can contribute to disparities in the serum liver enzymes levels. In our analysis, with increasing age, serum ALT, AST and GGT levels were found to be significantly increased shows their positive association with the age within the age range of 21–48 years in males. The AST and ALT levels for age group 31–40 and 41–50 year were higher than the 21–30 years age group in males. In females although AST and GGT were positively correlated with age between 21–51 years but no significant association of serum ALT with age could be observed in female. On age sub-group comparison, serum AST and ALT levels were higher in age group 31–40 years compared to age group 21–30 years in female group. Our data suggests that age is an important modulating factor for liver enzymes in healthy population. In a similar study, Lee et al. studied the healthy population comprising of 643 men and 462 women of Korea with biopsy proven histological normal livers. They concluded that age was independently correlated with ALT levels in both sexes [18].
In addition to age, gender plays a crucial role as modulating factor. For our population, gender wise analysis showed that ALT and GGT levels were higher in male than female. In other study focused on blood donors without a diagnosis of hepatitis from London, the ALT level was much higher in males than females [19]. Piton et al. observed that healthy females have lower serum ALT levels than healthy males. Serum ALT levels in women remain constant throughout their lifetime, while in men they steadily rise up to the fifth decade and then decline [4]. In another study, Sripanidkulchai et al. studied the Thailand population and concluded that the median serum GGT level of healthy females was significantly lower than the values for healthy males, i.e., 21 (range = 9–260) versus 32 (range = 9–459) U/L. In that study the serum GGT was not age dependent [20].
There is a strong possibility that these demographic factors can affect the healthy ranges of liver enzymes. So we attempted to re-evaluate the healthy ranges of serum ALT, AST and GGT levels for our population. Our study concluded that established RIs of liver enzymes were altered from current reporting ranges of our clinical centre. Major alteration was observed in ALT levels. Our study reports lower reference range of ALT (5.0–33.0 IU/L) for total population compared to the currently used reference range at our centre (40.0 IU/L). In this study it was also observed that serum ALT upper limit was lower in a sex specific manner (36.0 IU/L for male and 23.6 IU/L for female). The low ALT upper limit in women calls for separate RI to be used for male and female population. Lozano et al. analysed 579 male and 457 female blood donors. The mean ALT levels were 25.3 ± 14.5 IU/l for males and 16.3 ± 7.9 IU/l in females. They have also shown a need to have different cut-off values for ALT levels in males and females [21].
Although the median AST level was slightly high for female than male, (Table 1) the ULN of AST, was similar for both female and male population (39.0 IU/L). This ULN value was slightly lower compared to the value currently used in our hospital over the years (40.0 IU/L). In another Indian study, the AST reference ranges were reported by Sairam et al. for reference population comprising of four Indian cities (Chennai, Hyderabad, New Delhi and Ahmadabad). The RI established in this study for AST was—(12.0–37.0 IU/L for female and 14.0–42.0 IU/L for male) [22]. The RIs of GGT calculated in our study was 7.9–34.6 IU/L for female and 8.6–36.2 IU/L for male. The ULN of GGT for both male and female calculated by our study were lower than the value currently used in the laboratory (<50.0 IU/L). The low ULN of GGT can be explained by the fact that in our reference population only 14.5 % male were mild alcoholic and no women were alcoholic in our study. Our data suggests the gender based partitioning of serum ALT and GGT parameters.
Except demographic factors, body fat distribution can also have modulating effect on liver enzymes levels and alter their healthy ranges. BMI (measure of total body fat), WC and WHR (the measures of regional body fat) are good indicators of the risks of abdominal fat accumulation. Our study has attempted to identify association of these anthropometric parameters with liver enzymes. BMI was positively correlated to serum AST, ALT and GGT in both male and female study population. Further after partitioning of population into healthy and overweight using BMI criteria, the liver enzyme activity was significantly higher in over weight male compared to healthy weight male. Also the WC and WHR were significantly higher in over weight male compared to healthy weight male. In female, serum ALT, GGT levels, WC was significantly higher and WHR was significantly lower in overweight compared to healthy weight group. These results show the comparative pattern of fat distribution in overweight male and female for our population. In female, with increasing body weight (as measured by BMI), the fat deposition increased more on hip than on waist causing decrease in WHR in overweight female group. While in male, with increase in BMI, the fat deposition was relatively high on waist than hip causing increased WHR in overweight male. Our findings are in general agreement with previous studies which demonstrated that BMI is strongly associated with increased serum activities of liver enzymes, such as ALT, AST and GGT [11, 23, 24].
Some investigators also reported that serum ALT, AST, and GGT enzymes showed marked increases in mean levels with an increase in the BMI [25]. In our study the BMI even had visible effect on RIs of ALT AST and GGT liver enzymes. High RIs of AST, ALT and GGT were observed in overweight (BMI > 25) group (AST—15.0–39.0, ALT—5.0–27.0, GGT—7.9–34.6) as compared to normal weight (BMI ≤ 25) group (AST—14.6–39.0, ALT—5.0–38.0, GGT—8.9–37.6). Therefore BMI is a major factor contributing to the variability in the reference cohort used to establish ULN values. In our study the reference population comprised of 75.17 % (n = 796) healthy weight and 24.83 % (n = 263) overweight people.
So far BMI has been used to assess relative weight and obesity status and their relationship with hepatic enzymes levels but it does not suggest fatness homogeneously in all populations. Anthropometrical measures of central adiposity, such as WC can also be used to assess the relationship between body fat distribution and liver enzyme activity [12].
Our study attempted to identify healthy and overweight by BMI and WC and waist-hip ratio. As indicated by BMI > 25, 38.50 % female and 22.20 % male were overweight in our population. When WC (>80 cm for female and >85 cm for male) was used as obesity indictor, the percent of overweight female increased to 45.02 % and for male it increased to 46.51 %. These data indicates the percentage of over- weight individuals increased almost twofold in both genders when the classification of obesity was based on WC Statistical analysis showed that median BMI was significantly low for male than female which indicate that overall adiposity was high in female than male. In female WHR was significantly high compared to male population which indicates that in female more fat deposition was on hip area than waist. Thus large hip circumference lowered the WHR (as hip circumference is the denominator in the ratio) in female subjects compared to male subjects.
Except BMI, WC was used for dividing population into healthy weight and overweight to evaluate the difference in live enzyme’s RIs. Significant association of WC with AST, ALT and GGT was observed in both male and female. ULN of ALT and GGT were high in over weight group as compared to normal weight group classified according to WC (Table 4).
Pouliot et al. have shown that WC not only correlated better with BMI than WHR, but was also better related to the metabolic variables known to be associated with biochemical parameters such as serum lipid and glucose levels [26]. In our study WHR was positively correlated with AST, ALT and GGT in male but negatively correlated with ALT and GGT in female. Kumar et al. and Piton et al. also observed the negative correlation of WHR with serum ALT in female and positive correlation of ALT with WHR in male [4, 11]. In another study, WC, liver enzymes and pertinent demographic data were abstracted from NHANES III, There was a significant relationship observed between enzyme activity and WC (p < 0.001 for ALT, p < 0.01 for ALP, p < 0.01 for AST, p = 0.02 for GGT), and the relationship was stronger in females (p < 0.002) and Mexican Americans (p < 0.001) [27]. Thus WC can be used as comprehensible and physiologically valid indicator of obesity except BMI to study the effect of body fat distribution on liver enzymes.
ULNs of liver enzymes established in our study were lower as compared to north and south Indian population. Yadav et al. 2014 established reference ranges of liver function test parameters for north Indian population (AST—14.00–55.00, ALT—11.00–70.35, GGT—6.7–51.09 IU/L, for Male and AST—13.00–50.43 ALT—9.00–63.43, GGT—3.92–48.70 IU/L for Female). In another study conducted by Furruqh et al., ULNs of liver enzymes for urban south Indian population were also high. In this study the age range was 21–70 years and they observed increase in ULN of liver enzymes with increasing age. The explanation for wider reference range of liver enzymes given by the author was difference in the diet pattern of reference population. The one cause of difference in our RIs with north and south India may be the broad age range selected for north Indians (15–60 years) and south Indian (21–70 years) compared to our reference population (21–51 years). As the age increases RI may differ from younger adults which emphasize the importance of age in establishing RIs. Alternatively, anthropometric (BMI, WHR) and other clinical differences could also be responsible for such variations [28, 29].
In conclusion, our study suggests that the ULNs of liver enzymes are different from the currently used ULNs of our laboratory so they need to be standardized. Liver enzyme values are also significantly affected by gender, age, BMI, and WC/WHR in healthy population and an important relationship exists between RIs of liver enzymes and these demographic factors. Being surrogate marker in screening of liver diseases, AST, ALT and GGT reference values should be corrected for these factors to increase specificity of liver enzymes tests and to ensure their clinical utility. Updated RIs documented in our study for liver enzymes would help clinicians in better interpretation of these parameters for early diagnosis and prognosis of liver diseases in Western Indian population.
References
Pincus MR, Schaffner JR. Assessment of liver function in clinical diagnosis and management by laboratory methods. Philadelphia: Saunders; 1996.
Panteghini M, Falsetti F, Chiari E, Malchiodi A. Determination of aspartate aminotransferase isoenzymes in hepatic diseases—preliminary findings. Clin Chim Acta. 1983;128:133–40.
Dong MH, Bettencourt R, Barrett-Connor E, Loomba R. Alanine aminotransferase decreases with age: the Rancho Bernardo Study. PLoS One. 2010;5(12):e14254.
Piton A, Poynard T, Imbert-Bismut F, Khalil L, Delattre J, Pelissier E, et al. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. Hepatology. 1998;27(5):1213–9.
Dong MH, Bettencourt R, Brenner DA, Barrett-Connor E, Loomba R. Serum levels of alanine aminotransferase decrease with age in longitudinal analysis. Clin Gastroenterol Hepatol. 2012;10(285–290):e1.
Elinav E, Ben-Dov IZ, Ackerman E, Kiderman A, Glikberg F, Shapira Y, et al. Correlation between serum alanine aminotransferase activity and age: an inverted U curve pattern. Am J Gastroenterol. 2005;100:2201–4.
Liu Z, Hu Y, Yang X, Tan A, Gao Y, Qin X, Liang Y, Mo Z, Peng T. Combinative analysis of factors influence serum alanine aminotransferase activity in adult male population from southern China. Clin Biochem. 2012;45(18):1683–8.
Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–7.
Ichihara K, Itoh Y, Lam CWK, Poon PMK, Kim JH, Kyono H, et al. Sources of variation of commonly measured serum analytes in 6 Asian cities and consideration of common reference intervals. Clin Chem. 2008;54:356–65.
Malati T. Whether western normative laboratory values used for clinical diagnosis are applicable to Indian population? An overview on reference interval. Indian J Clin Biochem. 2009;24:111–22.
Kumar S, Amarapurkar A, Amarapurkar D. Serum aminotransferase levels in healthy population from western India. Indian J Med Res. 2013;138:894–9.
Stranges S, Dorn JM, Muti P, Freudenheim JL, Farinaro E, Russell M, et al. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology. 2004;39:754–63.
Kaur S, Walia I. Body mass index, waist circumference and waist hip ratio among nursing students. Nurs Midwifery Res. 2007;3(2):84–90.
Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in Asian Indian adults. Diabetes Care. 2003;26:1380–4.
Bergmeyer HU, Horder M, Rej R. International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 3. IFCC method for alanine aminotransferase (l-alanine: 2-oxoglutarate aminotransferase, EC 2.6.1.2). J Clin Chem Clin Biochem. 1986;24(7):481–95.
Burtis CA, Tietz ER. Fundamentals of clinical chemistry. Philadelphia: WB Saunders Company; 1996. p. 53–79.
Solberg HE. The IFCC recommendation on estimation of reference intervals. The RefVal program. Clin Chem Lab Med. 2004;42:710–4.
Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, et al. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2010;51:1577–83.
Aach R, Szmuness W, Mosley J, Hollinger F, Kahn R, Stevens C, et al. Serum alanine aminotransferase of donors in relation to the risk of non-A, non-B hepatitis in recipients. N Engl J Med. 1981;304(17):989–94.
Sripanidkulchai B, Areejitnusorn P, Sriamporn S, Sripanidkulchai K, Kamsa-ard S. Serum gamma-glutamyl transpeptidase and alkaline phosphatase of people in Khon Kaen, the northeastern Thailand. Asian Pac J Cancer Prev. 2004;5(1):54–7.
Lozano M, Cid J, Bedini JL, Mazzara R, Gimenez N, Mas E, et al. Study of serum alanine-aminotransferase levels in blood donors in Spain. Haematologica. 1998;83:237–9.
Sairam S, Domalapalli S, Muthu S, Swaminathan J, Ramesh V, Sekhar L, et al. Hematological and biochemical parameters in apparently healthy Indian population: defining reference intervals. Indian J Clin Biochem. 2013;29(3):290–7.
Burns CJ, Boswell JM, Olsen GW. Liver enzyme activity and body mass index. J Occup Environ Med. 1996;38:1248–52.
Choi JW. Association between elevated serum hepatic enzyme activity and total body fat in obese humans. Ann Clin Lab Sci. 2003;33:257–64.
Robinson D, Whitehead TP. Effect of body mass and other factors on serum liver enzyme levels in men attending for well population screening. Ann Clin Biochem. 1989;26(Pt 5):393–400.
Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–8.
Samadi N, Cembrowski GS, Chan J. Effect of waist circumference on reference intervals of liver-related enzyme tests in apparently healthy adult Mexican Americans, black and white Americans. Clin Biochem. 2007;40:206–12.
Yadav D, Mishra S, Gupta M, John PJ, Sharma P. Establishment of reference interval for liver specific biochemical parameters in apparently healthy north indian population. Indian J Clin Biochem. 2013;28:30–7.
Furruqh S, Anitha D, Venkatesh T. Estimation of reference values in liver function test in health plan individuals of an urban south Indian population. Indian J Clin Biochem. 2004;19(2):72–9.
Acknowledgments
The author would like to acknowledge all the laboratory staff of Central Clinical Biochemical Laboratory, Seth G. S. Medical College, Mumbai for their timely help and assistance.
Conflict of interest
The authors declare no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maksane, S.N., Dandekar, S.P., Shukla, A. et al. Hepatic Enzyme’s Reference Intervals and Their Modulating Factors in Western Indian Population. Ind J Clin Biochem 31, 108–116 (2016). https://doi.org/10.1007/s12291-015-0508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-015-0508-y