Abstract
With the success of post-transplant cyclophosphamide based platform and improved clinical care, the number of haploidentical stem cell transplants (HaploSCT) have surged over the last decade. However, data from India is scarce. We aimed to evaluate the outcome of haploSCT at our centre. Since the inception of government schemes, many patients at our centre are able to undergo transplantation at subsidized cost. We conducted a retrospective analysis of the haploidentical transplants performed between January 2015 and November 2022. Fifty patients were eligible for this study. Patient details were obtained from case files. The graft versus host disease (GVHD) prophylaxis was post-transplant Cyclophosphamide (PTCy) with Mycophenolate-mofetil and Cyclosporine/tacrolimus/sirolimus. All patients were transfused peripheral blood stem cells from donors. Post-transplant, patients continued regular follow up as per schedule. Supportive care was given as per unit protocol. Overall survival (OS) was calculated using the Kaplan–Meier method. Fifty patients underwent haploSCT. A total of fifty patients with a median age of 20 years (range 3–53 years) underwent haploidentical HSCT from a family donor. Twenty three (46%) patients were > 18 years age and 82% were males. Indications for transplant included both benign and malignant hematological diseases. Most common conditioning regimen used was Fludarabine + Busulphan + Cyclophosphamide (n = 38, 76%). Thirty five patients (70%) engrafted successfully. In the patients who had successful engraftment, the median time to neutrophil engraftment was 16 days (range 10–20 days) and platelet engraftment was 18 days (range 10–32). Fourteen patients developed acute GVHD (28%), and three patients developed chronic GVHD (6%). The median follow-up was 30 months and the two-year OS was 43% with a median OS of 17 months. Twenty-one (adult = 9, pediatric = 12) out of 50 patients (42%) are alive and on regular follow-up. HaploSCT with a PTCy platform is a cost-effective, promising modality of treatment in patients who have no suitable matched donors and are not affording matched unrelated transplants. At our centre, we were able to achieve acceptable results with use of generic medications at affordable cost. Transplant Related Mortality (TRM) rates were comparable to other centres, however, multi-drug resistant bacterial infection remains a challenge in performing haploidentical HSCT in developing countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogenic stem cell transplantation is the only curative therapy for high risk hematologic malignancies. Ideal donors include Human Leucocyte Antigen (HLA) matched related donors (MRD) or unrelated donors. For patients who do not have matched donors, alternate donor transplants are an option. In India, the probability of getting a MRD is only 20% to 30%. There is high degree of polymorphism in the HLA gene pool in the Indian subcontinent. This reduces the probability of finding matched unrelated donors (MUD) through registries [1]. Higher costs associated with MUD transplants also limits widespread applicability in India. In the year 2021 almost 2498 transplants were done in India of which 1499 were allogenic stem cell transplants and 999 were autologous transplants. Of the 1499 allogenic transplants done around 50% were matched related donor, 40% were haploidentical donors and 10% were matched unrelated donor stem cell transplants [2].
With the success of post-transplant cyclophosphamide (PTCy) based platform and improved clinical care, the number of haploidentical stem cell transplants (HaploSCT) have surged over the last decade. Overall lower costs involved as compared to MUD transplants also favours the increased popularity of haploSCTs. Moreover, the PTCy platform is more cost-effective than alpha–beta T cell depletion. However, this comes at the cost of increased incidence of infections and higher rates of Graft-versus host disease (GVHD). Current indications for haploSCTs include both malignancies such as leukemias and lymphomas as well as benign hematologic conditions like inherited marrow failure syndromes, severe aplastic anemia etc. Its use has been explored in both adult and pediatric patients [3].
Numerous transplant centres from India have shared their experiences with haploidentical stem cell transplant in both benign as well as malignant conditions to improve our understanding of the protocols followed and results obtained [4,5,6,7,8,9]. We performed a retrospective analysis of haploSCTs done at our government tertiary care centre over a period of 7 years and reported the outcomes. We especially emphasize that in limited resource setups too the option of haploidentical stem cell transplant is a readily available and feasible option for a patient who intend curative therapy.
Materials and Methods
We conducted a retrospective analysis of the haploSCT performed between January 2015 and September 2022. Patient details were collected from the case files submitted in the medical records section. Institute ethics committee approval was obtained prior to the conduct of the study. Recipients and donors underwent a detailed pre-transplant work up to assess organ function. Detailed counselling of both patient and donor was done prior to transplant as per protocol. Young donors with no comorbidities and absence of donor specific antibodies were preferred.
Stem Cell Source and Harvest
For stem cell mobilisation, all donors received Granulocyte colony stimulating factor (G-CSF) at a dose of 10 mcg/ kg/day in two divided doses for 5 days. On day 5, peripheral blood stem cells (PBSCs) were collected from the donors using apheresis machines via peripheral venous access. Mid-harvest CD34 cell count was assessed and accordingly, an adequate volume was harvested. A minimum CD34 cell dose of 5.5 × 106/kg recipient body weight was aimed.
Conditioning Regimen and GVHD Prophylaxis
Choice of conditioning regimen depended on the recipient’s age, comorbidities, performance status, indication of transplant and finances. Majority of the patients received a fludarabine (FLU) based conditioning regimen (Table 1). The GVHD prophylaxis comprised of PTCy, cyclosporine (CSA), and mycophenolate mofetil (MMF). PTCy was administered in two doses on Day + 3 and Day + 4 after transplant at a dose of 50 mg/ kg/day, along with mesna. CSA was administered intra- venously at a dose of 5 mg/kg/day in 2 divided doses starting from day + 5 and later on switched to oral formulation. Target CSA levels were between 200–300 ng/ml. MMF was tapered from Day + 35 onwards in all patients. In patients with no GVHD, CSA was tapered between day + 60 and day + 90 in patients with malignancies and after day + 180 in those with benign disorders. Injection GCSF was started on Day + 5 at a dose of 5 mcg/kg/day and was continued till engraftment.
Engraftment
Neutrophil engraftment was defined as an absolute neutrophil count of more than 500/mm3 for 3 consecutive days and platelet engraftment was defined as an unsupported platelet count more than 20,000/mm3 for 7 consecutive days [10]. Primary graft failure was defined as failure to achieve neutrophil engraftment by day 28 of stem cell infusion [10].
Supportive Care
Supportive treatment, including antimicrobials and blood products, was given according to the unit protocol. Patients were closely monitored for acute GVHD (aGVHD) and chronic GVHD (cGVHD) and graded as per standard guidelines. Cytomegalovirus (CMV) quantitative PCR monitoring was done at regular intervals both during and post-transplant.
HLA Typing and Chimerism Monitoring
HLA typing was performed by high resolution typing. Antibodies in the recipient to donor HLA class I and II antigens were tested using a single antigen bead assay. Post-transplant, chimerism was monitored by Fluorescence in situ hybridization FISH-XY (sex mismatch transplants) or Variable number tandem repeats (VNTR) (same sex transplants) on day + 30, + 60, + 90, and + 180.
Statistical Analysis
For data analysis, SPSS version 23.0 was used. Continuous variables were reported as median and categorical variables as frequencies. For survival analysis, Kaplan Meier method was used. P value was considered significant if < 0.05. Overall survival (OS) was calculated from the date of stem cell infusion to date of death or last follow-up and it was estimated using the Kaplan–Meier method. The cumulative incidence rates of non-relapse mortality (NRM), aGVHD, and cGVHD were also computed.
Results
Patient and Donor Characteristics
A total of fifty patients with a median age of 20 years (range 3–53 years) underwent haploidentical HSCT from a family donor. Twenty three (46%) patients were > 18 years age and 82% were males. Benign haematological disorders included aplastic anaemia (n = 6), Fanconi Anemia (n = 3), Dyskeratosis Congenita (n = 1) paroxysmal nocturnal hemoglobinuria (n = 1) and Hyper IgM syndrome in one case. Malignant disorders included relapsed acute lymphoblastic leukaemia (n = 14), relapsed acute myeloid leukaemia (n = 16), chronic myeloid leukaemia (n = 3), myelodysplastic syndrome (n = 4) and Hodgkin disease (n = 1). The patients with malignancies were in morphological complete remission prior to the transplant. Most common conditioning regimen used was Fludarabine + Busulphan + Cyclophosphamide (n = 38, 76%) (Table 2).
Sibling donors were used in 23 patients, parents in 20 and children in 7 patients (Table 3). The median age of donors was 25 years (range 6–48). Most of the donors were male (n = 33), the father being the most common (n = 16). A sex mismatch was present in 19 patients, of which 11 were female recipients and 7 were male recipients. Seventeen out of 50 donors (34%) had mis- matched ABO blood groups, among these 11 were major and 6 were a minor mismatch. Donor-specific antibodies were present in 74% of the patients. None of the donors developed any side effects during stem cell mobilisation or harvesting.
Engraftment and Chimerism
The mean dose of CD34+ cells transfused to patients was 5.7 × 106/kg/recipient body weight (range: 3.9–7.8 × 106/kg). Thirty five patients (70%) engrafted successfully. In the patients who had successful engraftment, the median time to neutrophil engraftment was 16 days (range 10–20 days) and platelet engraftment was 18 days (range 10–32). Primary graft failure occurred in 3 patients (6%) and secondary graft failure in 5 patients (10%). The median duration of hospital stay was 27 days (range 7–64 days). Fourteen patients developed aGVHD (28%), and three patients developed cGVHD (6%). Most patients had GVHD limited to one organ, whereas only one patient had GVHD in 3 organs. Most common organ involved was the skin (14 patients) followed by gastrointestinal system (5 patients) and liver (3 patients). Donor chimerism at day + 30 and day + 90 was greater than 90% in the surviving patients on regular follow up. Details are mentioned in Table 4.
Infections
Gram-negative multidrug-resistant (MDR) organism was cultured from 19 patients with sepsis (38%). Two patients had probable pulmonary fungal infection (radiological) and was managed with amphotericin B. Thirty five patients had hemorrhagic cystitis (70%). Cystitis was associated with BK virus infection in urine in 6 patients amongst the 18 patients it was tested for. CMV reactivation was observed in 33 patients (66%) and all responded to preemptive therapy with ganciclovir. (Table 5).
Follow up and Survival Outcomes
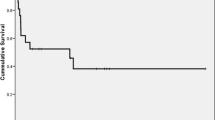
The median follow-up was 30 months and the two-year OS was 43% with a median OS of 17 months (Fig. 1). Twenty-one (adult = 9, pediatric = 12) out of 50 patients (42%) are alive and on regular follow-up. There OS rates of adults and children were similar (p = 0.58). The 2-year OS for malignant diseases was 51%.
Early TRM (< 30 days) was seen in 13 patients, sepsis being the most common reason. Late TRM (> 30 days) was seen in 7 patients. Seven patients died due to relapse, occurring within first 100 days post-transplant (Table 6).
Discussion
Due to paucity of randomized controlled trials comparing various alternative donor transplants and MSD, it is difficult to predict the best donor for any given patient in the absence of MSD. HaploSCT has the advantages of availability of donor in most cases, low costs with the use of PTCy and acceptable rates of graft failure, GVHD and NRM. With the advent of better conditioning regimens, supportive care, and trained staff, haploSCT has been utilised in a vast array of hematological disorders and this has also translated to improved survival outcomes [11].
Haploidentical stem cell transplant is an option with its own risks and benefits. While cost is definitely much less compared to matched unrelated transplants, the associated post-transplant complications such as Graft versus host disease has been life threatening. Initially haploidentical stem cell transplants have been reported to have a success rate of 37% (3-year OS) in high-risk groups with infection and GVHD being the most common causes of mortality [3]. However, over the years incorporation of PTCy to the haploidentical transplant protocol has significantly reduced complications and improved outcomes. HaploSCT is a feasible option that can be easily made available at the numerous transplant centres in India and improve patient’s chances of cure in both malignant as well as non-malignant hematological conditions. The ideal donor for allogeneic HCT is an HLA-matched sibling followed by an matched unrelated donor. However, < 30% of patients will have a matched sibling donor. The benefits of haploidentical over MUD HSCT are numerous, with arguably the most notable being that haploSCT extends donor availability to nearly all patients. However, data on haploidentical transplants from India is sparse.
Besides numerous scattered case reports, a few studies from India have been published describing the outcomes of haploSCT. Jaiswal et al. reported the aGVHD and cGVHD rates of 40.3% and 16.7% respectively in their cohort of 25 children who underwent haploSCT [5]. In contrast, we observed an aGVHD incidence of 28% and a cGVHD incidence of 6%. The platelet and neutrophil engraftment was 14 days in their study whereas it was 16 days and 18 days respectively in our cohort of patients. Their 2-year failure-free survival was 63.5% and 1 year NRM was 24%.
Batra et al., reported on outcomes of haploSCT in patients with acute leukemia and chronic myeloid leukemia. At a median follow up of 26 months, the 2 year overall survival was 38% for the 21 patients analysed. The TRM was 38% and 4 patients died due to subsequent relapse of the disease [4].
In the data from Christian Medical College, Vellore, India, 257 patients underwent 269 haploSCT between 2010 and 2020 [6]. Engraftment was seen in 76.2% patients which was comparable to our data. Acute GVHD and cGVHD was seen in 48% and 42% patients respectively which is higher than what was observed in our study. Atleast one documented infection was seen in more than 90% of patients, commonest being viral infections (71%), followed by bacterial (44%), and fungal infections (38%). The 2 year OS for the entire cohort was 40.5% and comparable to our 2 year OS of 43%. The outcomes in pediatric patients were better than the adults. The incidence of CMV reactivation was 33% followed by bacterial infections. In our study, there was no difference in overall survival for pediatric and adult patients (p = 0.58) and incidence of CMV reactivation was much higher (66%).
Nataraj et al. reported outcomes of 120 AML cases who underwent HSCT. Of these, 46 underwent haploSCT. The incidence of aGVHD (40.9% vs. 32.6%; p = 0.372) and cGVHD (16.7% vs. 15.2%; p = 0.837) were similar in MSD and haploSCT. Day-100 survival and OS were significantly better in the MSD cohort [7]. In another multicentre study on outcomes of haploSCT in Aplastic anemia was conducted on 79 cases. The primary graft failure rate was 16.43% whereas the rates of aGVHD and cGVHD were 26.4% and 18.9% respectively. The OS at a median follow up of 48 months was 61.6% [8].
HaploSCT performed with Alpha/Beta T-cell and CD19 B-cell depletion of donor stem cells ex-vivo, is associated with reduced incidence of GVHD. Bhatt et al. reported their outcomes using this technique [9]. In a total of 22 patients who underwent haploSCT, the aGVHD incidence was 5% and none of the patients had cGVHD. The one-year event-free survival was 77% with a TRM of 14%. With this technique, there is no need for GVHD prophylaxis but there is lesser graft versus leukemia effect and higher costs involved. At our institute, the average cost of the haploSCT from admission to Day + 60 ranged from INR 6–8 lakhs for pediatric patients and INR 10–12 lakhs for adult haploSCT.
There were few limitations of our study. It was retrospective in nature with fewer number of subjects. The viral testing for the aetiology of infections was not done in all patients due to cost constraints.
Conclusion
HaploSCT with a PTCy platform is a cost-effective, promising modality of treatment in patients who have no suitable matched donors and are not affording matched unrelated transplants. At our centre, we were able to achieve acceptable results with use of generic medications at affordable cost. TRM rates were comparable to other centres, however, multi-drug resistant bacterial infection remains a challenge in performing haploidentical HSCT in developing countries. CMV reactivation and hemorrhagic cystitis are also highly prevalent. Sharing of haploSCT data between various centres in India can help in improving patient outcomes.
References
Dedhia L, Gadekar S, Mehta P et al (2015) HLA haplotype diversity in the South Indian population and its relevance. Indian J Transplant 9:138–143
ISBMT Activity report (2021)
McCurdy SR, Kanakry JA, Showel MM et al (2015) Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood 125(19):3024–3031
Batra A, Perumal Kalaiyarasi J, Kannan K et al (2021) Haploidentical hematopoietic stem cell transplantation in leukemia’s: experience from a cancer center in India. Indian J Hematol Blood Transfus 37(3):463–471. https://doi.org/10.1007/s12288-020-01374-w
Jaiswal SR, Chakrabarti A, Chatterjee S et al (2016) Haploidentical transplantation in children with unmanipulated peripheral blood stem cell graft: the need to look beyond post-transplantation cyclophosphamide in younger children. Pediatr Transpl 20(5):675–682
George B, Kulkarni U, Lionel S et al (2022) Haploidentical transplantation is feasible and associated with reasonable outcomes despite major infective complications-A single center experience from India. Transplant Cell Ther 28(1):45.e1-45.e8
Nataraj KS, Prabhu S, Bhat S et al (2020) Hematopoietic stem cell transplant outcomes in patients with acute myeloid leukemia from a tertiary care center in South India. Biol Blood Marrow Transplant 26:123–124
Kharya G, Jaiswal SR, Bhat S et al (2023) Impact of conditioning regimen and graft-versus-host disease prophylaxis on the outcome of haploidentical peripheral blood stem cell transplantation for high-risk severe aplastic anemia in children and young adults: a report from the pediatric severe aplastic anemia consortium of India. Transplant Cell Ther 29(3):199.e1-199.e10
Bhat S, Ngangbam S, Iqbal W et al (2017) Outcomes of myeloablative haploidentical hematopoietic stem cell transplant in pediatric patients with TCR a/b; and CD 19 depletion. Biol Blood Marrow Transplant 23:S191–S192
Hutt D (2018) Engraftment, graft failure, and rejection. In: Kenyon M, Babic A (eds) The European blood and marrow transplantation textbook for nurses: under the auspices of EBMT. Springer, Cham, p 13
Aggarwal M, Agrawal N, Ahmad R et al (2016) Haploidentical stem cell transplant: established treatment, expanding horizons. Asian J Oncol 2:8–13
Funding
Nil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Ethical approval
Approved by the Institutional Review Board of the GCRI.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garg, A., Trivedi, M., Raj, A. et al. Haploidentical Stem Cell Transplantation: Half Match but More Hope!—Single Centre Experience from Western India. Indian J Hematol Blood Transfus 40, 385–391 (2024). https://doi.org/10.1007/s12288-023-01722-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-023-01722-6