Abstract
Radiotherapy is not usually a part of standard Burkitt lymphoma treatment. We aim to assess patient and treatment characteristics of Burkitt lymphoma, particularly RT use, and how they relate to survival. Retrospective cohort of adult patients treated from 2008 to 2019 in an academic hospital. All patients had biopsy-proven Burkitt’s lymphoma staged I to IV according to St. Jude’s/Murphy staging system. Patients were followed for at least six-months or until death. Forty-eight consecutive patients were selected. Median age at diagnosis was 36.9 years (18–62). Median follow-up was 7.78 months (0.5–187.5). Most were male (81.3%) and had good performance by ECOG scale on their first hematologist appointment (56.2% were ECOG 0). Median OS and PFS were 8.4 months (interquartile range Q1-Q3: 3.96–152.2) and 8.3 months (interquartile range Q1-Q3: 6.7-not reached), respectively, with 32 deaths. A total of 43 patients (89.6%) were HIV-positive and had a median CD4 + level of 193.5 cells/mm3 at diagnosis. Patients that did not present a drop in CD4 + levels after treatment had better OS than those that did (p = 0.020). 11 patients underwent radiotherapy (22.9%) who had better OS than those who did not (p = 0.015). Our findings show that adult patients living with HIV presenting Burkitt lymphoma who maintained their immune status throughout treatment had better prognosis than those who presented CD4 + cells drops. Also, patients treated with radiotherapy—either with palliative intent or as consolidation after chemotherapy—had statistically significant better OS than those not irradiated. Prospective data is warranted for radiotherapy as a consolidative and as a palliative treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Burkitt lymphoma’s treatment is still a challenge. The results are still more modest than those of other B-cell lymphomas and toxicities are frequently limiting [1]. The use of rituximab [2] in this disease has shown improvements in survival and changes in the cytotoxic chemotherapy, with special focus on the cytarabine-containing regimens such as Hyper-CVAD [3] and CODOX [4], and the persistent use of modifications of the EPOCH [5] regimen, has improved survival results. Toxicities, however, are still frequent and increased with the use of more toxic pediatric chemotherapy regimens in the adult population that frequently cannot be tolerated. Patients that are living with the HIV virus are particularly vulnerable to those effects [3].
Radiotherapy (RT) use in the treatment of Burkitt lymphoma has decreased. A recent SEER retrospective analysis [1] has shown that RT has been used less than in the past, notably due to the improvements in chemotherapy. Some still argue a place for RT at sanctuary sites such as the central nervous system (CNS) and the testis [6], but those patients are usually not referred to a radiation oncologist for consultation.
Even though seldom used, RT is effective against Burkitt lymphoma cells. It has been long proposed as part of its treatment [7], particularly in countries with the endemic form of the disease. Cell culture research has shown that the proliferative aspect of the Burkitt lymphoma cell make it especially sensitive to RT, and to combinations with drugs, such as rituximab [8]. Inside the field of radiobiology, Burkitt lymphoma cells have shown sensitivity to hyperfractionated [9] regimens and some have been clinically tested.
This research aims to retrospectively analyze how RT influenced oncological outcomes in the context of adult Burkitt lymphoma and how RT can be a confounding factor when the influence on survival results of different aspects and patients’ characteristics is assessed.
Methods
We retrospectively reviewed the charts of all adult patients treated for Burkitt lymphoma in our institution to analyze radiotherapy use from 2008 to 2019. Only patients with proven-biopsy diagnosis and at least six months of follow up were included. Three patients who were treated before 2008 (one in 2002 and two in 2004) who had late relapse (over 5 years) and were treated as de novo patients were also included because they met inclusion criteria of being referred to treatment between 2008 and 2019. Patients that did not receive intended treatment and died before any treatment could be given were excluded. All patients were staged accordingly to St Jude/Murphy staging system [10] and usually with 18FDG-PET scans when possible since HIV positive patients could not have the exam authorized.
Overall survival (OS) and progression-free survival (PFS) were measured from patients’ diagnostic biopsy date until event. Survival was analyzed with Kaplan-Meier method, univariate was made with Log-Rank test and multivariate analysis was made with Cox regression models. Toxicities were retrospectively assessed accordingly with the CTCAE 4.0 NCI criteria [11].
This research was submitted to local ethics committee and consent was given in June 2018. We were granted a waiver to request each patient consent due to the retrospective study nature by the ethics committee.
Results
Forty-eight patients were available for analysis after exclusion criteria was assessed. Median age at diagnoses was 36.9 years (18–62). The male/female ratio was 4.3 males to every female patient. Most patients had a good performance status at first appointment (56.2% were ECOG 0). Four patients did not have performance for chemotherapy and were treated with best life support only. Those were excluded from analysis. Most patients (91.6%) had advanced stage disease and most were stage IV (54.2%) and well balanced between the groups that did receive and did not receive radiotherapy. Risk stratification was done according to the SEER stratification system [12] and showed that one patient was low-intermediate risk, one high risk and all the other 46 patients were high-intermediate risk. Table 1 sums up patients’ demographics.
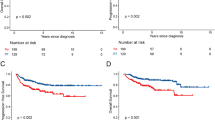
Mean follow-up was 31.5 months (0.5–187.5). Median overall survival and progression-free survival were 8.4 months (interquartile range Q1–Q3: 3.96–152.2) and 8.3 months (interquartile range Q1–Q3: 6.7-not reached), respectively, with 32 deaths reported. Table 2 contains results for univariate and multivariate analysis of variables impact on overall survival. Figure 1 describes progression-free survival.
Chemotherapy consisted mostly of schemes containing high doses of anthracyclines and cytarabine as recommended. The most common chemotherapy scheme was Hyper-CVAD (75.0%), a regimen highly recommended for aggressive non-Hodgkin lymphomas. A minority (17.7%) of patients received regimens not recommended as CHOP, primary older patients or patients with recently diagnosed HIV infection with active opportunistic diseases. Immunotherapy was seldom used and always in HIV negative patients, even though appropriate and feasible [13].
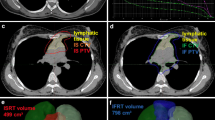
Radiotherapy (RT) was given to 11 patients (22.9%) who had better OS than those who did not receive it (p = 0.015, Fig. 2). 4 patients that received RT had curative intent, either consolidative RT or prophylactic to sanctuary sites. Patients that receive RT with curative intent were treated with 30–36 Gy involved-site in 1.8–2 Gy fraction. All patients were treated after chemotherapy was completed. The other 7 patients received it as part of their palliative treatment after progression and were treated with varied schedules of radiotherapy, from 30 to 40 Gy in fraction of 1.8–3 Gy/fraction. Table 3 describes radiotherapy schemes and doses.
Response to chemotherapy did not impact on radiotherapy selection neither comparing responses against progression (p = 0.072) nor comparing complete response against present disease (p = 0.970). Response to first line chemotherapy was assessed and it was a variable in the univariate and multivariate analysis. The multivariate analysis showed that, even though response to chemotherapy has a significant impact on survival, it was independent to the impact of radiotherapy. The small sample size could interfere in this assessment.
Patients living with HIV were 89.6% (43 patients). Patients that were HIV negative were classified as sporadic disease and did not have other immunodeficiency reported. Median CD4 + level was of 193.5 cells/mm3 at diagnosis. Patients that did not present a drop in CD4 + levels after treatment had better overall survival (OS) than those that did (p = 0.020, Fig. 3).
Toxicities were very high with chemotherapy, but not with radiotherapy. Table 4 details treatment toxicities. As for RT, toxicities were very limited and most frequently manageable skin and gastrointestinal (GI) toxicities. Only two patients had grade 2 GI toxicities and no patient had any grade 3 or greater toxicities related to RT.
People living with HIV were a large part of the sample. 43 patients (89.6%) were people living with HIV so HIV-related variables were analyzed. HIV itself did not impact survival (p = 0.181). HIV was an important selection bias considering staging system, since until 2014 18FDG-PET scans were not available to those patients at our public health system and there were also concerns about using rituximab in this population until proper prospective data was published [14]. Median CD4 + level at diagnosis was 193.5 cells/mm3. A comparison between CD4 + cell levels prior to oncological treatment and after treatment was made to assess whether the CD4 + cell count had fallen to levels inferior to those influenced only by HIV and HAART and not by chemotherapy and radiotherapy. This drop would represent a toxicity from oncological treatment in patients CD4 + cells and could impact negatively on survival. Patients that did not present any drop in CD4 + levels after treatment had better overall survival (OS) than those that did (p = 0.020). Most patients (52.1%) were long-time users of highly active anti-retroviral therapy (HAART) but that did not influence survival (p = 0.063). HIV characteristics can be found in Table 5.
Discussion
Radiotherapy is rarely given to Burkitt lymphoma patients. Prospective data shows that rituximab is standard of care and it is the drug that can mostly contribute to a satisfactory outcome in this disease [15]. After that, radiotherapy has not been tested in prospective trials. The role for radiotherapy in the prophylaxis of sanctuary sites, such as testis and central nervous system (CNS) has been traded for intrathecal chemotherapy after small prospective trials [16]. The use of RT among Burkitt lymphoma patients is already very rare and it is diminishing [1] due to the lack of positive data. Nevertheless, the role of radiotherapy has never been established in prospective data.
Our numbers show a large institutional cohort that has resorted to radiotherapy in two different situations: as a prophylaxis for sanctuary sites, especially testis since CNS has been primarily been treated with intrathecal chemotherapy and as part of palliative care. Burkitt lymphoma is known to have good responses to radiation, particularly because of its increased duplication rate, been particularly sensitive to hyperfractionated radiotherapy [7]. This can be an opportunity for the use of radiotherapy in this scenario, where quick responses with mild toxicities are the best choice of treatment. Our data has supported that hypothesis that should be tested in larger and prospective studies.
HIV has also been shown to be an important cause of morbidity in these patients. Cell count for CD4 + lymphocytes had an impact on survival and it is both a result of HIV infection and a consequence for oncological treatment. Patients that were long time user of HAART had a more regular testing on those levels but those who were recently diagnosed did not. Frequently HIV testing was performed after histological diagnosis was made and sometimes during chemotherapy. The main cause of CD4 + cell count drop not being reported was lack of the basal level of this variable before treatment and accounted for 68.7% of patients. Therefore, even though positive, those results should be seen with caution.
Conclusion
This is a relatively large retrospective institutional cohort on the use of radiotherapy in the treatment of Burkitt lymphoma. Our data has generated the hypothesis that radiotherapy can still have a role. Prospective data is warranted regarding the role of radiotherapy as a consolidative therapy, as a therapy for sanctuary sites and as a palliative treatment.
Data Availability
Data on this research is available on request to the corresponding author.
References
Liu ZL, Liu PP, Bi XW, Lei DX, Wang Y, Li ZM, Jiang WQ, Xia Y (2019) Trends in survival of patients with stage I/II Burkitt lymphoma in the United States: a SEER database analysis. Cancer Med 8(3):874–881
Hoelzer D, Walewski J, Döhner H, Viardot A, Hiddemann W, Spiekermann K, Serve H, Dührsen U, Hüttmann A, Thiel E, Dengler J (2014) Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial Blood. J Am Soc Hematol 124(26):3870–3879
Ribera JM, García O, Grande C, Esteve J, Oriol A, Bergua J, González-Campos J, Vall-llovera F, Tormo M, Hernández-Rivas JM, García D (2013) Dose-intensive chemotherapy including rituximab in Burkitt’s leukemia or lymphoma regardless of human immunodeficiency virus infection status: final results of a phase 2 study (Burkimab). Cancer 119(9):1660–1668
Lacasce A, Howard O, Li S, Fisher D, Weng A, Neuberg D, Shipp M (2004) Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymphoma 45(4):761–767
Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, Widemann B, Staudt LM, Jaffe ES, Little RF, Wilson WH (2013) Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med 369(20):1915–1925
Rizzieri DA, Johnson JL, Niedzwiecki D, Lee EJ, Vardiman JW, Powell BL, Barcos M, Bloomfield CD, Schiffer CA, Peterson BA, Canellos GP (2004) Intensive chemotherapy with and without cranial radiation for Burkitt leukemia and lymphoma: final results of Cancer and Leukemia Group B Study 9251. Cancer 100(7):1438–48
Norin T, Onyango J (1977) Radiotherapy in Burkitt’s lymphoma conventional or superfractionated regime—early results. Int J Radiat Oncol* Biol* Phys 2(5–6):399–406
Fengling M, Fenju L, Wanxin W, Lijia Z, Jiandong T, Zu W, Xin Y, Qingxiang G (2009) Rituximab sensitizes a Burkitt lymphoma cell line to cell killing by X-irradiation. Radiat Environ Biophys 48(4):371–378
Charafeddine K, Hilal L, Bazarbachi A, Salame N, Youssef B (2017) Hyperfractionated radiation therapy in Burkitt’s lymphoma: a reconsideration aspect. Hematol Oncol 35(4):856–860
Blum KA, Lozanski G, Byrd JC (2004) Adult Burkitt leukemia and lymphoma. Blood 104(10):3009–3020
Common terminology criteria for adverse events v4.0 (CTCAE). Publish Date: May 28, 2009. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
Castillo JJ, Winer ES, Olszewski AJ (2013) Population-based prognostic factors for survival in patients with Burkitt lymphoma: an analysis from the surveillance, epidemiology, and end results database. Cancer 119(20):3672–3679
Haidenberger A, Fromm-Haidenberger S, de Vries A, Popper BA, Steurer M, Skvortsova I, Kantner J, Gunsilius E, Lukas P (2011) Feasibility and toxicity of concomitant radio/immunotherapy with MabThera (Rituximab®) for patients with non-hodkin’s lymphoma. Strahlenther Onkol 187(5):300–305
Noy A, Lee JY, Cesarman E, Ambinder R, Baiocchi R, Reid E, Ratner L, Wagner-Johnston N, Kaplan L (2015) AMC 048: modified CODOX-M/IVAC-rituximab is safe and effective for HIV-associated Burkitt lymphoma Blood. J Am Soc Hematol 126(2):160–166
Ribrag V, Koscielny S, Bosq J, Leguay T, Casasnovas O, Fornecker LM, Recher C, Ghesquieres H, Morschhauser F, Girault S, Le Gouill S (2016) Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: a randomised, controlled, open-label, phase 3 trial. The Lancet 387(10036):2402–2411
Corazzelli G, Frigeri F, Russo F, Frairia C, Arcamone M, Esposito G, De Chiara A, Morelli E, Capobianco G, Becchimanzi C, Volzone F (2012) RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’highly aggressive B-cell lymphoma. Br J Haematol 156(2):234–244
Acknowledgements
We thank Dr. Antonio Brandão, hematologist, for his efforts and help.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
RF and GM were responsible for study design and ethics committee approval. RF, CM and LC were responsible for data collection. RF and GM have written project’s final draft. GM were responsible for statistics analysis. EW was responsible for overall orientation and manuscript review.
Corresponding author
Ethics declarations
Conflict of interests
The authors do not have any conflict of interest to declare.
Ethics Approval
Ethics committee authorization was obtained in the local ethics committee according to Brazilian law and the Declaration of Helsinki. All patients have given written consent to participate.
Consent for Publication
The author grants the publisher the sole and exclusive license of the full copyright. The authors guarantee that this manuscript has not been previously published elsewhere. The authors declare that any person named as co-author of the contribution is aware of the fact and has agreed to being so named.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pereira, R.F., Mauro, G.P., Medici, C.T.M. et al. Radiotherapy in Adult Burkitt Lymphoma: A Retrospective Analysis in a Large University Center. Indian J Hematol Blood Transfus 38, 508–515 (2022). https://doi.org/10.1007/s12288-021-01495-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-021-01495-w