Abstract
Approximately 40% of limited-stage (stage I and II) diffuse large B-cell lymphoma (LS-DLBCL) presents with extranodal disease. Extranodal LS-DLBCL may have significant biological differences and associated with worse outcomes than nodal disease. Although rituximab based chemoimmunotherapy is standard of first-line treatment, the role of consolidative radiotherapy (RT) in this particular subgroup is controversial. In this multicenter retrospective study, we evaluated the survival benefit of consolidative RT in patients diagnosed with extranodal LS-DLBCL and received rituximab-based chemoimmunotherapy with or without consolidative RT. A total of 328 patients were included, 129 patients (39.3%) received chemoimmunotherapy and consolidative RT, and 199 patients (60.7%) received chemoimmunotherapy alone. With a median follow-up of 5.1 years (range, 0.3–14.8 years), 5-year progression-free survival (PFS) and overall survival (OS) for all patients were 75.4% and 83.9%, respectively. In multivariate analyses, the addition of consolidative RT was associated with superior OS (P = 0.004) and PFS (P = 0.005). High stage-modified International Prognosis Index (SM-IPI) risk predicted worse OS (P = 0.001) and PFS (P = 0.005). Also, propensity score-matched analyses showed RT improved both OS (hazard ratio [HR] 0.228, 95% confidence index [CI] 0.111–0.467, P < 0.001) and PFS (HR 0.308, 95% CI 0.167–0.566, P < 0.001). Among patients who achieved CR, 49 patients (16.6%) developed disease relapse, of which 30.6% relapsed at local sites. Consolidative RT significantly reduced relapse risk (P = 0.002). Our results demonstrated that consolidative RT significantly improved outcomes in patients with extranodal LS-DLBCL in the rituximab era.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma, with 25–30% of patients presenting as a limited-stage disease (stage I and II) [1, 2]. Although most DLBCL arises in lymph nodes, approximately 40% of limited-stage DLBCL (LS-DLBCL) arises in a variety of organs, such as the gastrointestinal tract, sinus/nose, breast, testis, thyroid, and many others [3, 4]. Extranodal DLBCL exhibits heterogeneous clinical and molecular features, with a subset of patients showing a more aggressive course with relapses and inferior outcomes compared to nodal disease [3, 5,6,7,8]. Specifically, extranodal involvement in sites such as the breast and uterus demonstrate a high prevalence of the ABC phenotype and the MCD (MYD88/CD79b mutated) genomic subtype, which might be associated with an increased risk of central nervous system relapse [9,10,11].
Patients with LS-DLBCL generally have an excellent prognosis treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) [12,13,14,15,16], which has led to further question the role of consolidative radiotherapy (RT) in the rituximab era. The single-arm phase II study SWOG S0014 evaluated 3 cycles of R-CHOP with RT in LS-DLBCL patients with at least one stage-modified International Prognosis Index (SM-IPI) adverse risk factor. The 4-year progression-free survival (PFS) and 4-year overall survival (OS) rates were 92% and 88%, respectively [17]. Despite the inclusion of patients with more adverse risk factors, S0014 study appeared to improve outcomes compared to the historical SWOG cohort [18]. In addition, several retrospective studies have investigated the impact of consolidative RT in LS-DLBCL and observed continued benefit even in the rituximab era [19, 20]. However, the prospective LYSA/GOELAMS trial showed that R-CHOP alone is not inferior to R-CHOP followed by RT in patients with non-bulky LS-DLBCL [14]. The S1001 study prospectively evaluated a PET-CT adapted approach in patients with non-bulky LS-DLBCL. Interim PET (iPET) scanning was performed after three cycles of R-CHOP treatment. Patients with a negative iPET received an additional cycle of R-CHOP, while those with a positive iPET received radiotherapy (RT) plus ibritumomab tiuxetan radioimmunotherapy. The results indicated no statistical difference between the iPET-negative and iPET-positive groups [16]. It is important to note that in most studies, only 30–40% of patients included had extranodal involvement, or extranodal disease was not analyzed separately. Thus, extrapolating these findings to extranodal LS-DLBCL may not have the same outcomes.
Given recent studies have attempted to omit consolidative RT in LS-DLBCL and the benefit of consolidative RT in extranodal LS-DLBCL remains controversial, we undertook this multicenter retrospective study and aimed to clarify the role of consolidative RT in extranodal LS-DLBCL in the rituximab era.
Materials and methods
Patients
In this multicenter retrospective study, all patients with newly diagnosed extranodal LS-DLBCL treated with rituximab-based chemoimmunotherapy with or without consolidative RT were eligible for inclusion in the analysis. Data were collected from 5 institutes in China, with patients treated between January 2008 and December 2020. LS-DLBCL was defined as stage I or non-bulky disease stage II disease according to Ann Arbor classification [18]. The initial staging was assessed using positron emission tomography (PET-CT), contrast-enhance computed tomography (CT), or magnetic resonance imaging (MRI). All patients underwent a bone marrow biopsy. Lymphoma limited to lymph nodes, Waldeyer ring, thymus, or spleen was considered as a nodal disease and excluded. Patients with primary mediastinal lymphoma, primary central nervous system (CNS) lymphoma, and transformation of a previous indolent lymphoma were also excluded. Bulky disease was defined as any mass > 10 cm in maximum dimension. SM-IPI is based on age > 60 years, elevated lactated dehydrogenase (LDH), performance status ≥ 2, and stage II or IIE [18]. Cell of origin (COO) was classified as eighter germinal B-cell like (GCB) phenotype or non-GCB according to the Hans algorithm [21]. Staging workup and initial treatment for patients were performed according to local clinician discretion. Independent or centralized pathologic verification of diagnosis was not performed since all specimens had already undergone review by an expert hematopathologist. This study was approved by the institutional review board and was conducted in accordance with the Declaration of Helsinki.
Statistical methods
Patient characteristics were compared using the Pearson’s Χ2 test or Fisher’s exact test for categorical variables, and the Mann-Whitney U-test for continuous variables. OS was defined as the time from the initial diagnosis to death from any cause or to the last follow-up. PFS was defined as the time from the initial diagnosis to disease progression or death from any cause. Time to relapse was calculated from the date of diagnosis to the date of relapse, progression, or last follow-up. The median follow-up time was estimated by the reverse Kaplan-Meier method [22]. OS and PFS were calculated by the Kaplan–Meier method. Survival outcomes were compared using the log-rank test. Univariate and multivariate analyses for PFS and OS were performed using the Cox proportional hazard model. Competing risk analyses were performed using the Fine & Gray method, with death without relapse as competing events [23]. Significant variables (P < 0.1) in univariate analysis were included in multivariate analysis. Propensity score-matching with the nearest neighbor method with a caliper width of 0.2 was used to match patients treated with consolidative RT to those treated without. A two-tailed P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistics (version 26.0) and R version 4.3.1.
Results
Patient characteristics
We identified 328 patients with newly diagnosed extranodal LS-DLBCL. The baseline characteristics and a comparison of characteristics between patients who received consolidative RT and those who did not are summarized in Table 1. The median age at diagnosis was 54 years (range, 18–88 years). By Ann Arbor staging system, 129 patients (39.3%) were diagnosed with stage IE disease, and 199 patients (60.7%) with stage IIE disease. According to SM-IPI, 36.3% of patients were classified as high-risk [2,3,4]. The most common sites of extranodal disease at diagnosis were as follows: stomach (21.0%), intestine (18.6%), sinus/nose (17.7%), and breast (15.5%) (Supplementary Table S1). With the exception of patients who underwent complete resection, baseline characteristics in patients who received consolidative RT or those who did not were not significantly different.
Treatment and response
A total of 84 patients (25.6%) underwent complete surgical resection. In terms of the first-line chemotherapy regimen, 310 (94.5%) received R-CHOP, 12 (3.7%) received R-EPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin), and 6 (1.8%) received R-CHOPE (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone, and etoposide). The median number of chemoimmunotherapy cycles overall was 6, with 74.4% (n = 244) of patients receiving 6–8 cycles. At the end of chemoimmunotherapy, 296 patients (90.2%) achieved complete response (CR), 20 patients (6.1%) achieved partial response (PR) and 12 patients (3.7%) experienced progressive disease (PD) during the initial treatment. Consolidative RT was given to 114 of the 296 patients who achieved CR, and to 15 patients who achieved PR. The median RT dose for patients who achieved CR was 36.0 Gy (range, 30.0–40.0 Gy). Patients were most likely to receive consolidative RT with primary thyroid (77.3% of 22), sinus/nose (46.6% of 58), breast (45.1% of 51), and stomach (36.2% of 69) involvement.
Survival outcome
With a median follow-up time of 5.1 years (range, 0.3–14.8 years), the 5-year PFS rate among all patients was 75.4% (95% CI 70.4%-80.8%) and the OS rate was 83.9% (95% CI 79.6%-88.4%), respectively. Fifty-five patients died during the follow-up period. The cause of death was lymphoma related in 46 patients, and non-lymphoma related in 9 patients.
On univariate analysis of all patients, elevated LDH, SM-IPI score, and consolidative RT significantly affected both PFS and OS. Stage II disease is also associated with inferior PFS (Supplemental Table 2).
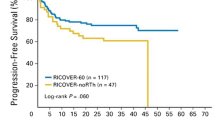
Consolidative RT significantly improved the 5-year OS (90.0% vs. 80.0%, P = 0.002) and PFS rates (84.6% vs. 68.7, P = 0.003) compared with no RT (Fig. 1A and B). In the subgroup of patients who achieved a CR, consolidative RT was still associated with better 5-year OS (93.7% vs. 87.6%, P = 0.011) and PFS (89.9% vs. 74.7%, P = 0.001) compared with no RT.
Survival outcomes for patients with extranodal LS-DLBCL. (A) Overall survival by the administration of consolidative RT or not. (B) Progression-free survival by the administration of consolidative RT or not. (C) Overall survival by SM-IPI risk groups. (D) Progression-free survival by SM-IPI risk groups
Patients with high SM-IPI risk had significantly worse OS (5-year, 77.3% vs. 88.1%, P = 0.005) and PFS (5-year, 68.9% vs. 79.8%, P = 0.005) compared to those with low SM-IPI risk (Fig. 1C and D).
In multivariate analyses (Table 2), patients who received consolidative RT had a superior OS (HR 0.399, 95% CI 0.213–0.747, P = 0.004) and PFS (HR 0.477, 95% CI 0.285–0.798, P = 0.005) compared to those who did not receive RT. High SM-IPI risk predicted inferior OS (HR 2.389, 95% CI 1.397–4.086, P = 0.001) and PFS (HR 1.929, 95% CI 1.224–3.039, P = 0.005).
Propensity score-matched analysis
Propensity score-matched analysis was performed to compare the outcome of patients treated with or without consolidative RT. A total of 127 patients in each group were matched based on sex, SM-IPI score, and COO. Among propensity score-matched patients, multivariate analysis showed the addition of consolidative RT still improved both OS (HR 0.228, 95% CI 0.111–0.467, P < 0.001) and PFS (HR 0.308, 95% CI 0.167–0.566, P < 0.001) than those who did not receive RT. The 5-year OS and PFS for patients who received consolidative RT and those who without were 90.0% vs. 78.9% and 84.6% vs. 67.2%, respectively. SM-IPI was an independent prognostic factor for OS (HR 4.738, 95% CI 2.380–9.430, P < 0.001) and PFS (HR 3.185, 95%CI 1.721–5.894, P < 0.001).
Pattern of relapses
During the follow-up period, 49 of 296 (16.6%) patients who achieved a CR developed disease relapse. Fifteen patients (30.6%) had relapsed at local sites, 31 patients (63.3%) at distant sites, and 3 patients (6.1%) at both local and distant sites. The majority of relapse (n = 45, 91.8%) occurred within 5 years, and 55.1% of patients (n = 27) developed relapse within the first 2 years. Consolidative RT significantly reduced the cumulative risk of relapse (P = 0.002). The 2- and 5-year cumulative risk of relapse in patients treated with consolidative RT and without RT were 3.6% vs. 12.8% and 10.0% vs. 24.6%, respectively (Fig. 2). Among the patients who received consolidative RT, no patients experienced failures within the radiation field.
Discussion
In present multicenter retrospective study, we specially evaluated the survival benefit of consolidative in patients with extranodal LS-DLBCL. Our results indicate that consolidative RT significantly improved survival outcomes and reduced relapse risk for patients with extranodal LS-DLBCL even in the rituximab era. We also confirmed that SM-IPI had robust prognostic utility for patients with extranodal LS-DLBCL.
Although R-CHOP with or without consolidative RT remains common practice for LS-DLBCL, no randomized trial has clearly demonstrated the survival benefit for extranodal LS-DLBCL in the rituximab era. The role of consolidative RT in this special subgroup is contentious. The UNFOLDER trial randomized patients to R-CHOP with or without RT to bulky and/or extranodal disease. However, the trial was stopped early due to a higher rate of treatment failure in the arm without RT, which indicated additional treatment were required in those patients [24]. Some retrospective studies have evaluated the role of consolidative RT on outcome in extranodal LS-DLBCL and the results were inconsistent [6, 19, 25, 26]. In a recent retrospective study restricted to patients with stage I DLBCL, a subgroup analysis of patients with extranodal disease suggested a benefit of adding RT to chemoimmunotherapy [6]. These discrepancies might be due to different patient populations including stage and extranodal disease sites, small sample size, and radiation dose. In the current study, we demonstrated that even in the rituximab era, the use of consolidative RT significantly improved both OS and PFS for patients with extranodal LS-DLBCL. After performing propensity score-matched analysis, consolidative RT was still associated with a significant improvement in outcome. In addition, consolidative RT showed better local control at the original extranodal site and reduced relapse risk compared to chemoimmunotherapy alone. Considering there is a tendency to forego RT in LS-DLBCL patients, our results suggest caution against the omission of consolidative RT in patients with extranodal LS-DLBCL.
Patients with LS-DLBCL generally have excellent survival and those at risk for inferior prognosis were difficult to identify, especially for extranodal LS-DLBCL. Only a few retrospective studies identified several prognostic factors with a small sample size and heterogeneous composition and treatment [6, 27, 28]. We confirmed SM-IPI was an independent factor for extranodal LS-DLBCL in the rituximab era, which was consistent with the previous study [6]. According to SM-IPI, we identified two main risk groups: patients with low SM-IPI risk (0–1) have excellent outcomes and 5-year OS and PFS rates of 88.1% and 79.8%, while patients with high SM-IPI risk [2,3,4] have worse 5-year PFS and OS rates of 77.3% and 68.9%.
In recent years, several studies reported a continuous pattern of relapse beyond 5 years in LS-DLBCL, which is in contrast to advanced disease with most relapses occurring within the first 2 years after frontline treatment [12, 15, 29]. However, in this study, we observed most relapses (91.8%) occurred within 5 years, and 55.1% of relapses occurred within the first 2 years. These distinct relapse patterns may indicate biological differences between nodal and extranodal LS-DLBCL, with the extranodal disease having more aggressive behavior. However, these observations need to be confirmed with longer follow-up and further generic study.
This study has several limitations. As its retrospective nature, selection bias may exist. The small number of each extranodal disease site makes it difficult to analyze the prognostic value of extranodal location. However, we performed propensity score-matched analysis to overcome potential bias between the groups. Given the lack of a prospective study, this real-world experience may support clinical management decisions and help to guide further prospective trials.
Our study showed that the addition of consolidative RT improved outcomes of patients with extranodal LS-DLBCL even in the rituximab era. A further prospective trial is necessary to confirm the role of consolidative RT in extranodal LS-DLBCL and better define the group of patients who will benefit from consolidative RT.
Data availability
No datasets were generated or analysed during the current study.
References
A clinical evaluation of the International Lymphoma Study Group (1997) Classification of non-hodgkin’s lymphoma. The Non-hodgkin’s lymphoma classification project. Blood 89(11):3909–3918
Liu Y, Barta SK (2019) Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol 94(5):604–616. https://doi.org/10.1002/ajh.25460
Møller MB, Pedersen NT, Christensen BE (2004) Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation–a population-based study of 1575 cases. Br J Haematol 124(2):151–159. https://doi.org/10.1046/j.1365-2141.2003.04749.x
Krol AD, Hermans J, Dawson L, Snijder S, Wijermans PW, Kluin-Nelemans HC et al (1998) Treatment, patterns of failure, and survival of patients with Stage I nodal and extranodal non-hodgkin’s lymphomas, according to data in the population-based registry of the Comprehensive Cancer Centre West. Cancer 83(8):1612–1619. https://doi.org/10.1002/(sici)1097-0142(19981015)83:8<1612::aid-cncr17>3.0.co;2-g
López-Guillermo A, Colomo L, Jiménez M, Bosch F, Villamor N, Arenillas L et al (2005) Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncology: Official J Am Soc Clin Oncol 23(12):2797–2804. https://doi.org/10.1200/jco.2005.07.155
Bobillo S, Joffe E, Lavery JA, Sermer D, Ghione P, Noy A et al (2021) Clinical characteristics and outcomes of extranodal stage I diffuse large B-cell lymphoma in the Rituximab era. Blood 137(1):39–48. https://doi.org/10.1182/blood.2020005112
Ryan G, Martinelli G, Kuper-Hommel M, Tsang R, Pruneri G, Yuen K et al (2008) Primary diffuse large B-cell lymphoma of the breast: prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Annals Oncology: Official J Eur Soc Med Oncol 19(2):233–241. https://doi.org/10.1093/annonc/mdm471
Deng L, Xu-Monette ZY, Loghavi S, Manyam GC, Xia Y, Visco C et al (2016) Primary testicular diffuse large B-cell lymphoma displays distinct clinical and biological features for treatment failure in Rituximab era: a report from the International PTL Consortium. Leukemia 30(2):361–372. https://doi.org/10.1038/leu.2015.237
Cao XX, Li J, Cai H, Zhang W, Duan MH, Zhou DB (2017) Patients with primary breast and primary female genital tract diffuse large B cell lymphoma have a high frequency of MYD88 and CD79B mutations. Ann Hematol 96(11):1867–1871. https://doi.org/10.1007/s00277-017-3094-7
Shen R, Xu PP, Wang N, Yi HM, Dong L, Fu D et al (2020) Influence of oncogenic mutations and tumor microenvironment alterations on extranodal invasion in diffuse large B-cell lymphoma. Clin Transl Med 10(7):e221. https://doi.org/10.1002/ctm2.221
Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ et al (2018) Genetics and Pathogenesis of diffuse large B-Cell lymphoma. N Engl J Med 378(15):1396–1407. https://doi.org/10.1056/NEJMoa1801445
Stephens DM, Li H, LeBlanc ML, Puvvada SD, Persky D, Friedberg JW et al (2016) Continued risk of Relapse Independent of treatment modality in Limited-Stage diffuse large B-Cell lymphoma: final and Long-Term Analysis of Southwest Oncology Group Study S8736. J Clin Oncology: Official J Am Soc Clin Oncol 34(25):2997–3004. https://doi.org/10.1200/jco.2015.65.4582
Poeschel V, Held G, Ziepert M, Witzens-Harig M, Holte H, Thurner L et al (2019) Four versus six cycles of CHOP chemotherapy in combination with six applications of Rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): a randomised, phase 3, non-inferiority trial. Lancet 394(10216):2271–2281. https://doi.org/10.1016/s0140-6736(19)33008-9
Lamy T, Damaj G, Soubeyran P, Gyan E, Cartron G, Bouabdallah K et al (2018) R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood 131(2):174–181. https://doi.org/10.1182/blood-2017-07-793984
Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P et al (2013) Rituximab plus Cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet 381(9880):1817–1826. https://doi.org/10.1016/s0140-6736(13)60313-x
Persky DO, Li H, Stephens DM, Park SI, Bartlett NL, Swinnen LJ et al (2020) Positron Emission Tomography-Directed therapy for patients with limited-stage diffuse large B-Cell lymphoma: results of Intergroup National clinical trials Network Study S1001. J Clin Oncology: Official J Am Soc Clin Oncol 38(26):3003–3011. https://doi.org/10.1200/jco.20.00999
Persky DO, Unger JM, Spier CM, Stea B, LeBlanc M, McCarty MJ et al (2008) Phase II study of Rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncology: Official J Am Soc Clin Oncol 26(14):2258–2263. https://doi.org/10.1200/jco.2007.13.6929
Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM et al (1998) Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-hodgkin’s lymphoma. N Engl J Med 339(1):21–26. https://doi.org/10.1056/nejm199807023390104
Phan J, Mazloom A, Medeiros LJ, Zreik TG, Wogan C, Shihadeh F et al (2010) Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncology: Official J Am Soc Clin Oncol 28(27):4170–4176. https://doi.org/10.1200/jco.2009.27.3441
Vargo JA, Gill BS, Balasubramani GK, Beriwal S (2015) Treatment selection and survival outcomes in early-stage diffuse large B-Cell lymphoma: do we still need consolidative Radiotherapy? J Clin Oncology: Official J Am Soc Clin Oncol 33(32):3710–3717. https://doi.org/10.1200/jco.2015.61.7654
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103(1):275–282. https://doi.org/10.1182/blood-2003-05-1545
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17(4):343–346. https://doi.org/10.1016/0197-2456(96)00075-x
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. Publications Am Stat Association 94(446):496–509
Pfreundschuh M, Murawski N, Ziepert M, Altmann B, Dreyling MH, Borchmann P et al (2018) Radiotherapy (RT) to bulky (B) and extralymphatic (E) disease in combination with 6xR-CHOP-14 or R-CHOP-21 in young good-prognosis DLBCL patients: Results of the 2x2 randomized UNFOLDER trial of the DSHNHL/GLA. J Clin Oncol 36(15suppl):7574. https://doi.org/10.1200/JCO.2018.36.15_suppl.7574
Stephens DM, Li H, Constine LS, Fitzgerald TJ, Leonard JP, Kahl BS et al (2022) Extranodal presentation in limited stage diffuse large B-cell lymphoma as a prognostic marker in three SWOG trials S0014, S0313 and S1001. Haematologica. https://doi.org/10.3324/haematol.2022.281004
Ermann DA, Vardell VA, Shah H, Tao R, Gaffney DK, Stephens DM et al (2021) Treatment Outcomes of Consolidative Radiation in Extranodal early-stage diffuse large B-Cell lymphoma. Blood 138:49. https://doi.org/10.1182/blood-2021-153197
Shen H, Wei Z, Zhou D, Zhang Y, Han X, Wang W et al (2018) Primary extra-nodal diffuse large B-cell lymphoma: a prognostic analysis of 141 patients. Oncol Lett 16(2):1602–1614. https://doi.org/10.3892/ol.2018.8803
Nijland M, Boslooper K, van Imhoff G, Kibbelaar R, Joosten P, Storm H et al (2018) Relapse in stage I(E) diffuse large B-cell lymphoma. Hematol Oncol 36(2):416–421. https://doi.org/10.1002/hon.2487
Pfreundschuh M, Kuhnt E, Trümper L, Osterborg A, Trneny M, Shepherd L et al (2011) CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) group. Lancet Oncol 12(11):1013–1022. https://doi.org/10.1016/s1470-2045(11)70235-2
Acknowledgements
We thank all the patients, their families, and the institutions for supporting this study.
Funding
This study was supported by the Outstanding Young Scientific and Technological Talents Fund of Sichuan Province (No. 2022JDJQ0059), the National Natural Science Foundation of China (No. 82003198 and No. 82270196), and the Cancer Innovative Research Program of Sun Yat-sen University Cancer Center [grant number CIRP-SYSUCC-0022].
Author information
Authors and Affiliations
Contributions
Tongyu Lin and Huangming Hong conceived the project and designed the research study. Huawei Weng and Le Yu analyzed and wrote the manuscript draft. Huangming Hong, Zegeng Chen, Huageng Huang, Liqun Zou, Xinggui Chen, Hongqiang Guo, and He Huang contributed participants and collected data. Tongyu Lin and Huangming Hong reviewed and editing the manuscript. All authors were involved in the review and revision of the manuscript. All authors approved the final version.
Corresponding authors
Ethics declarations
Ethical approval
This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (Guangzhou, China, No. B2021-470-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weng, H., Yu, L., Chen, Z. et al. Benefit of consolidative radiation in patients with extranodal limited-stage diffuse large B-cell lymphoma: a multicenter retrospective study in China. Ann Hematol (2024). https://doi.org/10.1007/s00277-024-05855-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00277-024-05855-0