Abstract
We aimed to demonstrate whether PET–CT can replace bone marrow biopsy in detecting bone marrow involvement in subtypes of lymphoma. In addition, we aimed to also reveal whether there is a difference between the mean survival of patients with bone marrow involvement via PET–CT or biopsy. A total of 276 newly diagnosed lymphoma patients who underwent bone marrow biopsy and PET–CT prior to the treatment were scanned retrospectively. Bone marrow biopsy was used as the standard method to investigate the presence of bone marrow involvement in PET–CT. The relationship between bone marrow involvement and mean survival was compared using both methods. Out of the 276 patients, bone marrow involvement was detected with PET–CT and with biopsy, respectively in 56 patients (20.2%) and in 78 patients (28.2%). In terms of PET–CT’s accuracy with respect to revealing bone marrow involvement, the highest rates were achieved respectively in diffuse large B cell lymphoma (DLBCL) (87.4%) and Hodgkin lymphoma (HL) (77.7%). In both the PET–CT and bone marrow biopsy methods, Overall Survival (OS) was found to be significantly shorter in patients with involvement than in patients without involvement (P: 0.001). PET–CT may replace bone marrow (BM) biopsy in detecting the bone marrow involvement in aggressive lymphoma subtypes such as DLBCL and HL. The presence of BM involvement at the time of diagnosis in both PET–CT and BM biopsy is associated with poor prognosis, and OS is short in this group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lymphomas, the prevalence of which increase nowadays, are clonal malignancies that originate from lymphocytes (T and B) and natural killer cells [1]. Lymphoma has various morphological, immunophenotypic and clinical prognoses depending on the cells from which it has originated. Demonstration of bone marrow involvement at the time of diagnosis is an important step in making the treatment decision. According to the Ann Arbor staging system, BM involvement increases the disease to a stage 4 disease and it is associated with short survival along with B symptoms [2]. PET–CT is widely used in staging of lymphoma patients with high sensitivity and specificity [3]. In addition, unilateral biopsy is routinely conducted on the posterior iliac crest to demonstrate the bone marrow involvement. However, the bone marrow involvement rate with bilateral biopsy is 10–60% more compared to the bone marrow involvement with unilateral biopsy [4, 5]. Screening of a limited region of the bone marrow leads to false negativity in patients with involvement pattern, such as focal involvement [6, 7]. Furthermore, bone marrow biopsy is a painful, hemorrhagic and anxiety-causing interventional procedure [8].
PET–CT is a non-invasive method. It demonstrates bone marrow involvement of various lymphoma subtypes with high sensitivity and specificity. Bone marrow infiltration manifests itself with greater FDG (fluorodeoxyglucose) uptake in the bone marrow than in the liver [9]. Both focal and diffuse bone marrow lesions can be detected by FDG-PET [10]. However, false positive results may occur in cases such as infection, inflammation, and anemia [11].
In this study, we aimed to compare the BM biopsy and PET–CT methods in the detection of bone marrow involvement in lymphoma patients, who have not been treated before, and to determine the lymphoma subtypes in which PET–CT can replace the BM biopsy. In this context, we wanted to determine whether there is a difference in survival between the groups, in which the bone marrow involvement was detected with either PET–CT or biopsy.
Materials and Methods
Patients
437 newly diagnosed lymphoma between July 2010 and December 2018 were retrospectively enrolled in this study from 1 tertiary center of Antalya. The inclusion criteria were recent diagnosis and histology proven Hodgkin lymphoma (HL) or non-Hodgkin lymphoma (NHL) and did not have other concomitant malignancies. Patients 18 years old and older were included in the study if both BMB and PET/CT were performed simultaneously as part of the routine pre-therapy. The maximum interval between PET/CT scan and BMB was 15 days. The staging of each patient was based on the Ann Arbor staging system. This retrospective study was approved by The Local Ethics Committee of our university and followed the principles of the Declaration of Helsinki.
Bone Marrow Biopsy
Unilateral BM biopsy was routinely obtained from the posterior iliac crest before any treatment was administered and interpretation was performed by experienced hematopathologist. The BMB specimens were analyzed by morphological and immunohistochemical studies. Flow cytometry or molecular analysis were not used for the analysis.
The 18F-FDG PET/CT Scanning
Patients were asked to fast for 4–6 h before 18F-FDG PET/CT scanning. Intravenous 0.1–0.2 mCi/kg 18F-FDG was administered to each patient with a blood glucose level below 120 mg/dL. Approximately 60 min after 18F-FDG injection, PET/CT scan from the vertex to the distal femur was performed with Siemens (Molecular Imaging, Siemens Medical Solutions Inc., Malvern, PA, USA) The result of each PET/CT was carefully reviewed by an experienced nuclear medicine physician. In visual assessment, bone marrow metabolic activity that is greater than the liver was considered to be bone marrow involvement.
Statistical Analysis
Clopper–Pearson exact confidence limits were calculated for sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy. The comparing difference between patients with PET–CT involvement according to diagnosis and overall survival values according to bone marrow biopsy involvement was performed by the Kruskal–Wallis test with post hoc analysis. The comparison of the subgroup data was performed using the Chi Square test. Overall survival analyzes were conducted by Kaplan–Meier method, whereas the difference between the groups was calculated by log-rank test. Statistical analysis was done using SPSS software (IBM SPSS Statistics 22, IBM Corporation, Chicago, IL). The P value for statistical significance was set to P < 0.05.
Results
Out of the 437 patients, whose files were retrospectively scanned, a total of 276 patients that underwent PET–CT for staging purposes and underwent unilateral BM biopsy prior to the treatment were included in the study. 127 of these patients had DLBCL (46.0%). The distribution of demographic and clinical characteristics according to the diagnoses is shown in Table 1. B symptoms were positive (71.1%) at most in patients diagnosed with DLBCL. At the time of diagnosis, low grade lymphomas are generally diagnosed as advanced stage (stage IV) (72.4%).
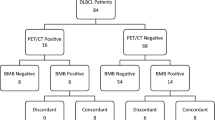
56 patients were found to have bone marrow involvement (20.2%) via the PET–CT and 78 patients were found to have bone marrow involvement (28.2%) via the bone marrow biopsy. When lymphoma subtypes were considered, PET–CT detected positive bone marrow involvement in more patients compared to biopsy, in the Hodgkin lymphoma group alone. Bone marrow involvement in terms of diagnoses via PET–CT and bone marrow biopsy is given in Table 2. Bone marrow biopsy didn’t detect any bone marrow involvement in 9 patients out of the 14 Hodgkin lymphoma patients, who were found to have bone involvement via PET–CT. On the other hand, PET–CT didn’t detect any bone marrow involvement only in 1 patient out of the 6 Hodgkin lymphoma patients, who were found to have bone involvement via the BM biopsy. The two methods have been found to be incompatible respectively in 16 of 127 patients diagnosed with DLBCL, in 17 of 46 patients diagnosed with FL, and in 19 of 58 patients diagnosed with low grade lymphomas [(NHL (Non-Hodgkin Lymphomas)].
Histopathological examination of bone marrow biopsy is the gold standard method in diagnosis in terms of specificity. Compared to the demonstration of bone marrow involvement using the bone marrow biopsy method, the specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV) and accuracy values calculated according to the patients’ diagnoses, in connection with the PET–CT method in terms of demonstration of bone marrow involvement are shown in Table 3. The subgroups, where PET–CT had the highest values in terms of sensitivity and specificity, were Hodgkin lymphoma (83.3%) and DLBCL (96.3%), respectively. The negative predictive value was found to be 96.7% in the Hodgkin lymphoma subgroup. When the accuracy of PET–CT in demonstrating the bone marrow involvement is considered, the highest rate was achieved in DLBCL (87.4%) and Hodgkin lymphoma (77.7%) subgroups.
In the group, in which bone marrow involvement was detected by both PET–CT and BM biopsy, overall survival (OS) rate in patients was found to be significantly short. (P:0.001). OS values that have been determined according to bone marrow involvement detected by both PET–CT and biopsy methods are given in Fig. 1. When the lymphoma subtypes were considered separately, the OS of the group, which has been diagnosed with DLBCL and detected to have bone marrow involvement via both PET–CT and biopsy, was found to be significantly shorter than it was in the other groups (P = 0.002). As summarized in Table 4, no significant relationship was found in the other groups.
The differences between the OS values determined on the basis of the patients’ diagnoses, according to whether the bone marrow involvement was detected by PET–CT and/or bone marrow biopsy were given in Table 4.
Discussion
The results of this single-center retrospective study revealed that PET–CT may have a high level of accuracy in detecting bone marrow involvement in patients with HL and DLBCL. Even though we accept bone marrow biopsy as the gold standard method in demonstrating the bone marrow involvement, the fact it yields false negative results in focal lesions is among the most important reasons that limit our study. However, biopsy is the only method that can be used to differentiate bone marrow involvement of lymphoma from the benign involvement.
The frequency of bone marrow involvement in the subtypes of lymphoma varies in various studies. Bone marrow involvement rate in HL patients was detected to be in the range of 6–14% in the previously conducted studies, whereas we found this rate to be 13.3% in our study [12, 13]. As it was the case in the previously conducted studies, bone marrow involvement rates detected via PET–CT in our studies were found to be higher than those detected via biopsy [12, 14,15,16]. Biopsy revealed bone marrow involvement in 6 patients, whereas PET–CT was positive in 5 (83.3%) of these patients. In our other studies, the sensitivity was detected as 7/9 (77.8%), 6/6 (100%) and 9/11 (81.8%), respectively. This indicates how good the sensitivity of the PET–CT method is. The high rates indicate that it is not an absolute must to perform a biopsy procedure in HL patients. Hence, the guideline as well no longer recommends the performance of bone marrow biopsy if PET–CT reveals any bone marrow involvement [17].
The BM involvement in patients diagnosed with DLBCL at the time of diagnosis varies between 11 and 27% [18, 19]; whereas in our study, we have detected 14.9% BM involvement in the DLBCL subgroup. Pakos et al. found as a result of their study that PET–CT had low sensitivity but high specificity in demonstrating bone marrow involvement in patients with DLBCL [20]. We, on the other hand, have determined the sensitivity to be very low, and the specificity to be high in line with the other studies available in the literature [21, 22]. In our study, NPV was found to be 89.6% and the accuracy was found to be 87.4%, which are both very high. These results support the studies of Teagle et al. and Voltin et al. In the light of these results, in the event that bone marrow involvement was observed in PET–CT, we are of the opinion that conducting a biopsy on these patients would not provide any additional contribution and would be the cause of an unnecessary morbidity instead. In a similar study, bone marrow biopsy of DLBCL patients is not recommended in centers with an experienced PET–CT team [23].
Bone marrow involvement of follicular lymphoma and other lymphoma subtypes is quite common. In some studies, this bone marrow involvement frequency was found to be over 50% [24,25,26]. In our study, we found the bone marrow involvement rates via biopsy and PET–CT respectively as 41.3% and 21.7% in follicular lymphoma and as 58% and 36.2% in other lymphoma subtypes. In two other studies involving follicular lymphoma patients, the rate of BM involvement via biopsy was found to be 44.4–49.3%, whereas the rate of BM involvement via PET–CT was found to be 23.9–28.9% [27, 28]. PET–CT has been found to have low sensitivity in two different studies involving patients of mantle cell lymphoma and marginal zone lymphoma, and it was concluded as a result that PET–CT cannot replace biopsy [29, 30]. In addition to all these studies, it has also been shown that BM involvement is more common in indolent lymphomas in general, and that PET–CT cannot replace biopsy in demonstrating BM involvement [31, 32].
FDG uptake in PET–CT increases as the histological grade of lymphomas increases [33]. Low or no FDG uptake in low grade lymphomas leads to false negative results on PET–CT. Lymphomas may have involvement patterns in the bone marrow such as paratrabecular, intrasinusoidal, diffuse, interstitial and nodular, and the frequency of these involvement patterns may vary according to subtypes [34]. The fact that diffuse pattern occurs more frequently in DLBCL increases the compatibility between PET–CT and biopsy results, whereas the more frequent occurrence of nodular pattern in marginal zone lymphoma or the more frequent occurrence of paratrabecular pattern in follicular lymphoma increases the incompatibility between PET–CT and biopsy results. If, in order to strengthen this compatibility, bilateral biopsy is performed instead of the biopsy conducted on unilateral iliac crest, BM involvement increases by 10–50% [6, 35, 36]. However, the drawback associated with DLBCL lymphomas is that diffuse BM involvement may occur in cases such as inflammation and cytokine release, and that it may lead to false positive PET–CT results [35]. To the contrary of this negative feature, a great advantage of PET–CT is its high sensitivity and specificity in demonstrating extranodal diseases [37, 38]. In addition to all these, PET–CT enables broad evaluation of the bone marrow and not just from a single region.
When the two methods were compared in all patients in terms of mean survival, OS was found to be significantly shorter in the group that had involvement via both PET–CT and biopsy. When the lymphoma subtypes were considered, OS was found to be significantly shorter only in the DLBCL group that had involvement via both PET–CT and biopsy. We think that it was the high number of patients in the DLBCL group, which has led to significant results only in this group. The presence of bone marrow involvement only in either one of the PET–CT or biopsy methods did not reveal any significant results in terms of OS in the entire lymphoma group. Karak et al. have found in their study they have conducted in patients with DLBCL that the fact that whether BM involvement is present on biopsy was not significant in terms of OS [39]. In addition, it was found in another study conducted in patients diagnosed with DLBCL that the fact that whether BM involvement is present in PET–CT was not significant in terms of OS [40]. Although our study was carried out in a high population in terms of number of patients, we think that it was the low number of patients with bone marrow involvement, which has led to insignificant results of BM involvement via biopsy in terms of OS. In a review including follicular lymphoma patients, OS was found to be significantly shorter in the group that had BM involvement via biopsy, compared to the group that did not have BM involvement via biopsy [26].
The biggest limitation of our study was that it was a retrospective study and that we had to use bone marrow biopsy as the gold standard method. In addition, the number of patients with bone marrow involvement in the subgroups other than the DLBCL subgroup was limited.
In conclusion, PET–CT may replace BM biopsy in demonstrating the bone marrow involvement in aggressive lymphoma subtypes such as DLBCL and HL. Bone marrow biopsy may not have to be performed in patients diagnosed with HL and DLBCL, who had BM involvement on PET–CT. On the other hand, biopsy remains the best method in patients with indolent lymphomas. The presence of BM involvement during diagnosis in both PET–CT and BM biopsy is associated with poor prognosis and OS is short in this group.
References
Swerdlow SH, Campo E, Pileri SA et al (2016) The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood 127(20):2375–2390. https://doi.org/10.1182/blood-2016-01-643569
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol 32(27):3059–3067. https://doi.org/10.1200/JCO.2013.54.8800
Schaefer NG, Hany TF, Taverna C et al (2004) Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging—do we need contrast-enhanced CT? Radiology 232(3):823–829. https://doi.org/10.1148/radiol.2323030985
Menon NC, Buchanan JG (1979) Bilateral trephine bone marrow biopsies in hodgkin and non-hodgkin lymphoma. Pathology 11(1):53–57. https://doi.org/10.3109/00313027909063538
Wang J, Weiss LM, Chang KL et al (2002) Diagnostic utility of bilateral bone marrow examination: significance of morphologic and ancillary technique study in malignancy. Cancer 94(5):1522–1531. https://doi.org/10.1002/cncr.10364
Moog F, Bangerter M, Kotzerke J, Guhlmann A, Frickhofen N, Reske SN (1998) 18-F-fluorodeoxyglucose-positron emission tomography as a new approach to detect lymphomatous bone marrow. J Clin Oncol 16(2):603–609. https://doi.org/10.1200/JCO.1998.16.2.603
Adams HJA, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RAJ, de Klerk JMH (2015) Diffusely increased bone marrow FDG uptake in recently untreated lymphoma: ıncidence and relevance. Eur J Haemato 95(1):83–89. https://doi.org/10.1111/ejh.12483
Bain BJ (2006) Morbidity associated with bone marrow aspiration and trephine biopsy—a review of UK data for 2004. Haematologica 91(9):1293–1294
Adams HJA, Kwee TC, De Keizer B, Fijnheer R, De Klerk JMH, Nievelstein RAJ (2014) FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 41(3):565–574. https://doi.org/10.1007/s00259-013-2623-4
Moog F, Bangerter M, Diederichs CG et al (1998) Extranodal malignant lymphoma: detection with FDG PET versus CT. Radiology 206(2):475–481. https://doi.org/10.1148/radiology.206.2.9457202
Sato M, Hiyama T, Kaito K, Hayashi Y, Okumura T (2009) Usefulness of F-18 FDG PET/CT in the assessment of disseminated Mycobacterium avium complex infection. Ann Nucl Med 23(8):757–762. https://doi.org/10.1007/s12149-009-0298-5
El-Galaly TC, D’Amore F, Mylam KJ et al (2012) Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol 30(36):4508–4514. https://doi.org/10.1200/JCO.2012.42.4036
O’Carroll DI, McKenna RW, Brunning RD (1976) Bone marrow manifestations of Hodgkin disease. Cancer 38(4):1717–1728. https://doi.org/10.1002/1097-0142(197610)38:4%3c1717:AID-CNCR2820380445%3e3.0.CO;2-9
Weiler-Sagie M, Kagna O, Dann EJ, Ben-Barak A, Israel O (2014) Characterizing bone marrow involvement in Hodgkin lymphoma by FDG-PET/CT. Eur J Nucl Med Mol Imaging 41(6):1133–1140. https://doi.org/10.1007/s00259-014-2706-x
Muzahir S, Mian M, Munir I et al (2012) Clinical utility of 18 F FDG-PET/CT in the detection of bone marrow disease in Hodgkin lymphoma. Br J Radiol 85(1016):490–496. https://doi.org/10.1259/bjr/29583493
Pelosi E, Penna D, Douroukas A et al (2011) Bone marrow disease detection with FDG-PET/CT and bone marrow biopsy during the staging of malignant lymphoma: results from a large multicentre study. Q J Nucl Med Mol Imaging 55(4):469–475
Eichenauer DA, Engert A, Federico M, Illidge T, Hutchings M, Ladetto M (2014) Clinical practice guidelines Hodgkin’s lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up † clinical practice guidelines. 25:1–6. https://doi.org/10.1093/annonc/mdu181
Chung R, Lai R, Wei P et al (2019) Concordant but not discordant bone marrow involvement in diffuse large B-cell lymphoma predicts a poor clinical outcome independent of the ınternational prognostic ındex. Blood J Am Soc Hematol 110(4):1278–1283. https://doi.org/10.1182/blood-2007-01-070300
Campbell J, Seymour JF, Matthews J, Wolf M, Stone J, Juneja S (2006) The prognostic impact of bone marrow involvement in patients with diffuse large cell lymphoma varies according to the degree of infiltration and presence of discordant marrow involvement. Eur J Haematol 76(6):473–480. https://doi.org/10.1111/j.1600-0609.2006.00644.x
Pakos EE, Fotopoulos AD, Ioannidis JPA (2005) 18F-FDG PET for evaluation of bone marrow infiltration in staging of lymphoma: a meta-analysis. J Nucl Med 46(6):958–963
Teagle AR, Barton H, Charles-Edwards E, Dizdarevic S, Chevassut T (2017) Use of FDG PET/CT in identification of bone marrow involvement in diffuse large B cell lymphoma and follicular lymphoma: comparison with iliac crest bone marrow biopsy. Acta Radiol 58(12):1476–1484. https://doi.org/10.1177/0284185117701305
Berthet L, Cochet A, Kanoun S et al (2013) In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med 54(8):1244–1250. https://doi.org/10.2967/jnumed.112.114710
Khan AB, Barrington SF, Mikhaeel NG et al (2013) PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood 122(1):61–67. https://doi.org/10.1182/blood-2012-12-473389
Canioni D, Brice P, Lepage E et al (2004) Bone marrow histological patterns can predict survival of patients with grade 1 or 2 follicular lymphoma: a study from the Groupe d’Etude des Lymphomes Folliculaires. Br J Haematol 126(3):364–371. https://doi.org/10.1111/j.1365-2141.2004.05046.x
Lee EYP, Gill H, Wang Y, Kwong YL, Khong PL (2015) Bone marrow uptake of indolent non-Hodgkin lymphoma on PET/CT with histopathological correlation. Nucl Med Commun 36(10):1035–1041. https://doi.org/10.1097/MNM.0000000000000361
Adams HJA, Nievelstein RAJ, Kwee TC (2015) Opportunities and limitations of bone marrow biopsy and bone marrow FDG-PET in lymphoma. Blood Rev 29(6):417–425. https://doi.org/10.1016/j.blre.2015.06.003
Luminari S, Biasoli I, Arcaini L et al (2013) The use of FDG-PET in the initial staging of 142 patients with follicular lymphoma: a retrospective study from the FOLL05 randomized trial of the Fondazione Italiana Linfomi. Ann Oncol 24(8):2108–2112. https://doi.org/10.1093/annonc/mdt137
Le Dortz L, De Guibert S, Bayat S et al (2010) Diagnostic and prognostic impact of 18F-FDG PET/CT in follicular lymphoma. Eur J Nucl Med Mol Imaging 37(12):2307–2314. https://doi.org/10.1007/s00259-010-1539-5
Hosein PJ, Pastorini VH, Paes FM et al (2011) Utility of positron emission tomography scans in mantle cell lymphoma. Am J Hematol 86(10):841–845. https://doi.org/10.1002/ajh.22126
Carrillo-Cruz E, Marín-Oyaga VA, de la Cruz Vicente F et al (2015) Role of 18F-FDG-PET/CT in the management of marginal zone B cell lymphoma. Hematol Oncol 33(4):151–158. https://doi.org/10.1002/hon.2181
Muslimani AA, Farag HL, Francis S et al (2008) The utility of 18-F-fluorodeoxyglucose positron emission tomography in evaluation of bone marrow involvement by non-Hodgkin lymphoma. Am J Clin Oncol Cancer Clin Trials 31(5):409–412. https://doi.org/10.1097/COC.0b013e318168d90b
Jerusalem G, Beguin Y, Najjar F et al (2001) Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) for the staging of low-grade non-Hodgkin lymphoma (NHL). Ann Oncol Off J Eur Soc Med Oncol 12(6):825–830. https://doi.org/10.1023/a:1011169332265
Okada J, Yoshikawa K, Imazeki K et al (1991) The use of FDG-PET in the detection and management of malignant lymphoma: correlation of uptake with prognosis. J Nucl Med 32(4):686–691
Sovani V, Harvey C, Haynes AP, McMillan AK, Clark DM, O’Connor SR (2014) Bone marrow trephine biopsy involvement by lymphoma: review of histopathological features in 511 specimens and correlation with diagnostic biopsy, aspirate and peripheral blood findings. J Clin Pathol 67(5):389–395. https://doi.org/10.1136/jclinpath-2013-201520
Carr R, Barrington SF, Madan B et al (1998) Detection of lymphoma in bone marrow by whole-body positron emission tomography. Blood 91(9):3340–3346
Coller BS, Chabner BA, Gralnick HR (1977) Frequencies and patterns of bone marrow involvement in non-Hodgkin lymphomas: observations on the value of bilateral biopsies. Am J Hematol 3:105–119. https://doi.org/10.1002/ajh.2830030201
Freudenberg LS, Antoch G, Schütt P et al (2004) FDG-PET/CT in re-staging of patients with lymphoma. Eur J Nucl Med Mol Imaging 31(3):325–329. https://doi.org/10.1007/s00259-003-1375-y
Fueger BJ, Yeom K, Czernin J, Sayre JW, Phelps ME, Allen-Auerbach MS (2009) Comparison of CT, PET, and PET/CT for staging of patients with indolent non-hodgkin lymphoma. Mol Imaging Biol 11(4):269–274. https://doi.org/10.1007/s11307-009-0200-9
El Karak F, Bou-Orm IR, Ghosn M et al (2017) PET/CT scanner and bone marrow biopsy in detection of bone marrow ınvolvement in diffuse large B-cell lymphoma. PLoS ONE 12(1):2–9. https://doi.org/10.1371/journal.pone.0170299
Adams HJA, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RAJ, de Klerk JMH (2014) Bone marrow 18F-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography cannot replace bone marrow biopsy in diffuse large B-cell lymphoma. Am J Hematol 89(7):726–731. https://doi.org/10.1002/ajh.23730
Funding
There is no source of financial support or funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest or relevant financial relationships related to this study.
Ethical Statement
The study protocol received institutional review board approval and all patient’s personal data have been secured.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Göçer, M., Kurtoğlu, E. Comparison of Bone Marrow Involvement with Bone Marrow Biopsy and PET–CT and Evaluation of Any Effects on Survival in Patients Diagnosed with Hodgkin and Non-Hodgkin Lymphoma. Indian J Hematol Blood Transfus 37, 52–59 (2021). https://doi.org/10.1007/s12288-020-01284-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-020-01284-x