Abstract
Chromosome 7 abnormalities in patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) heralds a poor prognosis. However its prevalence, morphological characteristics and clinical impact in MDS and AML in Indian subcontinent is sparsely reported. This was an observational cross-sectional study performed to evaluate the clinico-pathological profiles of MDS/AML patients with chromosome 7 abnormalities over a period of 4 years. 724 cases of MDS (n = 150) and AML (n = 574) were evaluated. Abnormal karyotype was detected in 49% (43/88) patients of MDS and 44% (127/289) cases of AML. Chromosome 7 abnormalities were detected in 18% cases of MDS (16/88) and 6.5% (19/289) cases of AML. Sole chromosome 7 abnormalities were detected in 5.7% (5/88) and 2.7% (8/289) and in adjunct to complex abnormalities in 7.9 and 3.1% cases of MDS and AML respectively. Morphologically, dyserythropoiesis, dysmyelopoiesis and eosinophilia were seen in 100, 66 and 56% cases of MDS and 38, 40 and 21% cases of AML. Majority of the patients had an aggressive natural course and outcome was dismal. Chromosome 7 abnormalities are strongly associated with the presence of morphological dysplasia and eosinophilia, irrespective of the type of aberration. It is invariably associated with very poor outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromosomal abnormalities are the key determinants for any disease initiation, progression, and risk-stratification in hematological disorders. Cytogenetic abnormalities in acute leukemia serve to discriminate between biologically distinct sub-groups of the disease and guide in predicting its behavior and optimizing therapy. Chromosome 7 is of particular interest as they are frequently altered in hematological malignancies. This chromosome spans about 159 million base pairs and represents more than 5% of the total DNA in the cells [1, 2]. Approximately 1150 protein-coding genes and 941 pseudogenes have been identified on chromosome 7 by using a clone-by-clone shotgun sequencing strategy [2]. The functional significance of many of these genes is yet to be elucidated, though some of these are implicated in the regulation of hematopoiesis which has been proven by in vitro and in vivo studies. Complete and interstitial losses of chromosome 7 (−7, 7q−) arenon-random anomalies which are frequently seen in de novo and therapy-induced MDS and AML [3,4,5]. Integrated data, using restriction fragment length polymorphism, fluorescent in situ hybridization and microarray-based comparative genome hybridization techniques have indicated the existence of at least three critical commonly deleted regions (CDRs) at bands 7q21–q22, 7q34, and 7q35–q36 which probably harbor one or more tumor-suppressor genes (TSGs) inactivated in MDS/AML [6, 7]. The candidate genes which are frequently inactivated in MDS and AML include Cux1, Ezh2, Samd9L, Dock 4 and LUC7L and their haplo-insufficiency is proposed to be the primary event in malignant transformation [8]. A diagrammatic illustration of some of the known genes on chromosome 7 [6,7,8,9,10,11,12,13,14,15] involved in leukemogenesis is shown in Fig. 1.
As per the western literature, they comprise the second most common cytogenetic abnormality in MDS, following deletion of long arm of chromosome 5 (5q−) (3). As sole abnormalities −7/7q − are observed in 5–15% of adults patients with denovo MDS and in approximately 50% of the patients with childhood MDS and therapy induced MDS [16, 17]. In contrast, its frequency is much lower in AML comprising approximately 5 and 3% of all cases in adult and pediatric population [18]. Loss of chromosome 7 (−7) indicate poor prognostic risk groups, in both these disorders [17, 18], though or 7q − has now been placed in the intermediate risk group according to the revised international prognostic risk stratification scoring system (R-IPSS) for MDS [19]. It is recommended to manage these patients on high-riskprotocols and they should be offered upfront hematopoietic stem cell transplantation after the first remission [20]. This underlies the importance to identify this sub-group of AML and MDS patients so as to facilitate early intervention and therapy optimization.
The morphological spectrum of chromosome 7 in MDS/AML is sparsely studied in Indian literature [21,22,23,24,25] and its true prevalence is thus largely unknown. In this study, we retrospectively analyzed all cases of acute leukemia and myelodysplastic syndrome with chromosome 7 alterations, with the aim to study its prevalence and impact on bone marrow morphology in patients with MDS and AML and also evaluate the treatment outcomein each subgroup.
Materials and Methods
This is a single center retrospective observational study over a period of 4 years (January 2013–December 2016). The clinical, morphological and cytogenetic data of all the patients with a diagnosis of de novo MDS and AML was retrieved from patient medical records. The study was approved by the Institute Ethics Committee.
Morphology
The May Grunwald Giemsa (MGG) stained bone marrow aspirate smears were reviewed by two independent pathologists (RG and KR). Key morphological features evaluated included, the presence of peripheral blood and bone marrow dyspoiesis, increased histiocytes, eosinophilia/basophilia, ring sideroblasts and marrow fibrosis.
Flow Cytometry
Immunophenotypic data was also collated for all the cases of acute myeloid leukemia. Flow cytometry was performed on bone marrow samples collected in EDTA vials using a panel of monoclonal antibodies against CD45, CD34, CD38, HLA-DR, CD13, CD33, CD117, CD10, CD19, CD20, CD79a, CD3, CD2, CD3, CD5, CD7, terminal deoxynucleotidyl-transferase (TdT) and myeloperoxidase. Monoclonal antibodies used were from Becton–Dickinson San Jose, CA. Samples were processed by a stain-lyse-wash protocol using 100 µl of the sample. A minimum of 50,000 blasts were gated on the using side scatter (SSC) versus CD45 dot plots. The data was analyzed with FACS Diva software V.6.1.1 (Becton–Dickinson, CA).
Cytogenetics
Cytogenetic analysis was performed on metaphases from the heparinized bone marrow aspirates taken at diagnosis with the use of the G-banding technique. A minimum of 20 metaphases were analyzed. Karyotypes were described with reference to the International System of Human Cytogenetic Nomenclature (ISCN) 2009 and 2013. Complex karyotype was defined as the presence of three or more structural abnormalities or monosomies. Monosomal karyotype was defined as the presence of at least 2 autosomal monosomiesor of a single monosomy associated with at least one additional structural abnormality [26]. Prognostic risk stratification was performed in all cases of MDS as per the R-IPSS scoring system [19].
Treatment
Patients of AML were offered standard induction chemotherapy with daunorubicin 60 mg/m2/day for 3 days along with cytosine arabinoside 200 mg/m2/day as a continuous infusion for 7 days (3 + 7 regimen), followed by consolidation therapy with high dose cytarabine. Complete remission was defined as the presence of 1.5 × 109/L granulocytes and 100 × 109/L platelets in the peripheral blood, and less than 5% blasts in the bone marrow. Treatment protocols for MDS varied amongst the patients. It included supportive therapy in the form of erythropoietin, hypomethylating agents like decitabine and azacytidine and allogenec stem cell transplantation for high risk patients.
Results
A total of 724 cases of MDS (n = 150) and AML (n = 574) were diagnosed during the study period. Cytogenetic data was available for 377 cases; 88/150 cases of MDS and 289/574 cases of AML. An abnormal karyotype was detected in 49% (43/88) patients with MDS and 44% (127/289) cases of AML. Cytogenetic abnormalities related to chromosome 7 were seen in 35 cases which included 18% (16/88) cases of MDS and 6.5% (19/289) cases of AML. The baseline hematological and cytogenetic data of the patients in both groups are shown in Table 1.
MDS with Chromosome 7 Abnormalities
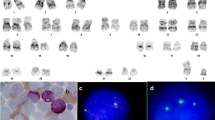
The median age of patients was 46 years (range 3–75 years), with a male predominance (Table 1). Eighty-one percent were less than 60 years of age and 37.5% were less than 40 years of age. There was prior history of breast cancer in one patient, which was managed by mastectomy and chemotherapy, 20 years back. Morphologically they were classified into MDS-SLD (n = 4), MDS–MLD (n = 6), MDS–EB-1 (n = 3), MDS–EB-2 (n = 2) and refractory cytopenias of childhood (n = 1). Dyserythropoiesis, dysmyelopoiesis and dysmegakaryopoiesis were seen in 100, 66 and 60% cases. Eosinophilia was seen in 56.5% (9/16) cases (Fig. 2a-c). Interestingly all four cases of MDS-SLD (mean age 44.5 years) revealed the presence of bone marrow hypoplasia with significant dyserythropoiesis.
May Grunwald Giemsa stained bone marrow smears, a hypoplastic marrow in a 30 year old male with dyspoietic megakaryocytes and hypogranular and hypolobate maturing myeloid cells with occasional blasts (inset), b increased blasts (long black arrow), eosinophils and basophils (red arrow) in a case of MDS–EB 2, c extensive eosinophilia, dyserythropoiesis and occasional blasts in a cases with MDS–MLD, d case of AML with dyspoietic megakaryocytes (blue arrow) and increased blasts
Isolated monosomy 7 was seen in 5.7% (5/88,) patients, while 7.9% (7/88) patients exhibited a complex karyotype (Table 2). A monosomal karyotype involving chromosome 7 was present in 4/88 (4.5%) cases of MDS. There was a single case each of deletion 7q and balanced translocation, which was seen in association with other complex abnormalities. Amongst the cases with complex karyotype, MDS–EB-1, was of particular interest due to presence of marked hyperdiploidy. Morphologically, the bone marrow smears in this case exhibited severe dyserythropoiesis with numerous multinucleate erythroid precursors besides proliferation of blasts (8%).
As per the IPSS-R, 13/16 (81%) cases belonged to intermediate risk groups, while 12.5% (2/16) and 6.25% (1/16) patients were in the good and high prognostic risk groups respectively.
AML with Chromosome 7 Abnormalities
The median age of patients was 39.5 years (age range 6–70 years), with a striking male preponderance (Table 1). There were only two cases of pediatric AML (age < 15 years). Morphologically, they were classified into FAB AML-M2 (11/19, 57.9%), AML-M4 (3/19, 15.8%) and AML M0 and AML-M1 (2/19, 10.5% each). Bone marrow examination revealed the presence of dyspoietic features involving at least two lineages (> 10%) in 47.3% (9/19) patients However, dysplastic changes in more than 50% cells in two lineages were appreciated in only three cases (15.7%). The presence of histiocytes with active phagocytosis and eosinophilia/basophilia (Fig. 2d) was noted in 21% (4/19) cases each (Table 3). Immunophenotypically, the majority of the cases showed expression of myeloid antigens CD13/CD33 and immaturity markers CD117/CD34/HLA-DR. Aberrant expression of CD7 was observed in 22% cases (n = 4).
Cytogenetically, isolated monosomy 7/del 7q was detected in 2.7% (8/289) cases and in association with complex abnormalities in 3.5% (10/289) cases (Table 3). Deletion 7q was seen in three cases, the breakpoints regions being 7q31.1, 7q12 and 7q36 two in isolation (1.5%) and one in conjunction with other complex abnormalities respectively. A monosomal karyotype involving chromosome 7 was observed in only 1.5% (2/127) patients with AML. None of the cases of AML showed balanced translocation of chromosome 7.
Outcome
Follow-up information could be collated for 16 patients (9 MDS and 7 cases of AML)
Seven patients (7/9) of MDS including 3 patients of MDS–MLD, 2-MDS–EB2, 1-MDS–EB1 and 1 hypoplastic MDS, died within 10 months of the diagnosis. They were predominantly on supportive therapy. Two of the patients progressed to AML; case of MDS–EB-2 and MDS–MLD (Fig. 3). The later had received three cycles of decitabine at a dose of 20 mg/m2 for 5 days, following failure of AML induction chemotherapy. She eventually succumbed to the disease and died due to septic complications. Two patients (2/9) are currently in regular follow-up, one of which underwent haploidentical bone marrow transplantation and is in remission day 200 + post-transplant. The second patient has been administered two cycles of azacytidine at a dose of 75 mg/m2 for 7 days each. He developed fever and is currently on antibiotics and antiviral therapy. The patient is planned for subsequent allogenic hematopoietic stem cell transplantation. In AML, all of the 7 patients died before the initiation of specific therapy.
Swimmer plot depicting the outcome of AML and MDS patients. Each bar represents one subject in the study. The bar length represents the follow up period in months, till the point of last contact. The red symbols represent death of the patient, the black dots represent progression to AML and arrows represent patients’ alive and maintaining response
Discussion
Overall chromosome 7 abnormalities were found in 35/377 (9.1%) of all MDS/AML. The incidence of chromosome 7 alterations was higher in MDS as compared to AML (18 vs. 6.5%). The results are comparable with those observed in studies form different parts of the country as well as from the West and Europe [21,22,23,24,25, 27,28,29,30,31,32,33,34]. Chromosome 7 abnormalities (−7/7q−) have been reported with a frequency of 11.7–14.2% in two large Indian studies, where the cytogenetic profile of MDS patients was evaluated with the help of conventional cytogenetics as well as fluorescent in situ hybridization [21, 22]. Our retrospective analysis of a cohort of MDS patients in the past revealed that chromosome 7 is the most frequently [32.5% (14/43)] involved in MDS as monosomy, interstitial deletion and rarely as balanced translocations [23]. A similar incidence has been reported by Hasse et al. [27] in their multi-institutional study from Austria and Germany, where they evaluated a total of 2124 patients with MDS. The monosomy 7/del(7q) anomaly was present in 8% of the patients in isolation and in 10% patients with a complex karyotype viz a viz 6.9 and 16.2% patients respectively, in the current study.
In AML, the overall incidence of chromosome 7 alteration was 6.5% (19/289) and sole −7 abnormalities were seen in 2.07% (6/289) cases in our study which are in concordance with the results obtained from two other large Indian studies on AML [24, 25]. In a large retrospective study at Mayo Clinic from 1989 to 2009, by Hussain et al., analyzed cytogenetic data of 24,262 patients, cytogenetic analysis was carried out in patients with suspected or known hematologic malignancies [35]. Twenty-seven percent (n = 6565) cases showed an abnormal karyotype and 49% of these (n = 3192) were sole chromosomal abnormalities. Fourteen percent of these (n = 900) included abnormalities of chromosome 7. Amongst these 900 patients, one-fourth patients (n = 230, 26%) had sole chromosome 7 structural abnormalities, which predominantly included monosomy 7 (43%), 7q − (22%), der(1;7)(q10;p10) (19%),balanced translocations (7%), 7p− (4%) and ring 7 (4%). Morphologically they were classified into MDS (28%), AML (17%), secondary MDS/AML (13%) primary myelofibrosis (7%), and chronic myelomonocytic leukemia (6%).
A strong association with morphological dysplasia was present in the majority of the cases of MDS and almost two-third cases of acute myeloid leukemia, irrespective of the presence of monosomy 7 as a sole abnormality or in association with other complex karyotypes, implying that partial or complete loss of chromosome 7 has specific morphological characteristics. Interestingly Honda et al. [8] demonstrated that homozygous and heterozygous loss of sterile motif (SAM) domain-9 (Samd9L) gene on chromosome 7, in mice, resulted in peripheral blood cytopenias and marked dysplasia indicating the direct impact of genes on chromosome 7 on the morphological spectrum of MDS/AML. Another striking observation was the presence of eosinophils, which was observed in more than one-half of the patients with MDS but not in AML. Bonemarrow eosinophils and or basophils have been shown to be significantly associated with abnormalities in chromosome 7, complex karyotypes, and i(17q) in previous studies as well [36, 37]. Thus it is evident that eosinophilia/basophilia in MDS is a strong predictor of underlying chromosomal abnormalities which carry intermediate or poor prognosis. None of the other morphological features evaluated were found to be significantly associated with chromosome 7 abnormality. Loss of chromosome 7 was equally distributed in the low risk as well high risk morphological sub groups of MDS. Complete loss of chromosome 7 (−7) was more frequent than 7q− in both disorders. The low frequency of 7q−, 6.25 and 15.7% in MDS and AML respectively did not permit statistically relevant comparison with −7 aberrations, in terms of differences in morphology or treatment outcome.
The prognosis of these myeloid disorders with chromosome 7 abnormalities is dismal. One of the major limitations of the study is lack of adequate follow-up data. The challenges in the management patients with malignant hematological disorders in an Indian scenario have also been aptly brought out by Philip et al. [25] while assessing the treatment outcome of AML patients at their center. Only one-third (29%, 109/380) of their patients opted for standard care of therapy at their center, with financial constraints being the major culpable reason for declining therapy. In our center as well, irrespective of the age at presentation, a definitive therapy was administered in a very small fraction and majority of the patients did not avail treatment. The natural course of the disease, in these patients was observed to be unambiguously fatal.
To conclude, this is a first descriptive study regarding chromosome 7 abnormalities among North Indian leukemic patients. It has specific morphological correlates like dyspoiesis and eosinophilia, particularly in MDS. Overall the frequency of deletion 7q is low in Indian patients with MDS as well as in AML. Though chromosome 7 alterations are more prevalent with complex karyotype, even as a sole abnormality, they predict an aggressive clinical course and invariably portend a poor prognosis in both these disease groups.
References
Scherer SW, Cheung J, MacDonald JR et al (2003) Human chromosome 7: DNA sequence and biology. Science 300:767–772
Hillier LW, Fulton RS, Fulton LA et al (2003) The DNA sequence of human chromosome 7. Nature 424:157–164
Heim S (1992) Cytogenetic findings in primary and secondary MDS. Leuk Res 16:43–46
Kardos G, Baumann I, Passmore SJ et al (2003) Refractory anemia in childhood: a retrospective analysis of 67 patients with particular reference to monosomy 7. Blood 102:1997–2003
Grimwade D, Hills RK, Moorman AV et al (2010) Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 116:354–365
Chen Z, Pasquini M, Hong B, DeHart S, Heikens M, Tsai S (2005) The human Penumbra gene is mapped to a region on chromosome 7 frequently deleted in myeloid malignancies. Cancer Genet Cytogenet 162:95–98
Johnson E, Cotter FE (1997) Monosomy 7 and 7q− associated with myeloid malignancy. Blood Rev 11:46–55
Honda H, Nagamachi A, Inaba T (2015) 7/7q− syndrome in myeloid-lineage hematopoietic malignancies: attempts to understand this complex disease entity. Oncogene 34:2413–2425
McNerney ME, Brown CD, Wang X, Bartom ET, Karmakar S, Bandlamudi C et al (2013) CUX1 is a haploinsufficient tumor suppressor gene on chromosome 7 is frequently inactivated in acute myeloid leukemia. Blood 121:975–983
Basiricò R, Pirrotta R, Fabbiano F et al (2003) Submicroscopic deletions in the 7q region are associated with recurrent chromosome abnormalities in acute leukemia. Haematologica 88:429–437
Cigognini D, Corneo G, Fermo E, Zanella A, Tripputi P (2007) HIC gene, a candidate suppressor gene within a minimal region of loss at 7q31.1 in myeloid neoplasms. Leuk Res 31:477–482
Brezinová J, Zemanová Z, Ransdorfová S et al (2007) Structural aberrations of chromosome 7 revealed by a combination of molecular cytogenetic techniques in myeloid malignancies. Cancer Genet Cytogenet 173:10–16
Graux C, Cools J, Michaux L, Vandenberghe P, Hagemeijer A (2006) Cytogenetics and molecular genetics of T-cell acute lymphoblastic leukemia: from thymocyte to lymphoblast. Leukemia 20:1496–1510
Döhner K, Brown J, Hehmann U et al (1998) Molecular cytogenetic characterization of a critical region in bands 7q35–q36 commonly deleted in malignant myeloid disorders. Blood 92:4031–4035
Kumar S, White DL, Takai S et al (1995) Apoptosis regulatory gene NEDD2 maps to human chromosome segment 7q34–35, a region frequently affected in haematological neoplasm. Hum Genet 95:641
Solé F, Espinet B, Sanz GF et al (2000) Incidence, characterization and prognostic significance of chromosomal abnormalities in 640 patients with primary myelodysplastic syndromes. GrupoCooperativoEspañol de CitogenéticaHematológica. Br J Haematol 108:346–356
Kardos G, Baumann I, Passmore SJ et al (2003) Refractory anemia in childhood: a retrospective analysis of 67 patients with particular reference to monosomy. Blood 102:1997–2003
Grimwade D, Hills RK, Moorman AV et al (2010) Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 116:354–365
Neukirchen J, Lauseker M, Blum S et al (2014) Validation of the revised international prognostic scoring system (IPSS-R) in patients with myelodysplastic syndrome: a multicenter study. Leuk Res 38:57–64
O’Donnell MR, Tallman MS, Abboud CN et al (2017) Acute myeloid leukemia, version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 15:926–957
Vundinti BR, Kerketta L, Jijina F, Ghosh K (2009) Cytogenetic study of myelodysplastic syndrome from India. Indian J Med Res 130:155–159
Varma N, Varma S (2008) Proliferative indices, cytogenetics, immunophenotype and other prognostic parameters in myelodysplastic syndromes. Indian J Pathol Microbiol 51:97–101
Gupta R, Rahman K, Singh MK, Kumari S, Yadav G, Nityanand S (2017) Clinico-pathological spectrum and novel karyotypic findings in myelodysplastic syndrome: experience of a tertiary care center in India. Mediterr J Hematol Infect Dis 9:e2017048
Amare PSK, Jain H, Setal K (2016) Cytogenetic profile in 7209 Indian patients with de novo acute leukemia: a single centre study from India. J Cancer Ther 7:530–544
Philip C, George B, Ganapule A et al (2015) Acute myeloid leukaemia: challenges and real world data from India. Br J Haematol 170:110–117
Kayser S, Zucknick M, Döhner K et al (2012) Monosomal karyotype in adult acute myeloid leukemia: prognostic impact and outcome after different treatment strategies. Blood 119:551–558
Haase D, Germing U, Schanz J et al (2007) New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood 110:4385–4395
Hasle H, Alonzo TA, Auvrignon A, Behar C, Chang M, Creutzig U et al (2007) Monosomy 7 and deletion 7q in children and adolescents with acute myeloid leukemia: an international retrospective study. Blood 109:4641–4647
Schanz J, Tuchler H, Sole F et al (2012) New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol 30:820–829
Gangat N, Patnaik NM, Begna K, Kourelis T, Kali A, Elliot MA (2015) Primary myelodysplastic syndrome: The Mayo clinic experience with 1000 patients. Mayo Clin Proc 90:1623–1638
Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC et al (2002) Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood 100:4325–4336
Cheng Y, Wang Y, Wang H, Chen Z, Lou J, Xu H et al (2009) Cytogenetic profile of de novo acute myeloid leukemia: a study based on 1432 patients in a single institution of China. Leukemia 23:1801–1806
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G et al (1998) The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 Trial. Blood 92:2322–2333
Qu S, Xu Z, Zhang Y, Qin T, Zhang T, Cui R et al (2012) Impacts of cytogenetic categories in the Revised International Prognostic Scoring System on the prognosis of primary myelodysplastic syndromes: results of a single-center study. Leuk Lymphoma 53:940–946
Hussain FT, Nguyen EP, Raza S, Knudson R, Pardanani A, Hanson CA et al (2012) Sole abnormalitiesof chromosome 7 in myeloid malignancies: spectrum, histopathologic correlates, and prognostic implications. Am J Hematol 87:684–686
Matsushima T, Handa H, Yokohama A, Nagasaki J, Koiso H, Kin Y et al (2003) Prevalence and clinical characteristics of myelodysplastic syndrome with bone marrow eosinophilia or basophilia. Blood 101:3386–3390
Matsushima T, Murakami H, Sawamura M et al (1993) Myelodysplastic syndrome with eosinophilia in bone marrow. Br J Haematol 84:636–638
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Gupta, R., Harankhedkar, S., Rahman, K. et al. Prevalence of Chromosome 7 Abnormalities in Myelodysplastic Syndrome and Acute Myeloid Leukemia: A Single Center Study and Brief Literature Review. Indian J Hematol Blood Transfus 34, 602–611 (2018). https://doi.org/10.1007/s12288-018-0941-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-018-0941-1