Abstract
Background
Triple-negative breast cancer (TNBC) is an aggressive type of breast cancer and associated with poor prognosis and shorter survival due to significant genetic heterogeneity, drug resistance and lack of effective targeted therapeutics. Therefore, novel molecular targets and therapeutic strategies are needed to improve patient survival. Serotonin (5-hydroxytryptamine, 5-HT) has been shown to induce growth stimulatory effects in breast cancer. However, the molecular mechanisms by which 5-HT exerts its oncogenic effects in TNBC still are not well understood.
Methods
Normal breast epithelium (MCF10A) and two TNBC cells (MDA-MB-231, BT-546) and MCF-7 cells (ER +) were used to investigate effects of 5-HT7 receptor. Small interfering RNA (siRNA)-based knockdown and metergoline (5-HT7 antagonist) were used to inhibit the activity of 5-HT7. Cell proliferation and colony formation were evaluated using MTS cell viability and colony formation assays, respectively. Western blotting was used to investigate 5-HT7, FOXM1 and its downstream targets protein expressions.
Results
We demonstrated that 5-HT induces cell proliferation of TNBC cells and expression of 5-HT7 receptor and FOXM1 oncogenic transcription factor. We found that expression of 5-HT7 receptor is up-regulated in TNBC cells and higher 5-HT7 receptor expression is associated with poor patient prognosis and shorter patient survival. Genetic and pharmacological inhibition of 5-HT7 receptor by siRNA and metergoline, respectively, suppressed TNBC cell proliferation and FOXM1 and its downstream mediators, including eEF2-Kinase (eEF2K) and cyclin-D1.
Conclusion
Our findings suggest for the first time that the 5-HT7 receptor promotes FOXM1, eEF2K and cyclin D1 signaling to support TNBC cell proliferation; thus, inhibition of 5-HT7 receptor/FOXM1 signaling may be used as a potential therapeutic strategy for targeting TNBC.
Graphical abstract

5-HT induces cell proliferation of TNBC cells through 5-HT7 receptor signaling. Also, genetic and pharmacological inhibition of 5-HT7 by RNAi (siRNA) and metergoline HTR7 antagonist, respectively inhibits FOXM1 oncogenic transcription factor and suppresses TNBC cell proliferation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer (TNBC) accounts for approximately 10–15% of all breast cancers and is defined by the lack of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 expression (HER2). TNBC is characterized by a highly invasive phenotype, early metastasis, poor prognosis, and limited response to conventional chemotherapies and lack of effective targeted treatment options [1]. Although, in 2020, a new targeted treatment strategy was approved by the FDA for patients with metastatic TNBC, the overall response rate was low with a median duration of response of 7.7 months [2,3,4]. Therefore, identification of novel molecular targets and development effective therapeutic strategies are urgently needed for treating TNBC.
Serotonin (5-hydroxytryptamine; 5-HT) is a biogenic monoamine neurotransmitter and, also, acts as a regulatory hormone involved in controlling various physiological processes [5]. Although 5-HT is mostly known as a neurotransmitter produced by tryptophan hydroxylase 2 (TPH2) in the central nervous system, it is also synthesized by epithelial cells in mammary gland through tryptophan hydroxylase 1 (TPH1) and acts in regulation of epithelial homeostasis in breast cancers [6]. The alteration of 5-HT and 5-HT receptor expression patterns leads to dysregulation of epithelial homeostasis which has been associated with breast cancer initiation, progression and tumorigenesis [6,7,8,9,10]. Furthermore, it is shown that 5-HT has been linked to the development of other cancers [11, 12]. These studies have suggested that 5-HT acts as a mitogenic signal for cancer cells and contributes to cell proliferation in various cancers including breast cancer [5, 9]. Moreover, it is shown that 5-HT activates oncogenic signal pathway such as JAK/STAT3/ERK [13], MAPK [7] and Akt/mTOR [10] in breast cancer cells. Furthermore, it was reported that free 5-HT levels in plasma may be used as a marker in the early detection of breast cancer, since 5-HT is produced in high levels in breast cancer cells [13].
Under physiologic conditions, 5-HT exerts its effects through involvement of 14 different receptors classified in 7 different families (5-HT-1-7) with several subtypes in each group [7, 9, 10, 14]. Expression of 5-HT receptor subtypes has been altered in cancer cell lines and tumor tissues compared to normal cells [9, 15]. In breast cancer, 5-HT1, 2, 3 and 7 receptors have been linked with cancer cell proliferation and progression [5, 8, 16,17,18,19,20]. In these receptors, 5-HT7 receptor has been shown to have an important role in actions of 5-HT in normal mammary gland homeostasis and function [20]. In addition, 5-HT stimulates cell proliferation and invasion by acting on 5-HT7 receptor, which is up-regulated in TNBC cells [8, 19, 20]. The signaling through 5-HT7 receptor is associated with PI3K/Akt, VEGF, ERK and MMP-9, and known to stimulate proliferation and invasion of breast cancer via the pathways [8, 19]. However, the exact molecular mechanism by which 5-HT7 receptor exerts oncogenic signaling in breast cancer cells is not understood. It is likely that multiple signaling pathways are involved in 5-HT7 receptor favorable effect on progression of breast cancer. Therefore, in the current study, we aimed to investigate the new molecular mechanisms of 5-HT7 receptor-mediated effects in TNBC cells.
Forkhead box M1 (FOXM1) is a multi-functional transcription factor, and an important molecular driver of TNBC metastasis and tumorigenesis [21, 22]. We recently showed that FOXM1 transcriptionally regulates expression of eukaryotic elongation factor-2 kinase (eEF2K) in TNBC cells, promoting TNBC tumor growth and metastasis [21], indicating that FOXM1 represents an important molecular target in TNBC [21, 22].
Given the proven effects of oncogenic signaling pathways of 5-HT/5-HT7 and FOXM1 signaling in TNBC cells, let us to hypothesize that the oncogenic signaling mediated through 5-HT7 receptor involves FOXM1 pathway leading to TNBC cells’ proliferation, invasion and progression. We found that 5-HT induced TNBC cell proliferation and expression of 5-HT7 and FOXM1. We also found that metergoline, a high-affinity 5-HT7 receptor antagonist [23], and 5-HT7-specific siRNA reduced TNBC cell proliferation and colony formation through inhibition FOXM1 and its downstream targets, including eEF2K and cyclin-D1 in TNBC cells.
In the current study, we demonstrated the existence of 5-HT/5-HT7/FOXM1 signaling axis promoting oncogenic effects cell proliferation and invasion of TNBC cells. Overall, our findings suggest for the first time that 5-HT/5-HT7 signaling is associated with FOXM1 and its downstream mediators including eEF2K/cyclin-D1 axis, representing a potential molecular target to control TNBC growth [21], demonstrating the significance of 5-HT7 as a driver of proliferation and disease progression.
Materials and methods
Cell lines, culture conditions, and reagents
Human TNBC cells (MDA-MB-231 and BT-549) and estrogen receptor positive (ER +) breast cancer cells (MCF7) and non-tumorogenic normal breast epithelium cells (MCF10A) were purchased from the American Type Culture Collection (Manassas, VA, USA). All breast cancer cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO). MCF10A cells were cultured in DMEM/F12 supplemented with 5% horse serum, epidermal growth factor, hydrocortisone, insulin, and cholera toxin (Calbiochem). Cells were cultured at 37 °C in a humidified incubator with 5% CO2 [21, 24, 25]. 5-HT (H9523) and Metergoline (M3668) were purchased from Sigma-Aldrich (St. Louis, MO). 5-HT and Metergoline were dissolved in %100 ethyl alcohol (EtOH) to prepare stock solutions. They were then diluted with FBS-free medium before applying to cells.
Transfection with siRNA
Small interfering RNAs (siRNAs) targeting Serotonin-receptor 7 (5-HT7) and Forkhead box M1 (FOXM1) genes and non-silencing control siRNA were purchased from Sigma-Aldrich. Exponentially growing cells were plated 24 h before transfection and transfected with 5-HT7 siRNA, two different FOXM1 siRNAs or control siRNA at a final concentration of 50 nM and 100 nM for 72 h, using HiPerFect Transfection Reagent (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Non-silencing control siRNA-transfected cells were used as negative controls. After treatment, the cells were harvested and processed for further analysis [21, 25, 26].
Cell viability and proliferation assays
Cell viability and proliferation were measured by MTS (3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay (Promega, Madison, WI) as described previously [22]. MDA-MB-231, BT-549 and MCF-7 cells were seeded in 96-well plates (1.20 × 103 cells/well) and treated with increasing doses of 5-HT (1, 2 and 4 µM, [8, 19] or metergoline (1, 10, 20, 30 and 35 µM, [23]. After treatments, a solution containing MTS and phenazine methosulfate (20:1 v/v) was added to the cells. After 3 h of incubation at 37 °C, the number of viable growing cells was estimated by measuring absorption at 490 nm, using Elisa Reader based on generation of formazan by the cells [21, 26].
Colony formation assays
To detect the effect of metergoline on cell proliferation and colony formation of breast cancer, we performed clonogenic assay as described previously [25]. TNBC cells were seeded in 6-well plates (1.5 × 103 cells/ well); treated with increasing doses of metergoline (1, 5, 10, 15, 20, 25 and 30 µM) for and incubated at 37 °C for 2 weeks to form colonies. Furthermore, cells were seeded in 6-well plates (1.5 × 103 cells/well) transfected with a non-silencing control siRNA or 5-HT7 siRNA (50 nM and 100 nM) and FOXM1 siRNAs (50 nM), and grown for 2 weeks. The cells were washed with PBS and stained with crystal violet, and visible colonies were counted [21, 26].
Western blot analysis
For western blot analysis, cells were seeded in T-25 culture flasks and treated with 5-HT or metergoline at indicated concentrations and time points. MDA-MB-231 cells were treated with 30–35 µM metergoline for 48 h and 20 µM metergoline for 72 h. BT-549 cells were treated with 20 and 30 µM metergoline for 48 h and 10 µM metergoline for 72 h. Furthermore, both MDA-MB-231 and BT-549 cells were transfected with siRNAs (50 nM and 100 nM) for 72 h. Then, the cells were collected, washed twice in ice-cold phosphate-buffered saline (PBS) and lysed in a lysis buffer at 4 °C. The protein concentrations were measured with a protein assay kit (DC kit; Bio-Rad, Hercules, CA). A total of 40 µg of protein from each sample was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis with a 4–20% gradient and transferred to polyvinylidenedifluoride membranes. The membranes were blocked with a blocking buffer (0.1 Triton X-100 with 5% dry milk in Tris-buffered saline-Tween 20 (TBS-T) for 60 min. After being washed with TBS-T, the membranes were probed with the following primary antibodies: FOXM1 (Cell Signaling), p-EF2 (Cell Signaling), EF2 (Cell Signaling), EF2K (Proteintech), 5-HT7 (YLBiont), Cyclin-D1 (Proteintech) and β-actin (Proteintech). After being washed with TBS-T, the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit (Bio-Rad, #170–6515) or anti-mouse secondary antibody (Bio-Rad). β-actin was used as a loading control. All antibodies were diluted in TBS-T containing 5% dry milk. Chemiluminescence detection was performed with Clarity Western ECL Substrate (Bio-Rad) and the blots were visualized with a ChemiDoc MP Imaging System (Bio-Rad) and quantified with a densitometer using the ChemiDoc MP Imager application program (Bio-Rad) [21, 24,25,26].
TCGA data analysis
Kaplan–Meier survival analysis were performed using breast cancer patient database The Cancer Genome Atlas (TCGA) Research Network (https://www.cancer.gov/tcga) that included mRNA-seq data of 404 TNBC/basal patients using https://kmplot.com to determine the association of 5-HT7 receptor (5HTR7) expression with overall patient survival. Based on gene expression analysis, patients with high 5-HT7 receptor gene expression (n = 122 patients) were compared to those with low high 5-HT7 expression (n = 282, cut-off value of 14 in the range of 1–124) for overall survival using a statistical analysis.
Statistical analysis
All experiments were conducted at least in triplicate, and the results were summarized as means with standard deviations. Statistical significance was determined using Student’s t test. p Values less than 0.05 were considered statistically significant. GraphPad Prism program was used to evaluate the data and plot the graphs.
Results
5-HT induces cell proliferation of TNBC cells
To determine the effects of 5-HT on TNBC (MDA-MB-231 and BT-549) and ER + (MCF-7) cells’ proliferation and viability, we performed MTS assay after 24 h and 48 h treatment with increasing doses of 5-HT (1, 2 and 4 μM). MTS assay revealed that TNBC cells were more sensitive to 5-HT and showed a significant increase in the number of viable cells at all concentrations and at different time points compared to untreated (NT) and EtOH-treated control cells (Fig. 1a–d). However, while no significant change was observed in number of MCF-7 cells treated with increasing concentration of 5-HT for 24 h (Fig. 1e), we found a significant increase in the number of cell viability at all concentrations after 48 h (Fig. 1f). These results indicated that TNBC cells more responsive to 5-HT induced cell proliferation compared to ER + MCF7 cells which were less sensitive to 5-HT-induced effects.
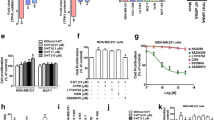
5-HT7 receptor is overexpressed in breast cancer cells and its expression is associated with shortened overall survival in BC patients
5-HT7 receptor has been shown to play a role in mediating 5-HT-induced mitogenic effects in TNBC [8, 19]. Therefore, we investigated the clinical significance of 5-HT7 expression in breast cancer patients, and we analyzed the NCI-The Cancer Genome Atlas (TCGA) breast cancer database using Kaplan–Meier survival curves. Our Kaplan–Meier survival analysis of the TCGA database included TNBC/basal subtype patients which included 404 patients. Kaplan–Meier survival analysis demonstrated that the overall survival rate was considerably shorter in patient tumors with high 5-HT7 gene expression (n = 122 patients) compared to those with low 5-HT7 expression (n = 282) (p = 0.022) (Fig. 2a). Kaplan–Meier analysis in ER + breast cancer patients did not have result in difference in the patient survival with the statistical significance, suggesting that HTR7 signaling is more important in TNBC patients compared to ER + BC patients. Next, we examined 5-HT7 receptor expression in two TNBC (MDA-MB-231 and BT-549) and one ER + (MCF-7) breast cancer cell line, and found that protein expression level of 5-HT7 receptor in TNBC was much higher than MCF-7 cells and MCF10A normal epithelial cells (Fig. 2b, c). We have previously demonstrated the significance of FOXM1 signaling in inducing TNBC tumor growth in mice [21]. Therefore, we focused on TNBC cells rather than ER + BC cells which have express higher 5-HT7 levels than ER + cells (MCF-7) (Fig. 2b, c).
5-HT7 expression is highly up-regulated in breast cancer cell lines and 5-HT7 expression is associated with shortened overall survival and poor prognosis in breast cancer patients a The analysis of Cancer Genome Atlas (TCGA) breast cancer database (n = 404 patients) using Kaplan–Meier survival analysis showed that increased 5-HT7 gene expression is associated with poor prognosis and significantly shorter overall survival (p = 0.022). b 5-HT7 protein are up-regulated in TNBC cells. β1-Actin was used as a loading control. c Band intensities were evaluated by densitometric analysis. MDA-MB-231 e and BT-549 cells g were treated with 5-HT (2 µM and 4 µM) or EtOH control for 48 h. Expressions of 5-HT7 and FOXM1 proteins were determined by Western blot. Protein expression intensities were evaluated by densitometric analysis in MDA-MB-231 (e, f) and BT-549 cells (h, i) (ns non-significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). All experiments were independently repeated at least twice
5-HT modulates 5-HT7 and FOXM1 signaling in TNBC cells
Although recent studies showed that 5-HT7 is also involved in proliferation of MDA-MB-231 cells [8, 19], the mechanisms by which it is responsible are still unclear. We and others have previously shown that FOXM1 is up-regulated in TNBC cells and required for cell proliferation and survival in TNBC [21, 27]. Also, according to analysis of The Cancer Genome Atlas breast cancer data base, we have reported that FOXM1 expression is associated with poor prognosis and shorter patient survival in breast cancer [25]. Therefore, we hypothesized that 5-HT/ 5-HT7 receptor signaling may induce FOXM1 to promote cell proliferation in TNBC cells. We found that 5-HT treatment (2 μM and 4 μM for 48 h) led to increase 5-HT7 and FOXM1 expression by western blot analysis in MDA-MB-231 (Fig. 2d–f) and BT-549 (Fig. 2g–i) cells compared to the control cells. These results indicated that 5-HT/5-HT7 signaling may modulate FOXM1 expression in TNBC cells.
Inhibition of 5-HT7 by an antagonist inhibitor suppresses cell proliferation and FOXM1 signaling in TNBC cells
To investigate effects of 5-HT7 on TNBC cells’ proliferation, we first treated cells with a 5-HT7 antagonist metergoline [23, 28, 29] with increasing concentration (1, 10, 20, 30 and 35 μM) for 48 h and 72 h. The MTS analysis revealed that metergoline treatment significantly reduced cell proliferation of MDA-MB-231 (Fig. 3a, b) and BT-549 cells (Fig. 3c, d) compared to untreated (NT) and EtOH-treated control cells. We then investigated the effects of metergoline on colony formation of MDA-MB-231 and BT-549 cells. Metergoline treatment (1–30 μM) led to a significant reduction in the number of colonies at doses starting from 1 μM both MDA-MB-231 and BT-549 cells compared to NT and EtOH-treated cells (Fig. 3e–h). BT-549 cells were more sensitive to metergoline treatment that completely inhibited cell proliferation starting from 15 μM in BT-549 cells, while the similar affects were observed in MDA-MB-231 cells at doses starting from 25 μM (Fig. 3e–h). More importantly, we found that metergoline treatment led to a marked reduction in the expression FOXM1 protein in TNBC cells (Fig. 4a–c, e–g). Moreover, both 5-HT7 receptor and FOXM1 expressions were inhibited in response to the treatment with metergoline (Fig. 4a–h). These findings suggested that 5-HT7 may promote cell proliferation by upregulating FOXM1 expression in TNBC cells.
Metergoline treatment inhibits cell proliferation and colony formation in TNBC cells. Cells were treated with increasing concentration of metergoline, and cell proliferation was evaluated after 48 h and 72 h by MTS assay (a–d). Cells were treated with increasing concentration of metergoline and evaluated for colony formation in MDA-MB-231 (e, f) and BT-549 (g, h). (ns non-significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). All experiments were independently repeated at least twice
Metergoline inhibits FOXM1 and 5-HT7 expressions in TNBC cells. Cells were treated with metergoline or EtOH as a control vehicle. Expressions of FOXM1 and 5-HT7 proteins were determined by Western blot in MDA-MB-231 (a, b) and BT-549 cells (e, f). Protein expression intensities were evaluated by densitometric analysis in MDA-MB-231 (c, d) and BT-549 cells (g, h) (*p < 0.05, ***p < 0.001, ****p < 0.0001). All experiments were independently repeated at least twice
Knockdown of 5-HT7 suppresses cell proliferation and FOXM1 expression in TNBC cells
Although metergoline has a high-affinity antagonist for the 5-HT7 receptor [23, 28, 30], it may also act as an antagonist for 5- HT1 and 5-HT2 serotonin receptors [31,32,33]. Therefore, to confirm the direct involvement of 5-HT7 in TNBC cell proliferation and FOXM1 expression, we knocked down 5-HT7 using specific siRNA targeting its mRNA in TNBC cells. To this end, the TNBC cells were transfected with 50 or 100 nM 5-HT7-siRNA, and about 2 weeks later, we examined colony formation. We found that knockdown of 5-HT7 resulted in a marked reduction of cell proliferation and colony formation in MDA-MB-231 (Fig. 5a, b) and BT-549 cells (Fig. 5c, d) compared to the control siRNA-transfected cells. BT-549 cells were more sensitive to 5-HT7 inhibition by siRNA and had much more reduced number of colonies compared to MDA-MB-231 cells treated with HT7 siRNA. Overall, these results indicated that 5-HT7 is involved in survival, proliferation of TNBC cells.
Next, we evaluated expressions of FOXM1 and 5-HT7 proteins in transfected TNBC cells with 5-HT7 siRNA (50 nM and 100 nM) by Western blot analysis. Knockdown of 5-HT7 by siRNA significantly inhibited expression of FOXM1 and 5-HT7 receptor in both MDA-MB-231 (Fig. 5e–g; supplementary (S) Fig. 1 (S1)a-c) and BT-549 cells (Fig. 5l–n; S2, a–c). Especially, 5-HT7 and FOXM1 expressions were significantly suppressed in the cells transfected with 100 nM 5-HT7-siRNA. These results indicated that 5-HT7 is involved in expression of FOXM1 in TNBC cells (Fig. 5e–g; Fig. 5l–n).
Knockdown of 5-HT7 inhibited downstream targets of FOXM1/eEF2K signaling in TNBC cells
We have previously demonstrated that eEF2K promotes cell proliferation and tumor growth and progression of TNBC [34]. We have also reported that FOXM1 transcriptionally regulates expression of eEF2K and FOXM1 inhibition leads to downregulation of eEF2K and suppresses cell proliferation and tumor growth in TNBC models [21]. Therefore, to elucidate the molecular mechanism of 5-HT7-induced cell proliferation, we also investigated whether knocked down of 5-HT7 leads to inhibition downstream targets of FOXM1 such as eEF2K, p-EF2 and cyclin D1. As expected, knockdown of 5-HT7 receptor by siRNA decreased the eEF2K expression and phosphorylation of EF2 (p-EF2) at Thr56 in MDA-MB-231 (Fig. 5, e, h, I; S1, a, d, e) and BT-549 cells (Fig. 5l–p; S2, a, d, e) compared to control cells. Interestingly, expression level of eEF2 was significantly decreased after transfection with 5-HT7-siRNA in BT-549 cells (Fig. 5l, r; S2, a, f). Cyclin-D1 promotes the cell cycle entry by inducing G1/S phase transition; also, it is downstream target of FOXM1 [21]. We found that Cyclin-D1 expression was significantly suppressed in MDA-MB- 231 (Fig. 5e, k; S1, a, g) and BT-549 (Fig. 5l, s; S2, a, g) cells transfected with 5-HT7-siRNA. Overall, our results indicated that 5-HT7 receptor regulates the FOXM1/eEF2K/cyclin-D axis, promoting cell proliferation in TNBC cells.
Inhibition of FOXM1 impairs proliferation of TNBC cells
To further demonstrate that the mechanism by which 5-HT7 regulates cell proliferation through FOXM1 expression, we investigated the effects of FOXM1 knockdown on cell proliferation of TNBC cells. Knockdown of FOXM1 by siRNA in MDA-MB-231 and BT-549 cells resulted in a marked reduc5tion of colony formation compared with control siRNA-transfected MDA-MB-231 (Fig. 5t–u) and BT-549 cells (Fig. v–y). Over all, these findings indicate that 5-HT7-induced TNBC cell proliferation is mediated by FOXM1 expression.
Knockdown of 5-HT7 inhibits expression of FOXM1 and its downstream targets, EF2K, p-EF2 and Cyclin-D1 in TNBC cells. Cells were transfected with 5-HT-7-siRNA (50 nM and 100 nM) targeting 5-HT-7 or control (non-targeting) siRNA. The colony areas were measured densitometrically at the end of the 14 days in MDA-MB-231 (a, b) and BT-549 (c, d). MDA-MB-231 and BT-549 cells were transfected with 5-HT-7 siRNA (100 nM) or control siRNA. Protein extracts were isolated 72 h after transfection. Knockdown of 5-HT-7 by siRNA (100 nM) inhibited expression levels of 5-HT7, FOXM1 and its downstream proteins in MDA-MB-231 (e) and BT-549 (l) cells. β-Actin was used as a loading control. Band intensities were evaluated by densitometric analysis in MDA-MB-231 (f–k) and BT-549 cells (m–s). Knockdown of FOXM1 by siRNA (50 nM) significantly inhibited colony formation in both MDA-MB-231 (t, u) and BT-549 (v, y) cells. (ns: non-significant, **p < 0.01, ***p < 0.001, ****p < 0.0001). All experiments were independently repeated at least twice
Discussion
Recent studies have demonstrated a potential stimulatory effect of 5-HT in promoting cell proliferation, angiogenesis, invasion, migration and metastasis in various of cancer types [8, 11, 21]. Interestingly, 5-HT is produced by several types of tumor cells, including breast cancer through upregulation of the 5-HT producing enzyme TPH1 [8].Therefore, 5-HT-mediated signaling is proposed as a potential therapeutic target. However, molecular mechanisms by which 5-HT acts as a growth factor and promotes mitogenic effects is not well understood [7, 9, 14]. Results of the current study demonstrated for the first time that 5-HT induces oncogenic FOXM1/eEF2K and cylin D1 signaling axis through HT7 receptor in TNBC cells.
5-HT has been shown to exert a mitogenic effect in various cancer cells. It is demonstrated that hepatocellular cancer cells exhibited increase in cell viability dose-dependent manner after treated with 5-HT, and predominantly promotes cell proliferation of hepatocellular cancer cells [11]. However, Ballou et al. [7] did not observe 5-HT mediated an increase in cell proliferation of breast cancer, but found that serotonin stimulation enhances activating phosphorylation of key mitogenic regulators such as Akt2, CREB, and MAPK. In another study showed that 5-HT stimulates cell proliferation, even, mitogenic effect of 5-HT is TNBC cells specific [8]. Similar results were reported by other authors, and where authors, indicated that an effect mitogenic of 5-HT had been for breast cancer cells [14]. The present study showed that 5-HT stimulated cell viability and proliferation in TNBC. As a result, both our study and other studies clearly indicate that 5-HT is signaling proliferative positive support to TNBC cells by increasing the rate of cell viability and proliferation.
It is known that 5-HT signaling through various 5-HT receptors regulates the survival and proliferation of cancer cells [9]. For instance, our previous study demonstrated that 5-HT1B and 5-HT1D receptors contribute in pancreatic cancer cells growth, invasion, and progression [35]. Also, in other, our study showed that 5-HT1B receptor promotes uterine leiomyoma cell survival and proliferation [36]. Liang et al. [37] reported that serotonin mediates the proliferation of hepatocellular carcinoma cells though 5-HTB2 receptor. Similarly, oncogenic effects of 5-HT on breast cancer cells are mediated through several receptors such as 5-HT1, 5-HT2, 5-HT2A and 5-HT3A [5, 7]. Eventually, all of studies reports that some 5-HT receptors subtypes are considered potential targets for treatment of several cancers [8, 9].
Although functions of many 5-HT receptors subtypes have been studied in various cancer types, studies regarding the role of 5-HT7 receptor in TNBC are very limited. Previous studies indicated that 5-HT7 receptor is associated with proliferation in prostate cancer [38], non-small cell lung cancer [39], hepatocellular carcinoma [11], and glioblastoma [40]. Although 5-HT7 receptor is expressed in the involution of the mammary gland [20] and breast cancer cells [7, 8, 14, 19, 20], there are only several studies investigating function of the 5-HT7 receptor in breast cancer cells. These studies reported that 5-HT induces MDA-MB-231 TNBC cell proliferation and invasion [14, 19, 20]. Our current study demonstrated that 5-HT7 receptor is overexpressed in TNBC and associated with poor patient prognosis and significantly shorter patient survival, suggesting that 5-HT7 is a clinically significant prognostic factor that contributes to an oncogenic signaling in breast cancer. Furthermore, 5-HT treatment dose-dependently increased 5-HT7 receptor and FOXM1 expression and cell proliferation in TNBC cells. Also, we showed that inhibition of 5-HT7 receptor by specific siRNA and metergoline, a 5-HT7 receptor antagonist, significantly suppressed TNBC cell proliferation, suggesting that 5-HT7 is promoting oncogenic signaling.
We have previously demonstrated that FOXM1 is overexpressed in TNBC cells and higher expression of FOXM1 is associated with shorter survival and worse prognosis of patients [21, 25]. In addition, FOXM1 regulates multiple oncogenic signaling pathways that are involved in cell proliferation, migration, invasion, and autophagy in TNBC [21, 26]. Presented study showed that FOXM1 expression correlates with levels of HT7, which also plays a critical role in cell proliferation and cell cycle progression in TNBC cells [21, 27].
Intracellular signaling pathways linked to mitogenic action of 5-HT are still unclear in breast cancer cells [9, 14]. Therefore, we investigated the downstream signaling molecules responsible for transmitting 5-HT/5-HT7 receptor signaling that leads to TNBC cell proliferation. We found a dose-depended induction in the FOXM1 expression levels by 5-HT. Moreover, we found that expression level of FOXM1 was significantly decreased, when 5-HT7 receptor is blocked by pharmacologically using 5-HT7 receptor antagonist or genetically using 5-HT7 receptor-specific siRNA. We demonstrated that 5-HT/HT7 axis promotes FOXM1 oncogenic signaling to induce TNBC cell proliferation and survival as inhibition of FOXM1 significantly suppressed cell proliferation in TNBC cell. We and others have previously shown that oncogenic transcription factor FOXM1 promotes TNBC proliferation, migration and invasion and tumor growth in mice models [21, 27]. Furthermore, we demonstrated that knockdown of 5-HT7 receptor by siRNA resulted in decreased expression level of eEF2K, which is a transcriptional target of FOXM1 [21] and one of the important oncogenic signaling pathways that promotes cell proliferation, invasion and tumorigenesis in TNBC [34]. Furthermore, we found that 5-HT7 knockdown reduced expression of cyclin-D1, which plays a key role in G1 phase, G1/S transition and oncogenesis, and recently shown to be regulated by FOXM1 and eEF2K in TNBC cells [21]. Overall, all of these findings suggest that 5-HT/ 5-HT7 receptor signaling may contribute to proliferation and survival of TNBC cells by regulating FOXM1 and its downstream mediators, including eEF2K and cyclin-D1. These results also suggest that 5-HT/ 5-HT7 signaling may be a promising therapeutic target for TNBC.
5-HT7 antagonists such as metergoline have been considered for the treatment of breast cancer, but there is no specific antagonist for the only 5-HT7 receptor [15]. Metergoline irreversibly blocks the 5-HT7 receptors and provides a profound inactivation of the 5-HT7 receptor [23, 28, 29]. In presented study, we showed that metergoline treatment led to a marked inhibition of 5-HT7 receptor expression in TNBC cells. Also, we found for the first time that the mitogenic effect of 5-HT7 receptor was blocked by metergoline treatment, which suppressed FOXM1 expression and downstream signaling, including eEF2K and cyclin D1 in TNBC cells. As expected, inhibition of FOXM1 with metergoline leads to significant reduced of cell proliferation and survival in TNBC cells. Therefore, in this study, we suggest that metergoline may be as a potential anti-cancer agent that suppresses TNBC progression, by inhibiting FOXM1 expression, and, thus, suppressing survival and proliferation of TNBC cells promoted by 5-HT. Considering the fact that expression level of 5-HT7 was lower in MCF10A normal breast epithelial cells and 5-HT does not stimulate the cell proliferation [8], suggesting that HT7 targeted therapeutics may be more selective toward TNBC cells.
Conclusions
Our findings demonstrated that 5-HT/ 5-HT7 receptor signaling plays an important role in TNBC cell proliferation by regulating FOXM1/eEF2K/Cyclin-D1 axis in TNBC cells. Thus, inhibition of 5-HT7/FOXM1 signaling may be used as a potential therapeutic approach for controlling TNBC. Further studies are needed to explore this therapeutic strategy in vivo TNBC tumor models to determine whether this therapeutic approach is valid for controlling TNBC tumor growth and progression.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lye L, Zhang S, Deng Y, Wang M, Deng X, Yang S, Wu Y. Dai Z Regulatory mechanisms, functions, and clinical significance of CircRNAs in triple-negative breast cancer. J Hematol Oncol. 2021. https://doi.org/10.1186/s13045-021-01052-y.
Agostinetto E, Eiger D, Punie K, de Azambuja E. Emerging therapeutics for patients with triple-negative breast cancer. Curr Oncol Rep. 2021. https://doi.org/10.1007/s11912-021-01038-6.
Emami F, Pathak S, Nguyen TT, Shrestha P, Maharjan S, Kim JO, Jeong JH. Yook S Photoimmunotherapy with cetuximab-conjugated gold nanorods reduces drug resistance in triple negative breast cancer spheroids with enhanced infiltration of tumor-associated macrophages. J Control Release. 2021;329:45–664.
Keihan Shokooh M, Emami F, Jeong JH, Yook S. Bio-Inspired and Smart Nanoparticles for Triple Negative Breast Cancer. Microenvironment Pharmaceutics. 2021. https://doi.org/10.3390/pharmaceutics13020287.
Olfati Z, Rigi G, Vaseghi H, Zamanzadeh Z, Sohrabi M, Hejazi SH. Evaluation of serotonin receptors (5HTR2A and 5HTR3A) mRNA expression changes in tumor of breast cancer patients. Med J Islam Repub Iran. 2020. https://doi.org/10.34171/mjiri.34.99.
Horseman ND, Collier RJ. Serotonin: a local regulator in the mammary gland epithelium. Annu Rev Anim Biosci. 2014;2:353–74.
Ballou Y, Rivas A, Belmont A, Patel L, Amaya CN, Lipson S, Khayou T, Dickerson EB, Nahleh Z, Bryan BA. 5-HT serotonin receptors modulate mitogenic signaling and impact tumor cell viability. Mol Clin Oncol. 2018;9:243–54.
Gautam J, Banskota S, Regmi SC, Ahn S, Jeon YH, Jeong H, Kim SJ, Nam TG, Jeong BS, Kim JA. Tryptophan hydroxylase 1 and 5-HT (7) receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Mol Cancer. 2016;15:75. https://doi.org/10.1186/s12943-016-0559-6.
Balakrishna P, George S, Hatoum H, Mukherjee S. Serotonin pathway in cancer. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22031268.
Jose J, Tavares CDJ, Ebelt ND, Lodi A, Edupuganti R, Xie X, Devkota AK, Kaoud TS, Van Den Berg CL, Anslyn EV, Tiziani S, Bartholomeusz C, Dalby KN. Serotonin analogues as inhibitors of breast cancer cell growth. ACS Med Chem Lett. 2017;8:1072–6.
Mamdouh F, Abdel Alem S, Abdo M, Abdelaal A, Salem A, Rabiee A, Elsisi OJ. Serum serotonin as a potential diagnostic marker for hepatocellular carcinoma. Interferon Cytokine Res. 2019;39:780–5.
Zhou J, Geng KK, Ping FF, Gao YY, Liu L, Feng BN. Cross-talk between 5-hydroxytryptamine and substance P in the melanogensis and apoptosis of B16F10 melanoma cells. Eur J Pharmacol. 2016;775:106–12. https://doi.org/10.1016/j.ejphar.2016.02.026.
Sola-Penna ML, Paixão P, Branco JR, Ochioni AC, Albanese JM, Mundim DM, Baptista-de-Souza D, Figueiredo CP, Coelho WS, Marcondes MC, Zancan P. Serotonin activates glycolysis and mitochondria biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively. Br J Cancer. 2020;122:194–208.
Fröbe A, Čičin-Šain L, Jones G, Soldič Ž, Lukač J, Bolanča AKusič, Z. Plasma free serotonin as a marker for early detection of breast cancer recurrence. Anticancer Res. 2014;34:1167–9.
Ye D, Xu H, Tang Q, Xia H, Zhang C, Bi F. The role of 5-HT metabolism in cancer. Biochim Biophys Acta Rev Cancer. 2021;1876: 188618. https://doi.org/10.1016/j.bbcan.2021.188618.
Hejazi SH, Ahangari G, Deezagi A. Alternative viewpoint against breast cancer based on selective serotonin receptors 5HTR3A and 5HTR2A antagonists that can mediate apoptosis in MCF-7 cell line. Curr Drug Discov Technol. 2015;12:240–9.
Kopparapu PK, Tinzl M, Anagnostaki L, Persson JL, Dizeyi N. Expression and localization of serotonin receptors in human breast cancer. Anticancer Res. 2013;33:363–70.
Pud D, Har-Zahav G, Laitman Y, Rubinek T, Yeheskel A, Ben-Ami S, Kaufman B, Friedman E, Symon Z, Wolf I. Association between variants of 5-hydroxytryptamine receptor 3C (HTR3C) and chemotherapy-induced symptoms in women receiving adjuvant treatment for breast cancer. Breast Cancer Res Treat. 2014;144:123–31.
Gautam J, Bae YK, Kim JA. Up-regulation of cathepsin S expression by HSP90 and 5-HT7 receptor-dependent serotonin signaling correlates with triple negativity of human breast cancer. Breast Cancer Res Treat. 2017;161:29–40.
Pai VP, Hernandez LL, Stull MA. Horseman ND The type 7 serotonin receptor, 5-HT7, is essential in the mammary gland for regulation of mammary epithelial structure and function. Biomed Res Int. 2015. https://doi.org/10.1155/2015/364746.
Hamurcu Z, Ashour A, Kahraman N. Ozpolat B FOXM1 regulates expression of eukaryotic elongation factor 2 kinase and promotes proliferation, invasion and tumorgenesis of human triple negative breast cancer cells. Oncotarget. 2016;7:16619–35.
Borhani S, Gartel AL. FOXM1: a potential therapeutic target in human solid cancers. Expert Opin Ther Targets. 2020;24:205–17.
Toohey N, Klein MT, Knight J, Smith C, Teitler M. Human 5-HT7 receptor-induced inactivation of forskolin-stimulated adenylate cyclase by risperidone, 9-OH-risperidone and other “inactivating antagonists.” Mol Pharmacol. 2009;76:552–9.
Hamurcu Z, Kahraman N, Ashour A, Ozpolat B. FOXM1 transcriptionally regulates expression of integrin β1 in triple-negative breast cancer. Breast Cancer Res Treat. 2017;163:485–93.
Hamurcu Z, Sener EF, Taheri S, Nalbantoglu U, Kokcu ND, Tahtasakal R, Cınar V, Guler A, Ozkul Y, Dönmez-Altuntas H, Ozpolat B. MicroRNA profiling identifies Forkhead box transcription factor M1 (FOXM1) regulated miR-186 and miR-200b alterations in triple negative breast cancer. Cell Signal. 2021. https://doi.org/10.1016/j.cellsig.2021.109979.
Hamurcu Z, Delibaşı N, Nalbantoglu U, Sener EF, Nurdinov N, Tascı B, Taheri S, Özkul Y, Donmez-Altuntas H, Canatan H, Ozpolat B. FOXM1 plays a role in autophagy by transcriptionally regulating Beclin-1 and LC3 genes in human triple-negative breast cancer cells. J Mol Med (Berl). 2019;97:491–508.
Tan Y, Wang Q, Xie Y, Qiao X, Zhang S, Wang Y, Yang Y, Zhang B. Identification of FOXM1 as a specific marker for triple-negative breast cancer. Int J Oncolb. 2019;54:87–97.
Dogrul A, Seyrek M. Systemic morphine produce antinociception mediated by spinal 5-HT7, but not 5-HT1A and 5-HT2 receptors in the spinal cord. Br J Pharmacol b. 2006;149:498–505.
Krobert KA, Levy FO. The human 5-HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects. Br J Pharmacol. 2002;135:1563–71.
Knight JA, Smith C, Toohey N, Klein MT, Teitler M. Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-Hydroxytryptamine7 receptor by risperidone, 9-OH-Risperidone, and other inactivating antagonists. Mol Pharmacol. 2009;75:374–80.
Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ , Bromidge SM Thomas DR. Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br J Pharmacol 2000; 130: 539–48.
Bagdy G, Sved AF, Murphy DL, Szemeredi K. Pharmacological characterization of serotonin receptor subtypes involved in vasopressin and plasma renin activity responses to serotonin agonists. Eur J Pharmacol. 1992;210:285–9.
Aulakh CS, Hill JL, Murphy DL. Effects of various serotonin receptor subtype-selective antagonists alone and on m-chlorophenylpiperazine-induced neuroendocrine changes in rats. J Pharmacol Exp Ther. 1992;210:285–9.
Tekedereli I, Alpay SN, Tavares CD, Cobanoglu ZE, Kaoud TS, Sahin I, Sood AK, Lopez-Berestein G, Dalby KN, Ozpolat B. Targeted silencing of elongation factor 2 kinase suppresses growth and sensitizes tumors to doxorubicin in an orthotopic model of breast cancer. PLoS One 2012; 7e41171.
Gurbuz N, Ashour AA, Alpay SN, Ozpolat B. Down-regulation of 5-HT1B and 5-HT1D receptors inhibits proliferation, clonogenicity and invasion of human pancreatic cancer cells. PLoS ONE. 2014;9(8): e105245.
Gurbuz N, Asoglu MR, Ashour AA, Salama S, Kilic GS, Ozpolat B. A selective serotonin 5-HT1B receptor inhibition suppresses cells proliferation and induces apoptosis in human uterine leiomyoma cells. Eur J Obstet Gynecol Reprod Biol. 2016;206:114–9.
Liang C, Chen W, Zhi X, Ma T, Xia X, Liu H, Zhang Q, Hu Q, Zhang Y, Bai X, Liang T. Serotonin promotes the proliferation of serum-deprived hepatocellular carcinoma cells via upregulation of FOXO3a. Mol Cancer. 2013;12:4. https://doi.org/10.1186/1476-4598-12-14.
Cinar I, Sirin B, Halici Z, Palabiyik-Yucelik SS, Akpinar E, Cadirci E. 5-HT7 receptors as a new target for prostate cancer physiopathology and treatment: an experimental study on PC-3 cells and FFPE tissues. Naunyn Schmiedebergs Arch Pharmacol. 2021. https://doi.org/10.1007/s00210-021-02051-z.
Du XT, Wang Z, Wu X, Gu Y, Huang Q, Wang J, Xie J. 5-HT7 receptor contributes to proliferation, migration and invasion in NSCLC cells. Onco Targets Ther. 2020;13:2139–51.
Mahé C, Bernhard M, Bobirnac I, Keser C, Loetscher E, Feuerbach D, Dev KK, Schoeffter P. Functional expression of the serotonin 5-HT7 receptor in human glioblastoma cell lines. Br J Pharmacol. 2004;143:404–10.
Acknowledgements
This study was supported by Erciyes University Research Fund (Project Number: TYL-2019-9187).
Funding
This study was supported by Erciyes University Research Fund (Project Number: TYL-2019–9187).
Author information
Authors and Affiliations
Contributions
All authors analyzed the results and approved the final version of the manuscript. Zuhal Hamurcu conceptualized, coordinated, administered the study, performed experiments, and wrote the paper. Bulent Ozpolat conceived, conceptualized, supervised the study, and revised and edited the manuscript. Venhar Cınar, Ahsen Guler, and Nursultan Nurdınov helped performing the experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper, and approved the final version of the manuscript being submitted. The results presented in this paper have not been published previously in whole or part.
Consent for publication
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Cınar, V., Hamurcu, Z., Guler, A. et al. Serotonin 5-HT7 receptor is a biomarker poor prognostic factor and induces proliferation of triple-negative breast cancer cells through FOXM1. Breast Cancer 29, 1106–1120 (2022). https://doi.org/10.1007/s12282-022-01391-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-022-01391-9