Abstract
Background
Accumulating studies have identified that microRNAs (miRNAs) are novel regulators acting as tumor suppressors or oncogenes in tumor progression. The aim of the study is to investigate the functional roles of miR-138-5p in breast cancer (BC) cells and explore the underlying mechanisms by identifying its target gene.
Methods and results
Our results first showed that miR-138-5p expression was remarkably decreased in BC tissues and cells using quantitative real-time PCR analysis. Forced expression of miR-138-5p significantly suppressed cell migration and invasion ability of BC using transwell assay. Moreover, miR-138-5p overexpression suppressed cell epithelial–mesenchymal transition (EMT) phenomenon of BC by upregulating E-cadherin expression, but downregulating N-cadherin and Vimentin expression. More importantly, rhomboid domain-containing protein 1 (RHBDD1) was predicted as the direct target of miR-138-5p by TargetScan and miRanda, which was subsequently confirmed by luciferase reporter assay in BC cells. RHBDD1 was up-regulated in BC tissues and negatively correlated with miR-138-5p expression. Furthermore, forced expression of miR-138-5p could down-regulate the expression of RHBDD1, but overexpression of RHBDD1 reversed the suppressive effects of miR-138-5p in BC cell migration, invasion and EMT.
Conclusions
Our findings revealed the tumor-suppressive role of miR-138-5p in regulating BC migration by targeting RHBDD1, suggesting that miR-138-5p negatively regulating EMT might be a therapeutic target in BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is one of the most frequently diagnosed malignant diseases worldwide [1]. Of the 316,120 new cases of BC expected in the United States (US) in 2017, about 252,710 are invasive cases, while 63,410 are in situ cases [2]. Even though technical progress in the management of BC over the last 30 years, mortality rates remain alarmingly high, approximately 40,290 deaths from BC are projected to occur in US women in 2017 [2]. Metastatic lesions to the regional lymph nodes, bone marrow, lung and liver are usual when BC cells adapt to a tissue microenvironment of distant organs that disseminate from the primary tumor [3]. The ability to prospectively identify molecular mechanisms controlling growth and metastasis of tumorigenic cancer cells will facilitate to design more effective therapies for patients with BC.
More than 20 years later since the discovery of the first microRNA (miRNAs), there has been growing interest in the analysis of the role of miRNAs in developmental and disease [4]. miRNAs are a large set of endogenous small RNAs of 21–24 nucleotides, playing critical roles of the human transcriptome through a number of cellular processes and pathways, including development, tumorigenesis, and aging [5]. Biological studies have revealed that multiple miRNAs can function as oncogene or tumor suppressor gene whose expression is amplified or lost during malignant progression [6]. Until now, human genome has been shown to harbor ~ 3000 miRNAs and the list is growing [7]. MiRNA-based therapy has potential clinical advantages, such as MRX, a liposomal miR-34a mimic, has shown inhibitory effects on hepatocellular carcinoma in clinical trials, and several more await entering clinical phases [8]. As a member of a large class of miRNAs, miR-138-5p has been shown to suppress cell growth and metastasis via targeting PD-L1 in colorectal cancer [9], FOXC1 in pancreatic cancer [10] and EIF4EBP1 in nasopharyngeal carcinoma [11]. Moreover, targeting G protein-coupled receptor 124 [12] and ZEB2 [13] by miR-138-5p has recently been reported in non-small cell lung cancer. However, the biological role of miR-138-5p remains unknown in BC.
Rhomboids are a family of well-conserved intramembrane serine proteases that are widely distributed in biological systems [14]. These proteins exhibit unusual properties of cleaving proteins within their transmembrane domains [15]. Previous studies revealed that they control many important biological processes, such as activation of Drosophila EGFR signaling [16], modulation remodeling of mitochondrial membrane [17], host cell invasion by apicomplexan parasites [18], and intracellular growth of Toxoplasma gondii [19]. Ten years ago, a new member of the Rhomboids family was reported as rhomboid domain-containing protein 1 (RHBDD1) [20]. RHBDD1 is expressed at high levels in the testis and is responsible for apoptosis via cleaving pro-apoptotic Bcl2 protein BIK [20]. It is now well recognized that RHBDD1 is dysregulated in many cancers and is a potent regulator of cellular-biological behaviors of cancer cells [21]. It is noteworthy that RHBDD1 functioned as an inhibitor of apoptosis and inducer of proliferation, migration and invasion of BC cells [22].
In the current study, we explored the biological role of miR-138-5p in BC and the mechanism by which it acts using BC MDA-MB-231 and ZR-75-30 cells. We focused on RHBDD1 as a putative miR-138-5p target and investigated the consequences of RHBDD1 restoration in BC cells. Tumor-suppressive miRNA-regulated cancer pathways could deepen our understanding of the role of miR-138-5p in BC and suggest a new therapeutic target in BC treatment.
Materials and methods
Expression datasets
To investigate the expression level of miR-138-5p in BC, the mRNA expression data were downloaded from the Cancer Genome Atlas project (TCGA dataset, https://portal.gdc.cancer.gov/) dataset. The t test method was used to compare the expression of miR-138-5p between BC and adjacent normal tissues (n = 1000).
Tissue samples
This study was approved by the Ethics Committee of the First Hospital of Lanzhou University. A total of 20 paired BC tissues and matched adjacent normal tissues were collected from patients who did not receive any radiotherapy or chemotherapy before operation. Written informed consent was obtained from all patients. All surgically resected tissue samples were immediately frozen in liquid nitrogen and then stored at − 80 °C until further analysis.
Cell culture and transfection
BC cell lines (MDA-MB-231, MDA-MB-468, T47D and ZR-75-30) and the normal breast epithelial cell line MCF-10A were obtained from American Tissue Culture Collection (ATCC, Manassas, VA, USA). All cell lines were cultured in Dulbecco’s Modified Eagle Medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (Gibco) at 37 °C in 5% CO2. Synthetic miR-138-5p mimic and scrambled negative control (miR-NC) were purchased from GenePharma (Shanghai, China). RHBDD1 coding sequences were sub-cloned into pcDNA3.1 (Sangon Biotech, China) to construct RHBDD1 overexpression vector (RHBDD1). Empty vector was used as negative control. For miR-138-5p overexpression, MDA-MB-231 and ZR-75-30 cells were transfected with miR-138-5p mimic or miR-NC at a final concentration of 50 nM. In the rescue experiments, MDA-MB-231 cells were co-transfected with miR-138-5p mimic or miR-NC together with RHBDD1 or empty vector. All cell transfection was performed for 48 h using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions.
Quantitative real-time PCR
Total RNA was extracted from tissues or cells by TRIzol reagent (Life Technologies, Grand Island, NY, USA) and complementary DNA (cDNA) was synthesized using a commercial Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions. Transcript levels of miR-138-5p and U6 snRNA were determined on a 7500 Real-time PCR System with TaqMan Gene Expression assays (Applied Biosystems). Forward and reverse primer sequences synthesized by Sangon (Shanghai, China) were as follows: miR-138-5p (forward, 5′-TGCAATGGGTTTGGCGTAGAAC-3′; reverse, 5′-CCAGTGCCGCAGGGTAGGT-3′), U6 (forward, 5′-CTCGCTTCGGCAGCACA-3′; reverse, 5′-AACGCTTCACGAATTTGCGT-3′). The fold changes of miR-138-5p expression using U6 as an endogenous control were analyzed by the \(2^{{ - \Delta \Delta C_{\text{t}} }}\) method.
Transwell migration and invasion assay
The migratory and invasive ability of BC cells were analyzed using transwell chambers with 8 μm pores (Corning Costar Corp., USA). For migration assay, approximately 2 × 104 stable transfected BC cells re-suspended with serum-free medium were seeded in the upper chamber. Then 500 μl medium containing 10% FBS was added to the lower chamber. After 24-h incubation, cells that migrated to the lower chamber were fixed with 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet for another 30 min at room temperature. Stained cells were observed under a microscope (Olympus, Tokyo, Japan) and counted in five randomly selected fields (magnification 200 ×). The invasion assay was the same as the migration assay except that the upper chambers pre-coated with Matrigel (BD Biosciences, USA).
Luciferase reporter assay
A search for putative targets of miR-138-5p was performed with TargetScan Human7.2 (www.targetscan.org/vert_72/) and miRanda (www.microrna.org/) software. For luciferase reporter assay, sequences containing wild-type (WT) or mutant (MUT) miR-138-5p-binding site in the 3′UTR of RHBDD1 were inserted into the pGL3 luciferase reporter vector (Promega, USA) to generate Luc-RHBDD1-WT or Luc-RHBDD1-MUT constructs, respectively. After verified by DNA sequencing, BC cells were co-transfected with pGL3 control vector and the Luc-RHBDD1-WT or Luc-RHBDD1-MUT vectors together with miR-138-5p mimic and miR-NC vectors in 12-well plates using Lipofectamine 2000 according to the manufacturer’s protocol. After 48-h transfection, the Firefly and Renilla luciferase activities were determined using luciferase reporter assay system (Promega, USA) and relative luciferase activities were calculated in three independent experiments.
Western blotting
Total proteins were extracted from cells using RIPA lysis buffer containing 1% protease inhibitor (Beyotime, Shanghai, China) and quantified with a BCA protein assay kit (Beyotime) according to the instructions. After denatured, protein (30 µg/well) was separated by 10% SDS-PAGE gel electrophoresis and then transferred onto polyvinylidene difluoride (PVDF, Millipore) membranes. The membranes were blocked with 5% non-fat milk in TBST for 2 h at room temperature and next incubated with primary antibodies against RHBDD1, E-cadherin, N-cadherin, Vimentin or GAPDH overnight at 4 °C, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) for 2 h at room temperature. The protein signals were detected using an enhanced chemiluminescence kit (GE Healthcare, Chicago, IL, USA).
Statistical analysis
Data are analyzed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA) and presented as mean ± standard deviation (SD) from three independent experiments. Differential comparisons between two groups were conducted using two-tailed Student’s t test and multiple group comparisons were conducted via one-way analysis of variance with Tukey’s post hoc test. Correlations between the expression of miR-138-5p and the expression of RHBDD1 in BC tissues were analyzed by Spearman’s correlation. The values of p less than 0.05 were considered to be a significant difference.
Results
Expression of miR-138-5p was down-regulated in BC tissues and cell lines
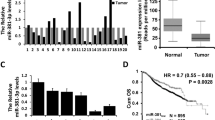
The expression of miR-138-5p was first evaluated in TCGA database. As shown in Fig. 1a, the expression of miR-138-5p was significantly down-regulated in BC (p = 0.0369) in comparison with normal tissues. Using quantitative real-time PCR analysis, we further determined the expression of miR-138-5p in 20 pairs of BC tissues and adjacent normal tissues. As shown in Fig. 1b, a significant decrease in miR-138-5p levels was observed in BC tissues, compared with that in matched adjacent tissues. Subsequently, the expression of miR-138-5p was further examined in BC cell lines and normal breast epithelial cell line MCF-10A. Similarly, the expression of miR-138-5p was very less in BC cells than in MCF-10A cells (Fig. 1c), particularly in MDA-MB-231 and ZR-75-30 cells. Hence, MDA-MB-231 and ZR-75-30 cells were used for the in vitro experiments.
The expression of miR-138-5p in BC tissues and cells. a The expression levels of miR-1247 in bladder cancer tissues from TCGA dataset (n = 1000, p = 0.0369). b The expression levels of miR-138-5p in 20 pairs of human BC tissues and adjacent normal tissues were detected using quantitative real-time PCR. c The expression levels of miR-138-5p in BC cells and MCF-10A were examined by quantitative real-time PCR. Each bar represents the mean ± SD values. **p < 0.01; ***p < 0.001 vs. MCF-10A
Overexpression of miR-138-5p inhibited BC cell migration and invasion ability
To characterize the function of miR-138-5p on cell migration and invasion in BC, miR-138-5p mimic and corresponding miR-NC were synthesized and transfected into MDA-MB-231 and ZR-75-30 cells. After transfection for 48 h, quantitative real-time PCR monitored the efficiency of transfection. As expected, miR-138-5p expression was obviously enhanced in the miR-138-5p mimic-transfected group compared with the miR-NC transfected group in both MDA-MB-231 (Fig. 2a) and ZR-75-30 cells (Fig. 2b). Next, transwell assay was performed to investigate the effects of miR-138-5p expression on cell migration and invasion. As shown in Fig. 2c, the migrated cells were significantly decreased in miR-138-5p mimic group compared with miR-NC group in MDA-MB-231 (164.0 ± 8.7 vs. 286.3 ± 7.4) and ZR-75-30 (258.0 ± 17.7 vs. 366.0 ± 6.2) cells. Similarly, miR-138-5p overexpression significantly reduced the invasive cells from 340.3 ± 5.5 to 173.3 ± 8.4 in MDA-MB-231 and 157.7 ± 19.1 to 68.7 ± 5.9 in ZR-75-30 cells (Fig. 2d). These results indicate that overexpression of miR-138-5p significantly impaired the migration and invasiveness of BC cells in vitro.
Effects of miR-138-5p overexpression on BC cell migration and invasion ability. Quantitative real-time PCR was used to detect miR-138-5p mimic transfection efficiency in a MDA-MB-231 and b ZR-75-30 cells. c Cell migration and d invasion abilities in MDA-MB-231 and ZR-75-30 cells were tested with transwell assay. Each bar represents the mean ± SD values. **p < 0.01; ***p < 0.001 vs. miR-NC
RHBDD1 is a direct target of miR-138-5p
To better understand the pathogenesis of BC, we performed miR target analysis using the websites TargetScan and miRanda to explore the potential targets of miR-138-5p. Among these predicted targets, RHBDD1 was selected as a potential target gene of miR-138-5p. As shown in Fig. 3a, bioinformatics analysis described the putative binding sites of miR-138-5p to the RHBDD1 3′UTR. Hence, luciferase reporter assay was performed to validate this interaction. The reporter plasmids were co-transfected with miR-138-5p mimic or miR-NC in BC cells. The luciferase reporter assay revealed that miR-138-5p mimic transfection significantly decreased the luciferase activity of the WT 3′UTR of RHBDD1, without effects on the MUT or control plasmids in both MDA-MB-231 (Fig. 3b) and ZR-75-30 (Fig. 3c) cells. Moreover, accumulation of miR-138-5p resulted in reductions in RHBDD1 protein in MDA-MB-231 and ZR-75-30 cells (Fig. 3d). Furthermore, we determined the expression of RHBDD1 in BC tissues. As depicted in Fig. 3e, RHBDD1 had a significantly higher expression in BC tissues compared with adjacent tissues (p < 0.001). Through a two-tailed Pearson’s correlation analysis, we discovered that the expression of miR-138-5p was inversely correlated with RHBDD1 expression in BC tissues (Fig. 3f, p = 0.0491). This finding suggested that miR-138-5p directly binds to the 3′-UTR of the RHBDD1 transcript to inhibit the expression of RHBDD1 in BC cells.
RHBDD1 is a target of miR-138-5p. a Putative binding sites of miR-138-5p and RHBDD1 were predicted by miRTarBase and miRanda. b, c The luciferase activity was investigated in MDA-MB-231 and MCF-7 cells co-transfected with WT, MUT or control luciferase vectors and miR-138-5p mimic or miR-NC. Each bar represents the mean ± SD values. **p < 0.01 vs. miR-NC. d The effect of miR-138-5p on RHBDD1 protein expression was examined in MDA-MB-231 and ZR-75-30 cells. e The mRNA expression level of RHBDD1 relative to GAPDH in human BC tissues and adjacent tissues were detected using quantitative real-time PCR. f A negative correlation was found between expression of miR-138-5p and RHBDD1 in BC tissues
Addition of RHBDD1 ablated miR-138-5p-mediated suppression of cell migration and invasion
Since RHBDD1 was validated as a target of miR-138-5p, we further explored whether RHBDD1 was required for the miR-138-5p-mediated effect on migration and invasion in BC cells by conducting rescue experiments. An overexpression plasmid of RHBDD1 (pcDNA3.1-RHBDD1) was transfected into MDA-MB-231 cells treated with miR-138-5p mimic. Unsurprisingly, transwell assay demonstrated that inhibitory effects of cell migration (Fig. 4a) and invasion (Fig. 4b) caused by miR-138-5p mimic were partly abolished by overexpressing RHBDD1 in MDA-MB-231 cells. These data suggest that RHBDD1 and miR-138-5p appear to play an opposite role in BC cell migration and invasion.
Addition of RHBDD1 reverses miR-138-5p-mediated suppression on cell migration and invasion of BC cells. MDA-MB-231 cells were co-transfected with miR-138-5p mimic/miR-NC and with RHBDD1 overexpression plasmid/empty vector. The effects of miR-138-5p and RHBDD1 on a migration and b invasion ability were detected in MDA-MB-231 cells by transwell analysis. Each bar represents the mean ± SD values. **p < 0.01 vs. miR-NC; ##p < 0.01 vs. miR-138-5p mimic + vector
Addition of RHBDD1 counteracted the effects of miR-138-5p on EMT-related gene expression
To deeply investigate the molecular mechanisms underlying the effects of miR-138-5p and RHBDD1 on the migration and invasion of BC cells, EMT-related proteins in the above three groups were detected using western blotting. As shown in Fig. 5, the abundance of RHBDD1, N-cadherin and Vimentin was reduced, while E-cadherin was elevated in MDA-MB-231 cells with transfection of miR-138-5p mimics. Moreover, the introduction of RHBDD1 overexpression vector reversed the effect of miR-138-5p on the expression of RHBDD1 and EMT-related molecules in MDA-MB-231 cells.
Effects of miR-138-5p and RHBDD1 on EMT-related gene expression of MDA-MB-231 cells. MDA-MB-231 cells were co-transfected with miR-138-5p mimic/miR-NC and with RHBDD1 overexpression plasmid/empty vector. The expression levels of RHBDD1, E-cadherin, N-cadherin, and Vimenin protein were detected using Western blotting
Discussion
Mounting evidence suggests that miRNAs play key roles in proliferation, survival, differentiation, invasion, migration and metabolism; therefore, abnormal expression of miRNAs would impact the normal cell growth and development, leading to increased disease progression including cancers [23]. In the present study, we found miR-138-5p was significantly down-regulated in BC samples derived from TCGA database and fresh BC tissue from 20 patients with BC. Besides, it was found to be down-regulated in four BC cell lines in comparison with normal breast mammary epithelial cell line MCF-10a.
Several literature studies have established the critical role of miR-138-5p, showing that the gene is down-regulated in colorectal cancer and pancreatic cancer, and that enforced expression of miR-138-5p favors suppression of cancer cell proliferation in vitro and inhibition of tumor formation in vivo [9, 10]. In malignant retinoblastoma, miR-138-5p was found to be lowly expressed and the upregulation of this gene correlates with decreased cell viability, migration, invasion, and increased apoptosis of tumor cells [24]. MiR-138-5p is markedly down-regulated in bladder cancer and endogenous overexpression of miR-138-5p also led to significant inhibition of proliferation and invasion [25]. According to these studies, miR-138-5p is suspected to have a functional role as a tumor suppressor in BC. Expectedly, restoration of miR-138-5p attenuated proliferation, invasion and migration in two BC cell lines, MDA-MB-231 and ZR-75-30. To our knowledge, this is the first investigation of miR-138-5p in BC biology.
MiRNAs are known to silence translation of their target mRNAs through pairing to “seedless” 3′UTR miRNA recognition sequences, which interacts mainly with the 5′-UTR of miRNAs in mammalians [26]. Bioinformatic predictions suggest that more than 30% of protein-coding genes can be regulated by miRNAs. In our opinion, identification of key genes regulated by anti-oncogenic factor miR-138-5p is required to reveal the molecular mechanisms of miR-138-5p in BC progression. We identified putative targets of miR-138-5p using two bioinformatics approach, including TargetScan Human7.2 and miRanda software. We narrowed down the targets of miR-138-5p to one gene, RHBDD1, according to the known role of RHBDD1 in BC and some other types of malignancies. Using luciferase reporter assay, we show that the miR-138-5p 5′UTR sequence “GUGGUCGA” could directly bind to RHBDD1 3′UTR sequence “CACCAGCA”. Western Blot analysis revealed that miR-138-5p is a powerful posttranscriptional regulator of RHBDD1. Further, increased expression of RHBDD1 ablated miR-138-5p-mediated suppression of cell migration and invasion of BC cells. These studies demonstrated that miR-138-5p exerted anti-oncogenic functions in BC through negatively regulating RHBDD1.
The EMT is a key determinant in malignant cancer cells in the acquisition of invasive and migration features [27]. What’s more, loss of E-cadherin and amplification of N-cadherin and Vimentin are frequently associated with cancer migration and invasion [28]. To gain a deeper insight into the molecular mechanisms responsible for miR-138-5p and RHBDD1-mediated regulation of migration and invasion of BC cells, we investigated expression levels of E-cadherin, N-cadherin and Vimentin. Our results revealed that overexpression of miR-138-5p significantly increased E-cadherin and decreased N-cadherin and Vimentin, dysregulation of these proteins could partially be restored by re-introduction of RHBDD1. These results strongly suggest that miR-138-5p blocks migration and invasion of BC cells by regulating of EMT markers (E-cadehrin, N-cadherin and Vimentin) through repressing RHBDD1. It has been shown that RHBDD1 functioned as an inducer of colorectal cancer cells invasion and migration via regulating phosphorylation of β-catenin at the site Ser 675 and Ser 552 to trigger the Wnt signaling pathway [29]. In a further, ZEB1, a potent activator of EMT, can also be regulated by RHBDD1 and is responsible for RHBDD1-mediated invasion and migration of colorectal cancer cells [29]. In the future, we will focus on whether RHBDD1 can directly or cooperatively with some key factors such as β-catenin and ZEB to regulate the expression of E-cadherin, N-cadherin and Vimentin in BC cells with overexpression of miR-138-5p.
In summary, loss of miR-138-5p was a frequent event in BC. MiR-138-5p suppressed proliferation, invasion and migration via directly targeting RHBDD1, which indicates that miR-138-5p is an anti-oncogenic factor in BC growth and metastasis. The identification and characterization of miR-138-5p target will deepen our understanding of molecular mechanisms behind BC development and progression.
References
DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67:439–48.
Rheinbay E, Parasuraman P, Grimsby J, Tiao G, Engreitz JM, Kim J, et al. Recurrent and functional regulatory mutations in breast cancer. Nature. 2017;547:55–60.
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6.
Peng Y, Croce CM. The role of microRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004.
Hata A, Kashima R. Dysregulation of microRNA biogenesis machinery in cancer. Crit Rev Biochem Mol Biol. 2016;51:121–34.
Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12.
Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer: a brief overview. Adv Biol Regul. 2015;57:1–9.
Beg MS, Brenner AJ, Sachdev J, Borad M, Kang YK, Stoudemire J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig New Drugs. 2017;35:180–8.
Zhao L, Yu H, Yi S, Peng X, Su P, Xiao Z, et al. The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget. 2016;7:45370–84.
Yu C, Wang M, Li Z, Xiao J, Peng F, Guo X, et al. MicroRNA-138-5p regulates pancreatic cancer cell growth through targeting FOXC1. Cell Oncol (Dordr). 2015;38:173–81.
Gao W, Lam JW, Li JZ, Chen SQ, Tsang RK, Chan JY, et al. MicroRNA-138-5p controls sensitivity of nasopharyngeal carcinoma to radiation by targeting EIF4EBP1. Oncol Rep. 2017;37:913–20.
Gao Y, Fan X, Li W, Ping W, Deng Y, Fu X. miR-138-5p reverses gefitinib resistance in non-small cell lung cancer cells via negatively regulating G protein-coupled receptor 124. Biochem Biophys Res Commun. 2014;446:179–86.
Zhu D, Gu L, Li Z, Jin W, Lu Q, Ren T. miR-138-5p suppresses lung adenocarcinoma cell epithelial-mesenchymal transition, proliferation and metastasis by targeting ZEB2. Pathol Res Pract. 2019;215:861–72.
Lohi O, Urban S, Freeman M. Diverse substrate recognition mechanisms for rhomboids: thrombomodulin is cleaved by mammalian rhomboids. Curr Biol. 2004;14:236–41.
Wolf EV, Zeissler A, Vosyka O, Zeiler E, Sieber S, Verhelst SH. A new class of rhomboid protease inhibitors discovered by activity-based fluorescence polarization. PLoS One. 2013;8:e72307.
Yogev S, Schejter ED, Shilo BZ. Drosophila EGFR signalling is modulated by differential compartmentalization of rhomboid intramembrane proteases. EMBO J. 2008;27:1219.
McQuibban GA, Saurya S, Freeman M. Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature. 2003;423:537–41.
Buguliskis JS, Brossier F, Shuman J, Sibley LD. Rhomboid 4 (ROM4) affects the processing of surface adhesins and facilitates host cell invasion by Toxoplasma gondii. PLoS Pathog. 2010;6:e1000858.
Brossier F, Starnes GL, Beatty WL, Sibley LD. Microneme rhomboid protease TgROM1 is required for efficient intracellular growth of Toxoplasma gondii. Eukaryot Cell. 2008;7:664–74.
Wang Y, Guan X, Fok KL, Li S, Zhang X, Miao S, et al. A novel member of the Rhomboid family, RHBDD1, regulates BIK-mediated apoptosis. Cell Mol Life Sci. 2008;65:3822–9.
Liu XN, Tang ZH, Zhang Y, Pan QC, Chen XH, Yu YS, et al. Lentivirus-mediated silencing of rhomboid domain containing 1 suppresses tumor growth and induces apoptosis in hepatoma HepG2 cells. Asian Pac J Cancer Prev. 2013;14:5–9.
Zhang X, Zhao Y, Wang C, Ju H, Liu W, Zhang X, et al. Rhomboid domain-containing protein 1 promotes breast cancer progression by regulating the p-Akt and CDK2 levels. Cell Commun Signal. 2018;16:65.
Kohlhapp FJ, Mitra AK, Lengyel E, Peter ME. MicroRNAs as mediators and communicators between cancer cells and the tumor microenvironment. Oncogene. 2015;34:5857.
Wang Z, Yao YJ, Zheng F, Guan Z, Zhang L, Dong N, et al. Mir-138-5p acts as a tumor suppressor by targeting pyruvate dehydrogenase kinase 1 in human retinoblastoma. Eur Rev Med Pharmacol Sci. 2017;21:5624–9.
Yang R, Liu M, Liang H, Guo S, Guo X, Yuan M, et al. miR-138-5p contributes to cell proliferation and invasion by targeting Survivin in bladder cancer cells. Mol Cancer. 2016;15:82.
Gosline SJC, Gurtan AM, JnBaptiste CK, Bosson A, Milani P, Dalin S, et al. Elucidating microRNA regulatory networks using transcriptional, post-transcriptional, and histone modification measurements. Cell Rep. 2016;14:310–9.
Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGF-beta and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–74.
Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C, et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat. 2010;124:317–26.
Zhang M, Miao F, Huang R, Liu W, Zhao Y, Jiao T, et al. RHBDD1 promotes colorectal cancer metastasis through the Wnt signaling pathway and its downstream target ZEB1. J Exp Clin Cancer Res. 2018;37:22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Patient samples were collected with written informed consent in accordance with the Declaration of Helsinki and under Ethics Committee of the First Hospital of Lanzhou University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Zhao, C., Ling, X., Li, X. et al. MicroRNA-138-5p inhibits cell migration, invasion and EMT in breast cancer by directly targeting RHBDD1. Breast Cancer 26, 817–825 (2019). https://doi.org/10.1007/s12282-019-00989-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-019-00989-w