Abstract
Background
Breast cancer growth is generally expected to differ between tumor subtypes. We aimed to evaluate tumor doubling time (DT) using ultrasonography and verify whether each tumor subtype has a unique DT.
Methods
This retrospective study included 265 patients with invasive breast cancer who received serial ultrasonography between diagnosis and surgery. Tumor diameters were measured in three directions and DTs were calculated according to an exponential growth model using the volume change during serial ultrasonography. We investigated the relationships between DT, tumor subtype, and histopathological factors.
Results
Volumes did not change in 95 (36%) of 265 tumors and increased in 170 (64%) tumors during serial ultrasonography (mean interval, 56.9 days). The mean volume increases of all tumors and volume-increased tumors were 22.1% and 34.5%, respectively. Triple-negative tumors had greater volume increases (40% vs. 20%, p = 0.001) and shorter DT (124 vs. 185 days, p = 0.027) than estrogen receptor (ER)+/human epidermal growth factor receptor 2 (HER2)– tumors. Volume-increased tumors had higher Ki-67 indices than those of volume-stable tumors in ER+/HER2− (p = 0.002) and ER+/HER2+ tumors (p = 0.011) and higher histological grades in all tumors except triple-negative tumors (p < 0.001). Triple-negative tumors with DTs < 90 days (short-DT) showed higher Ki-67 indices than those with DTs > 90 days (long-DT) (p = 0.008). In ER+/HER2− tumors, histological grades were higher for short-DT than for long-DT tumors (p = 0.022).
Conclusion
Differences in tumor DT depending on breast cancer subtype, Ki-67 index, and histological grade were confirmed using serial ultrasonography even during preoperative short interval.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Institutional or patient’s personal circumstances sometimes prolong the waiting time between the diagnosis of breast cancer and subsequent surgery. The waiting time at our institution is usually > 2 months mostly due to crowded surgical schedule, which is longer than the time of a study in the United States [1]. Several studies have shown that the survival outcomes in patients with early stage breast cancer are affected by the length of the interval between diagnosis and surgery [2, 3]. Waiting for treatment also causes anxiety because both patients and physicians are uncertain about the rate of patient-specific tumor progression. Actually, we sometimes encounter a rapidly progressing tumor during the waiting time for surgery because breast cancer is a heterogeneous disease presenting a wide range of tumor growth rates and variable clinical courses [4].
Breast cancer growth is generally expected to differ between tumor subtypes; however, the tumor growth cannot usually be observed prospectively because patients are required to be treated as soon as possible after the diagnosis. In previous studies, tumor growth rate has been mainly evaluated retrospectively by screening mammography [5,6,7,8]. However, mammography is not sufficiently reliable for the measurement of tumor size, particularly in mammographically dense breasts and small tumors [9,10,11]. In contrast, ultrasonography is more accurate in measuring tumor size even in dense breasts, and repetitive evaluation in a short interval is feasible because ultrasonography examination is a non-ionizing and non-invasive method. Recently, two reports described the breast cancer growth rate between diagnosis and surgery using ultrasonography [12, 13]; however, the two reports showed conflicting results with respect to the differences of tumor growth rate depending on tumor subtypes. Yoo et al. reported that tumor growth rate did not significantly differ according to the tumor molecular subtypes. On the contrary, Lee et al. showed that breast cancers with aggressive molecular subtypes showed faster tumor growth rate. If we can predict tumor growth rate at the time of diagnosis, we can design a more personalized precision treatment plan allowing an acceptable treatment waiting time for each patient. The aim of this study was to evaluate the tumor doubling time (DT) of breast cancers using serial ultrasonography between diagnosis and surgery and to verify whether each tumor subtype has a unique DT.
Materials and methods
Study population
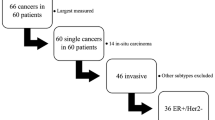
This retrospective study was approved by our institutional review board and the requirement to obtain informed consent was waived. We reviewed 309 patients with invasive breast cancer who had preoperatively received serial ultrasonographic examinations between diagnosis and surgery from April 2014 to September 2016. The second ultrasonographic examinations immediately before surgery were performed for marking the tumor position and extent for surgery planning. Patients who received neoadjuvant chemotherapy or hormonal therapy were not included from the study objective. Of 309 patients, 44 patients who received 11–13G vacuum-assisted biopsy (VAB) between serial ultrasonographic examinations were excluded because multiple tissue sampling by VAB might reduce tumor size. Finally, we included 265 patients (mean age, 60.1 years; range, 33–85 years). If patients had more than two preoperative ultrasonographic examinations, the initial and final preoperative ultrasonography was reviewed.
Ultrasonographic examinations and evaluation of tumor growth rate
All ultrasonographic examinations were performed using F75, ARIETTA 70, or ARIETTA 850 instruments (Hitachi Ltd., Tokyo, Japan) with a 5–18 MHz linear transducer or an Aplio 500 instrument (Toshiba Medical Systems, Tokyo, Japan) with a 5–12 MHz linear transducer. One of the four certified sonographers who had 10–15 years of experience in breast ultrasonography performed the examinations. The sonographers prospectively measured the tumor diameters during the examinations in three directions: the longest axis and the other two orthogonal axes (Figs. 1, 2). The tumor volumes were calculated using ellipsoid approximation with the formula: V = 4/3π abc, where a, b, and c were the three radii of the tumor. The volume increase (%) was defined as [(V2–V1)/V1] × 100, where V1 and V2 represent lesion volume on the initial and second preoperative ultrasonography, respectively. The tumor that showed even a slight increase in volume on second ultrasonography was classified as “volume-increased tumor” and the tumor which did not show size change was classified as “volume-stable tumor.” When tumor volume decreased on second ultrasonography, the volume increase was assumed to be 0% and the tumor was classified into “volume-stable tumor.” A tumor DT was calculated using the Collins method [14, 15], the interval in days (t) between the initial and second ultrasonographic examinations, and the change in tumor volume: DT = t · log 2/(log V2–log V1). We compared these size factors with the molecular subtype, Ki-67 index, histological grade, and lymph node status. Surgically evaluated axillary lymph nodes (263 of 265 cases, 99%) were included for comparison.
Ultrasonographic images of a 52-year-old woman with an ER–/HER2+ invasive ductal carcinoma. a, b On the first ultrasonographic examination, the diameters were measured in three directions using the largest plane of the tumor (a, 14 × 9 mm) and its orthogonal plane (b, 10 mm). Then, the tumor volume was calculated using ellipsoid approximation (0.7 cm3). c, d After 56 days and on the second ultrasonographic examination prior to surgery, the tumor volume increased to 1.2 cm3, and the doubling time was determined to be 61 days
Histopathological evaluation
All lesions were histologically diagnosed by surgery and all surgical specimens were evaluated by a dedicated pathologist. The histological grade, estrogen receptor (ER), human epidermal growth factor receptor 2 (HER2), Ki-67 index, and axillary lymph node status were included in the evaluation. The histological grade was determined as the summation of scores for the tubular formation, nuclear pleomorphism, and mitotic counts according to the Elston–Ellis modification of the Scarff–Bloom–Richardson grading system [16]. The expressions of ER, HER2, and Ki-67 index were examined by immunohistochemical analysis. ER positivity was defined as detection of ≥ 1% positively stained invasive tumor cells. HER2-positivity was defined as 3 + staining for HER2 protein overexpression or 2 + staining with HER2 gene amplification by fluorescent in situ hybridization. Ki-67 staining was evaluated as a percentage of nuclei showing a positive reaction. The molecular breast cancer subtypes were classified into four groups on the basis of ER and HER2 status as follows: ER-positive and HER2-negative (ER+/HER2−), ER-positive and HER2-positive (ER+/HER2+), ER-negative and HER2-positive (ER–/HER2+), and ER-negative and HER2-negative (triple-negative tumors).
Statistical analysis
All statistical analyses were performed using SPSS v22.0 (IBM Japan, Tokyo). The Kruskal–Wallis test was used to compare the differences among the four tumor subtypes. Subsequently, a post hoc test in SPSS was performed to detect differences between each pair of subtypes. The Mann–Whitney U test was used to compare the Ki-67 indices, histological grades, and lymph node status between the volume-increased and the volume-stable tumors and between tumors with a DT of < 90 days and tumors with a DT of > 90 days. Two-sided p values < 0.05 were considered as indicating significant differences.
Results
Patient and tumor characteristics (Table 1)
Of the 265 breast cancers examined, 209 (79%) were ER+/HER2−, 15 (6%) were ER+/HER2+, 13 (5%) were ER–/HER2+, and 28 (11%) were triple-negative tumors. Significant differences in patient ages among tumor subtypes were observed (p = 0.006). Subsequent post hoc tests showed that patients with triple-negative tumors were significantly older than those with ER+/HER2− tumors (p = 0.003). Histological types were invasive ductal carcinomas of no special type (n = 206), invasive lobular carcinomas (n = 28), and other special types (n = 31). Significant differences in histological grades and Ki-67 indices among tumor subtypes were observed (p for both < 0.001). In post hoc tests, the ER+/HER2− tumors showed significantly lower histological grades and Ki-67 indices than those of other tumor subtypes (p < 0.05 and p < 0.005, respectively). There were no significant differences in axillary lymph node statuses among tumor subtypes.
Tumor volume changes and DTs (Table 2)
The mean time between the initial and second ultrasonographic examination was 56.9 days. The initial tumor diameters and volumes of ER+/HER2+ (28.4 mm and 7.1 cm3) and ER–/HER2+ subtypes (34.0 mm and 9.7 cm3) were significantly greater than those of ER+/HER2− tumors (17.8 mm and 2.3 cm3) (post hoc tests, p < 0.05 for all comparisons). The initial tumor diameters and volumes of ER+/HER2+ tumors were also greater than those of triple-negative tumors (18.0 mm and 2.3 cm3) (post hoc tests, p for both < 0.05). The mean volume increase of all 265 breast cancers was 22.1% (range 0–215.2). In volume-increased tumors (170 of 265), the mean volume increase was 34.5%. The triple-negative tumors showed greater volume increases than the ER+/HER2− tumors (40% vs. 20%, p = 0.001). The median DT of the volume-increased tumors was 174.1 days. The DT was shortest in the ER–/HER2+ tumors (85.4 days), followed in order by triple-negative (123.6 days), ER+/HER2+ (165.3 days), and ER+/HER2− tumors (185.0 days). There was a significant difference between the triple-negative and the ER+/HER2− tumors (p = 0.027). There was no significant difference between the ER–/HER2+ and the ER+/HER2− tumors, probably because of the small numbers of ER–/HER2+ tumors. The DT of volume-stable tumors could not be calculated from the Collins method formula because the calculation results became “infinity.”
Comparison between the volume-increased tumors and volume-stable tumors (Table 3)
The calculated tumor volumes appeared stable between the initial and second ultrasonography in 95 (36%) of 265 tumors and increased in 170 (64%) of 265 tumors. Volume increases were more frequent in the triple-negative tumors than in the ER+/HER2− tumors (86% vs. 60%, p = 0.044).
The Ki-67 indices were higher in the volume-increased tumors than in the volume-stable tumors among all tumors (median, 20% vs. 12%, p < 0.001), ER+/HER2− tumors (18% vs. 11%, p = 0.002), and ER+/HER2+ tumors (59% vs. 16%, p = 0.011). Histological grades were higher in the volume-increased tumors than in the volume-stable tumors among all tumors (p < 0.001), ER+/HER2− (p = 0.002), ER+/HER2+ tumors (p = 0.029), and ER–/HER2+ tumors (p = 0.027).
There was no significant difference in axillary lymph node statuses between the volume-increased and volume-stable tumors.
Comparison between tumors with a DT of < 90 days (short DT) and tumors with a DT of > 90 days (long DT) (Table 4)
Short DTs were more frequent in the triple-negative tumors than in the ER+/HER2− tumors [8/28 (29%) and 23/209 (11%), respectively, p = 0.037]. The short-DT tumors had significantly higher Ki-67 indices than those of the long-DT tumors among all tumors (median, 33% vs. 16%, p = 0.001) and triple-negative tumors (89% vs. 27%, p = 0.008). Furthermore, the short-DT tumors had significantly higher histological grades than those of the long-DT tumors among all tumors (p < 0.001) and ER+/HER2− tumors (p = 0.022). There was no significant difference in axillary lymph node statuses between the short and long DT tumors.
Discussion
The time between the diagnosis of breast cancer and surgery should be minimized [2, 3], but the waiting time for surgery is often prolonged for unavoidable reasons, such as patient’s desire for a second opinion, limitations in the patient’s or physician’s schedule, additional testing, or crowded surgical schedule. The waiting time at our institution is usually > 2 months, which is longer than the time of a study in the United States [1]. If we can predict the tumor growth rate at diagnosis, we can manage surgical schedules more effectively according to the patient-specific tumor growth rate, which will also result in reducing a patient’s and physician’s preoperative anxiety.
Several possible growth patterns of human cancer have been proposed [5, 14, 17, 18]. The exponential growth model has been classically used since Collins et al. reported that a specific growth rate quantified as DT was a characteristic of an individual tumor [14]. The Gompertz model assumes that a tumor initially grows rapidly but slows with increasing size [17]. For short observation periods, the exponential model is commonly used because the Gompertz model lacks necessary parameters [19, 20]. We used the classical exponential growth model because it is a widely accepted and established simple method. Kusama et al. [6] calculated the DT (median, 3.5 months) for 199 patients with primary or metastatic breast cancers. They reported associations of rapid tumor growth with poor prognosis and of slow growth with relatively favorable prognosis. They estimated the DT as infinite when the tumors showed no growth during observation periods and excluded such cases from DT calculations. Heuser et al. [21] calculated DTs (mean, 325 days) of primary breast cancers from serial mammograms in a screening population and excluded no-growth tumors. Fournier et al. [19] calculated DT (mean, 212 days) by measuring the growth of the tumor shadow in serial mammograms, and the results corresponded with the exponential model. They found no correlation between DT and histological differentiation of the tumors. Previous mammographic studies commonly evaluated tumor shadow on serial screening mammograms retrospectively. Consequently, slow-growing tumors tended to be detected, and fast-growing tumors not detected on previous mammograms, so-called “interval cancers,” were excluded. Hence, the growth rate distribution probably skewed toward slow growth.
Recent molecular characterization of breast cancer has enabled personalized therapeutic strategies. However, most previous mammographic studies referring to breast cancer growth rate were performed > 20 years ago and did not discuss molecular subtypes. Recently, Ryu et al. [15] evaluated DT by serial ultrasonography. They apparently were the first to report a significant difference in tumor growth rates between tumor molecular subtypes. The DT of ER-positive tumors was 2.4-fold higher than that of the triple-negative tumors. Most of their patients had been initially assessed as having benign lesions by ultrasonography. A selection bias was inevitable because only the tumors with benign-like appearance that had been initially recommended observation were included, and the tumors treated without observation were excluded. However, their study is meaningful for demonstrating the difference in tumor growth rates among tumor subtypes and providing a clue for breast cancer surveillance.
Ultrasonography can be repeatedly performed to evaluate tumor growth because it is facile, less expensive, non-invasive, and non-ionizing. There have been only two reports describing breast cancer growth rate between diagnosis and surgery using serial ultrasonography [12, 13]. Yoo et al. [12] analyzed growth rates of 957 patients by performing two preoperative breast ultrasonography. The initial and second examination interval (median, 28 days) was remarkably shorter than those of previous mammographic studies. They found that 55% of the patients showed increased size during the short ultrasonography intervals and tumor growth rate did not significantly differ according to the tumor molecular subtypes. On the contrary, Lee et al. [13], from the same institution with Yoo et al. but using different data and method, found that breast cancers with aggressive molecular subtypes showed faster tumor growth during wait times for surgery. One of the causes of the different results of the two studies was that Yoo et al. used only single diameter of tumors based on the medical records assuming the tumor shape as a sphere. Lee et al. used three perpendicular diameters, which were the same with our method, assuming the tumor as a spheroid. Breast cancers often grow to extend in one axis forming an oblate spheroid or a cylinder [21]. Heuser et al. reported that using a different geometric formula such as sphere, spheroid, or cylinder significantly affected the calculated DT [21]. Moreover, Yoo et al. reported much shorter DTs (mean, 15 days) than those of other studies because they incorporated negative DTs of apparent size-decreased tumors into the calculation without adjustment [12]. Although Lee et al. also included apparent size-decrease into calculation, they could reduce the measurement deviation by three radiologists measurement in the same probe directions on PACS workstations [13]. Spontaneous regression of breast cancer without any treatment is extremely rare and possibly caused by immunological response [22, 23], so we assumed that tumor size reductions on ultrasonography during the relatively short interval were measurement variations and interobserver variabilities. Hence, we excluded the tumors showing no growth from the DT calculation, similar to previous mammographic studies [6, 21]. Although our DT results might skew toward fast-growing tumors, the median DT in our study (174 days) was within previously reported ranges assessed by mammography or ultrasonography [4, 6, 19, 20, 24, 25]. Our’s and Lee’s [13] studies showed that it would be possible to show the difference of tumor growth rate depending on tumor subtypes even during short preoperative interval. In addition, we found that triple-negative tumors with short-DT showed higher Ki-67 indices than those with long-DT (p = 0.008). It suggested that although tumor growth rates of the triple-negative subgroup were generally fast, very fast tumors with higher Ki-67 expression and less fast tumors with relatively lower Ki-67 were mixed. Triple-negative tumors show genetic, histological, and clinical differences [26,27,28,29]. Such heterogeneity of triple-negative tumors may explain why they had longer DTs than those of the ER–/HER2+ tumors.
Similar to previous studies [12, 25], higher histological grade was associated with higher growth rate in our study. Higher Ki-67 index was also associated with higher growth rates, which was reasonable because Ki-67 is a surrogate marker of the cell proliferation rate and histological grade is strongly correlated with Ki-67 index [30]. Although higher growth rate has reportedly been associated with axillary lymph node metastasis [12, 25, 31, 32], the significant association was not found in our study. Breast cancers with lymph node involvement are subjected to be candidate for neoadjuvant chemotherapy. Since we did not include patients who received neoadjuvant chemotherapy, the association might be obscured.
Ultrasonography appears to be more accurate than mammography, at least in dense breasts, because tumor size is not influenced by density [10, 11]. Although serial magnetic resonance imaging (MRI) may be more objective, repetitive contrast-enhanced MRI during a short period is too expensive and time consuming. Recently developed automated breast volume scanners may become standard for objectively monitoring tumor volume changes [33, 34].
This study had several limitations. First, because we focused on natural tumor size changes, we excluded patients who received neoadjuvant chemotherapy. Hence, the number of HER2-positive and triple-negative tumors and the tumors with lymph node metastasis decreased. The exclusion of such aggressive tumors may have caused an underestimation in the differences in DTs among tumor subtypes. Second, slight volume increases might be difficult to identify by ultrasonography over relatively short intervals, particularly in non-mass lesions. Consequently, 36% of the tumors did not show increases on second ultrasonography. However, the tumor volume change frequency itself is meaningful. Slight volume increases during the preoperative period are not clinically important. The critical issue is to detect rapidly growing tumors with very short DTs that affect surgical or chemotherapy outcomes and planning. Our preoperative serial ultrasonography method is practical and applicable to daily clinical examinations.
In conclusion, differences in tumor DT depending on breast cancer subtype, Ki-67 index, and histological grade were confirmed using serial ultrasonography even during preoperative short interval. Triple-negative or ER–/HER+ tumors, high histological grade, and high Ki-67 index were valid predictors of fast tumor growth, which possibly indicates the need for immediate treatment and possibly considers surgery more preferential than other tumors if the surgical waiting time is long.
References
Richardson LC, Royalty J, Howe W, Helsel W, Kammerer W, Benard VB. Timeliness of breast cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program, 1996–2005. Am J Public Health. 2010;100:1769–76.
Bleicher RJ, Ruth K, Sigurdson ER, Beck JR, Ross E, Wong YN, et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol. 2016;2:330–9.
Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112(Suppl 1):92–107.
Friberg S, Mattson S. On the growth rates of human malignant tumors: implications for medical decision making. J Surg Oncol. 1997;65:284–97.
Hart D, Shochat E, Agur Z. The growth law of primary breast cancer as inferred from mammography screening trials data. Br J Cancer. 1998;78:382–7.
Kusama S, Spratt JS Jr, Donegan WL, Watson FR, Cunningham C. The gross rates of growth of human mammary carcinoma. Cancer. 1972;30:594–9.
Spratt JS, Greenberg RA, Heuser LS. Geometry, growth rates, and duration of cancer and carcinoma in situ of the breast before detection by screening. Cancer Res. 1986;46:970–4.
Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, Aalen OO, Tretli S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008;10:R41.
Ho JM, Jafferjee N, Covarrubias GM, Ghesani M, Handler B. Dense breasts: a review of reporting legislation and available supplemental screening options. AJR Am J Roentgenol. 2014;203:449–56.
Bosch AM, Kessels AG, Beets GL, Rupa JD, Koster D, van Engelshoven JM, et al. Preoperative estimation of the pathological breast tumour size by physical examination, mammography and ultrasound: a prospective study on 105 invasive tumours. Eur J Radiol. 2003;48:285–92.
Fasching PA, Heusinger K, Loehberg CR, Wenkel E, Lux MP, Schrauder M, et al. Influence of mammographic density on the diagnostic accuracy of tumor size assessment and association with breast cancer tumor characteristics. Eur J Radiol. 2006;60:398–404.
Yoo TK, Min JW, Kim MK, Lee E, Kim J, Lee HB, et al. In vivo tumor growth rate measured by US in preoperative period and long term disease outcome in breast cancer patients. PLoS One. 2015;10:e0144144.
Lee SH, Kim YS, Han W, Ryu HS, Chang JM, Cho N, et al. Tumor growth rate of invasive breast cancers during wait times for surgery assessed by ultrasonography. Medicine (Baltimore). 2016;95:e4874.
Collins VP, Loeffler RK, Tivey H. Observations on growth rates of human tumors. Am J Roentgenol Radium Ther Nucl Med. 1956;76:988–1000.
Ryu EB, Chang JM, Seo M, Kim SA, Lim JH, Moon WK. Tumour volume doubling time of molecular breast cancer subtypes assessed by serial breast ultrasound. Eur Radiol. 2014;24:2227–35.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Norton L. A Gompertzian model of human breast cancer growth. Cancer Res. 1988;48:7067–71.
Mehrara E, Forssell-Aronsson E, Ahlman H, Bernhardt P. Specific growth rate versus doubling time for quantitative characterization of tumor growth rate. Cancer Res. 2007;67:3970–5.
von Fournier D, Weber E, Hoeffken W, Bauer M, Kubli F, Barth V. Growth rate of 147 mammary carcinomas. Cancer. 1980;45:2198–207.
Peer PG, van Dijck JA, Hendriks JH, Holland R, Verbeek AL. Age-dependent growth rate of primary breast cancer. Cancer. 1993;71:3547–51.
Heuser L, Spratt JS, Polk HC. Jr. Growth rates of primary breast cancers. Cancer. 1979;43:1888–94.
Dussan C, Zubor P, Fernandez M, Yabar A, Szunyogh N, Visnovsky J. Spontaneous regression of a breast carcinoma: a case report. Gynecol Obstet Invest. 2008;65:206–11.
Tokunaga E, Okano S, Nakashima Y, Yamashita N, Tanaka K, Akiyoshi S, et al. Spontaneous regression of breast cancer with axillary lymph node metastasis: a case report and review of literature. Int J Clin Exp Pathol. 2014;7:4371–80.
Kuroishi T, Tominaga S, Morimoto T, Tashiro H, Itoh S, Watanabe H, et al. Tumor growth rate and prognosis of breast cancer mainly detected by mass screening. Jpn J Cancer Res. 1990;81:454–62.
Tabbane F, Bahi J, Rahal K, el May A, Riahi M, Cammoun M, et al. Inflammatory symptoms in breast cancer. Correlations with growth rate, clinicopathologic variables, and evolution. Cancer. 1989;64:2081–9.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67.
Lehmann BD, Jovanovic B, Chen X, Estrada MV, Johnson KN, Shyr Y, et al. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS One. 2016;11:e0157368.
Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–98.
Pareja F, Geyer FC, Marchio C, Burke KA, Weigelt B, Reis-Filho JS. Triple-negative breast cancer: the importance of molecular and histologic subtyping, and recognition of low-grade variants. NPJ Breast Cancer. 2016;2:16036.
Ono M, Tsuda H, Yunokawa M, Yonemori K, Shimizu C, Tamura K, et al. Prognostic impact of Ki-67 labeling indices with 3 different cutoff values, histological grade, and nuclear grade in hormone-receptor-positive, HER2-negative, node-negative invasive breast cancers. Breast Cancer. 2015;22:141–52.
Galante E, Gallus G, Guzzon A, Bono A, Bandieramonte G, Di Pietro S. Growth rate of primary breast cancer and prognosis: observations on a 3- to 7-year follow-up in 180 breast cancers. Br J Cancer. 1986;54:833–6.
Heuser LS, Spratt JS, Kuhns JG, Chang AF, Polk HC Jr, Buchanan JB. The association of pathologic and mammographic characteristics of primary human breast cancers with “slow” and “fast” growth rates and with axillary lymph node metastases. Cancer. 1984;53:96–8.
Zhang S, Ding Y, Zhou Q, Wang C, Wu P, Dong J. Correlation Factors Analysis of Breast Cancer Tumor Volume Doubling Time Measured by 3D-Ultrasound. Med Sci Monit. 2017;23:3147–53.
Girometti R, Zanotel M, Londero V, Linda A, Lorenzon M, Zuiani C. Automated breast volume scanner (ABVS) in assessing breast cancer size: A comparison with conventional ultrasound and magnetic resonance imaging. Eur Radiol. 2017.
Acknowledgements
We thank Enago (http://www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Nakashima, K., Uematsu, T., Takahashi, K. et al. Does breast cancer growth rate really depend on tumor subtype? Measurement of tumor doubling time using serial ultrasonography between diagnosis and surgery. Breast Cancer 26, 206–214 (2019). https://doi.org/10.1007/s12282-018-0914-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-018-0914-0