Abstract
Background
Aromatase inhibitors (AI) have been established as the gold-standard therapy for postmenopausal patients. Worldwide, adjuvant denosumab at a dose of 60 mg twice per year reduces the risk of clinical fractures in postmenopausal patients with breast cancer who received AI. However, the efficacy of denosumab in the treatment of AI-associated bone loss had not been prospectively evaluated in Japan. Previously, we reported the 12-month effect of denosumab in Japanese patients for the first time; the primary endpoint was the change in the percentage of bone mineral density (BMD) of the lumbar spine from baseline to 12 months.
Methods
This secondary follow-up study prospectively evaluated the change in the percentage of BMD of the lumbar spine from baseline to 24 months. Postmenopausal women with early-stage, histologically confirmed, hormone receptor-positive, invasive breast cancer who were receiving or scheduled to receive AI were included. Denosumab was administered subcutaneously on day 1 of the study and then 6, 12, 18, and 24 months. The lumbar spine and bilateral femoral neck BMD was measured at baseline and 6, 12, 18, and 24 months.
Results
At 18 and 24 months, the lumbar spine BMD increased by 5.9 and 7.0%, respectively. The femoral neck BMD also increased. Grade ≥ 2 hypocalcemia, osteonecrosis of the jaw, and atypical femoral fractures did not occur.
Conclusions
Our prospective study showed that semiannual treatment with denosumab was associated with continuously increased BMD in Japanese women receiving adjuvant AI therapy for up to 24 months, regardless of prior AI treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the second most common cancer worldwide. The age standardized mortality rates of breast cancer showed a generally increasing trend in Japan [1]. Aromatase inhibitors (AIs) are commonly used in the treatment of postmenopausal women with hormone receptor-positive breast cancer and have been shown to decrease bone mineral density (BMD) and increase the risk of bone fragility fractures [2]. AI-associated bone loss leads to a marked increase in bone resorption, with a two- to fourfold increase in bone loss compared with physiologic postmenopausal BMD loss [3,4,5,6,7,8,9,10,11,12].
The International Osteoporosis Foundation (IOF) recommends that all patients who receive AI therapy and have any two of the following risk factors should receive antiresorptive therapy: T score < − 1.5, age > 65 years, low body mass index (< 20 kg/m2), family history of hip fracture, personal history of fragility fracture after age 50, oral corticosteroid use for > 6 months, and current or history of smoking. Furthermore, the IOF recommends that any patient with a T score < − 2.0 in whom an AI is initiated should receive antiresorptive therapy, regardless of the presence of other risk factors. We previously reported the results of a 12-month, nonrandomized, prospective study which demonstrated that denosumab, a fully human monoclonal antibody against the receptor activator of nuclear-factor kappa-B ligand, increased the BMD of the lumbar spine and femoral neck in patients with hormone receptor-positive breast cancer who received adjuvant AI therapy and demonstrated evidence of low bone mass [13]. In this follow-up study, we described the 24-month results of the percentage of change in BMD, bone remodeling markers, and side effects in the same population.
Materials and methods
Ethics
The research ethics committees of all participating study centers provided their approval of the study protocol. All patients provided written informed consent. The study was approved by the Institutional Review Board of Kyoto Prefectural University of Medicine on August 2, 2013. This study was also registered in the UMIN Clinical Trial Registry (UMIN-CTR, UMIN 000016173).
Patients
The complete inclusion and exclusion criteria have been described previously. Briefly, the data of postmenopausal women with early-stage, histologically confirmed, hormone receptor-positive, invasive breast cancer who were scheduled to receive an AI as adjuvant endocrine therapy or were in the process of receiving AI adjuvant therapy were included in the analysis. We also included those who had completed a chemotherapy regimen ≥ 4 weeks before entering the study and patients with evidence of low bone mass (lumbar spine, right femoral neck, and left femoral neck BMD corresponding to a T-score classification of − 1.0 to − 2.5). We excluded patients with osteoporosis (T score < − 2.5), prior vertebral diseases, and current active dental problems including infection of the teeth or jawbone.
Study design
This non-randomized, prospective study was conducted at three institutions in Japan. Patients received 60 mg of denosumab subcutaneously every 6 months. Daily supplements containing 500 mg of elemental calcium, and at least 400 international units of vitamin D were highly recommended throughout the study period. No changes in AI therapy were mandated by the study protocol.
Assessment of outcomes
Denosumab was administered subcutaneously on day 1 of the study and then at 6, 12, 18, and 24 months. BMD was measured with dual-energy X-ray absorptiometry (DXA) using a Hologic (Hologic Inc., Bedford, MA) or Lunar (General Electric Lunar Corp., Madison, WI) densitometer. All DXA devices were standardized and cross-calibrated using four Bio-Imaging Bona Fide Phantoms. The BMD of the lumbar spine and bilateral femoral neck was measured at baseline and at 6, 12, 18, and 24 months.
The levels of bone turnover markers, serum tartrate-resistant acid phosphatase isoform 5b (TRAP5b), and bone alkaline phosphatase (BAP) were determined at baseline and 6, 12, 18, and 24 months. The albumin-corrected serum calcium concentration was measured at baseline and 1, 6, 12, 18, and 24 months. Hypocalcemia was defined as a corrected calcium level < 8.0 mg/dL that corresponded to grade 2 hypocalcemia according to the Common Terminology Criteria for Adverse Events.
Endpoints
The primary endpoint was the change in percentage of the BMD of the lumbar spine (L1–L4) from baseline to 12 months. The secondary endpoints were (1) the percentage of change in the BMD of the lumbar spine (L1–L4) from baseline to 6, 18, and 24 months; (2) the percentage of change in the BMD of the bilateral femoral neck from baseline to 6, 12, 18, and 24 months; and (3) changes in the serum markers of bone turnover, TRAP-5b, and BAP from baseline to 6, 12, 18, and 24 months.
Statistical analysis
According to preliminary calculations, a sample size of 74 patients was required to obtain a power of 80% and detect a 4% difference in the percentage of change in the BMD of the lumbar spine (L1–L4) from baseline to 12 months. To allow for a 20% dropout rate, at least 90 patients were required. Paired t tests were used to compare the two groups. The reported P values were based on a two-sided comparison. A P value ≤ 0.05 was considered to represent a statistically significant difference. All statistical analyses were performed using the JMP software, version 12.
Results
Patients
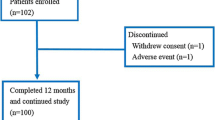
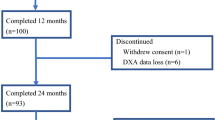
A total of 103 patients were enrolled (Fig. 1). Ninety-three patients completed the study. Ten patients dropped out. Two patients withdrew consent, one developed grade 2 arthralgia, one had disease progression (bone metastasis), and six were removed because of DXA data loss. The baseline characteristics of the patients who were included are shown in Table 1. The majority of these patients (68.8%) had received AI therapy before the denosumab treatment was initiated (mean period: 24 months).
BMD
At 24 months, the BMD of the lumbar spine increased by 7.0% [95% confidence interval (CI), 5.9–8.0] (Fig. 2). The BMD of the right and left femoral neck increased by 3.4% (95% CI 2.4–4.5) and 3.6% (95% CI 2.6–4.6), respectively. The BMD of the right femoral neck increased more than the BMD of the left femoral neck did at 12 months (1.9% mean difference, P = 0.0516), but this trend did not appear at 24 months (0.2% mean difference, P = 0.7745) (Fig. 3). The change in the percentage of the BMD of the lumbar spine from baseline to 24 months was 6.8% (95% CI 4.9–8.6) in patients who started to receive AI and denosumab simultaneously and 7.0% (95% CI 5.7–8.3) in patients who had received AI before the initiation of the denosumab therapy, with no significant difference (0.2% mean difference; P = 0.8519) (Fig. 4).
Percentage of change in BMD in a the right femoral neck and b left femoral neck from baseline (± 95% CI) over 24 months for all treatment groups. 1.9% mean difference between right and left: P = 0.0516 (12 months). 0.2% mean difference between right and left: P = 0.7745 (24 months). CI confidence interval, BMD bone mineral density
Percentage of change in BMD of the lumbar spine from baseline (± 95% CI) over 24 months in the denosumab and before denosumab groups. 0.2% mean difference between the denosumab and before denosumab groups: P = 0.8385 (24 months). 0.2% mean difference between the denosumab and before denosumab groups: P = 0.8519 (24 months). BMD bone mineral density, CI confidence interval, AI aromatase inhibitor
Fractures
We did not estimate lumbar fractures by imaging diagnosis such as plain X-P for evaluation of vertebral compression fractures. At 24 months, symptomatic clinical fractures did not occur in patients receiving AI and denosumab.
Safety
A safety analysis of the administered drugs was conducted in 100 patients. Adverse effects with an occurrence rate of 5% or more are shown in Table 2. Arthralgia occurred in approximately 40% of the study participants, but in almost all patients, it was grade 1 and controllable with nonsteroidal anti-inflammatory drugs. One patient withdrew from the study because of arthralgia. No patients had osteonecrosis of the jaw, and grade ≥ 2 hypocalcemia did not occur.
Markers of bone remodeling
With the use of denosumab, the levels of the markers of bone remodeling (TRAP5b and BAP) were rapidly reduced at 6 months, decreased at 12 months, and slightly increased at 18 and 24 months (Fig. 5). The percentages of change reductions of the TRAP5b from baseline to 6, 12, 18, and 24 months were 59.6% (95% CI, 45.6–65.6), 60.1% (95%CI, 54.6–65.6), 57.8% (95%CI, 53.4–63.4), and 48.7% (95%CI, 41.1–55.7), respectively. The percentages of change reductions of the BAP from baseline to 6, 12, 18, and 24 months were 52.2% (95% CI, 49.1–55.4), 55.0% (95%CI, 51.8–58.2), 50.4% (95%CI, 46.1–54.6), and 48.0% (95%CI, 51.3–52.2), respectively. The mean percentages of reductions in the TRAP5b and BAP levels at 24 months were 48.7 and 48.0%, respectively.
Discussion
We observed a 7.0% increase in lumbar spine BMD (baseline T-score − 1.0 to − 2.5) at 24 months, and Gnant et al. reported a 5.9% increase in ABCSG-18 trial (baseline T-score classification of < − 1.0) [14]. We did speculate that the difference in BMD improvement between these two studies might be dependent on baseline BMD.
Osteoporosis, which is estimated to occur in more than 13 million patients in Japan, mainly occurs in postmenopausal women. An estrogen deficiency that is induced by menopause can disturb endocrine feedback and is followed by bone loss due to increased bone resorption with a high bone turnover [15,16,17,18,19]. These patients are thought to be at a high risk of developing vertebral and hip fractures, and anti-resorptive drugs such as bisphosphonate and denosumab are well indicated as the standard therapies.
Generally, denosumab inhibits bone resorption more strongly than bisphosphonate does. This is because denosumab controls osteoclasts at the time of differentiation and maturity, and all stages of its function, whereas bisphosphonate takes in mature osteoclasts and binds them to the mineralized surface of the bone [20,21,22,23].
Zoledronic acid (ZOL) is a nitrogen-containing bisphosphonate that has the strongest and most persistent anti-resorptive activity. The Randomized Second Extension to the Health Outcomes and Reduced Incidence with Zolodronic Acid Once Yearly-Pivotal Fracture Trial demonstrated that the benefit of 9 years of ZOL was similar to that of 6 years of ZOL [24]. Therefore, we think that bisphosphonate may have a ceiling effect. In contrast, the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months Extension Study demonstrated that the benefit of denosumab continued to increase up to 10 years. In fact, the BMD curve of ZOL reached a plateau phase over the course of 6 years, but a plateau has not been observed with the use of denosumab, even over the course of 10 years [25]. Modeling-based bone formation after the use of denosumab might be associated with this difference [26,27,28,29,30]. Therefore, we think that denosumab is a more beneficial treatment for patients with osteoporosis than ZOL. Many studies and case reports indicate that atypical femoral fracture and osteonecrosis of the jaw could occur frequently in osteoporotic patients receiving anti-resorptive drugs such as bisphosphonate and denosumab [31,32,33]. In our study, no such cases were reported, but more studies with a longer follow-up period are needed.
Recently, two case reports described that multiple vertebral fractures occurred after denosumab treatment ended [34, 35]. Of 1001 participants who discontinued denosumab during FREEDOM or Extension, the vertebral fracture rate increased from 1.2 per 100 participant-years during the on-treatment period to 7.1 [36]. Rebound-associated vertebral fractures that occur after denosumab is discontinued are currently critical issues. The effects of denosumab withdrawal have not been studied sufficiently, but the position statement of the IOF about the management of AI-associated bone loss recommended sequential treatment with an intravenous dose of bisphosphonate after denosumab is stopped. The efficacy after denosumab in the treatment of AI in the Japanese population has not been evaluated in a prospective study. Therefore, we are conducting an ongoing clinical trial (UMIN000030328) in which we stop denosumab when the BMD of every three regions (lumber spine and bilateral femoral neck) increase from normal (> − 1.0SD) and re-administer denosumab when the BMD of at least one region decrease to osteopenia (< − 1.0SD). This trial will reveal the influence of denosumab after it is stopped and the usefulness of re-administering denosumab in Japanese patients.
Although the findings of this clinical study are important, it has several limitations. First, this was a non-randomized study, which limited the availability of data and did not allow us to collect additional data to facilitate further investigation. However, a placebo-controlled study is impossible from an ethical standpoint because AI treatment clearly decreases BMD. Therefore, we felt that a non-randomized, prospective study was the best option in this case. The second limitation was the small sample size of the clinical study on which this analysis was based. A larger sample size could have provided more reliable results. The final limitation of our study is that a centralized DXA data review was not performed, as we felt that this was beyond the scope of this investigation. In future studies, a more extensive review of the literature could provide additional data to support our findings.
In this study, we described the 24-month results of changes in the percentage of BMD, bone remodeling markers, and side effects in postmenopausal Japanese women receiving denosumab with adjuvant AIs for non-metastatic breast cancer. At 24 months, semiannual treatment with denosumab was associated with consistently increased BMD in Japanese women receiving adjuvant AI therapy, regardless of whether they underwent prior AI therapy.
References
Wang J, Lv H, Xue Z, Wang L, Bai Z, et al. Temporal trends of common female malignances on breast, cervical, and ovarian cancer mortality in Japan, Republic of Korea, and Singapore: application of the age-period-cohort model. Biomed Res Int. 2018;5307459:13
Gradishar W, Salerno KE. NCCN guidelines update: breast cancer. J Natl Compr Canc Netw. 2016;14:641–4.
Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol. 2009;69:73–82.
Lee SJ, Kim KM, Brown JK, Brett A, Roh YH, Kang DR, et al. Negative impact of aromatase inhibitors on proximal femoral bone mass and geometry in postmenopausal women with breast cancer. Calcif Tissue Int. 2015;97:551–9.
Kim W, Chung Y, Kim SH, Park S, Bae JH, Kim G, et al. Increased sclerostin levels after further ablation of remnant estrogen by aromatase inhibitors. Endocrinol Metab (Seoul). 2015;30:58–64.
Hadji P, Ziller M, Kieback DG, Dornoff W, Tessen HW, Menschik T, et al. Effects of exemestane and tamoxifen on bone health within the tamoxifen exemestane adjuvant multicentre (TEAM) trial: results of a German, 12-month, prospective, randomised substudy. Ann Oncol. 2009;20:1203–9.
Kyvernitakis I, Rachner TD, Urbschat A, Hars O, Hofbauer LC, Hadji P. Effect of aromatase inhibition on serum levels of sclerostin and dickkopf-1, bone turnover markers and bone mineral density in women with breast cancer. J Cancer Res Clin Oncol. 2014;140:1671–80.
Kyvernitakis I, Knoll D, Struck M, Hars O, Bauer T, Hadji P. Impact of BMI on serum estradiol and bone turnover markers in postmenopausal women with hormone-sensitive early breast cancer treated with anastrozole. J Cancer Res Clin Oncol. 2014;140:159–66.
Howell A. Adjuvant aromatase inhibitors for breast cancer. Lancet. 2015;366:431–33.
Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup exemestane study (IES): a randomised controlled study. Lancet Oncol. 2007;8:119–27.
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–71.
Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25:486–92.
Nakatsukasa K, Koyama J, Ouchi Y, Sakaguchi K, Fujita Y, Matsuda T, et al. Effects of denosumab on bone mineral density in Japanese women with osteoporosis treated with aromatase inhibitors for breast cancer. J Bone Miner Metab. 2018. https://doi.org/10.1007/s00774-018-0917-0.
Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesez R, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicenter, randomized, double-blind, placebo-controlled trial. Lancet. 2015;386:433–43.
Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal hormone therapy for the primary prevention of chronic conditions: a systematic review to update the US preventive services task force recommendations. Ann Intern Med. 2012;157:104–13.
Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–61.
Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, et al. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone. 2002;30:18–25.
Callewaert F, Venken K, Ophoff J, De Gendt K, Torcasio A, van Lenthe GH, et al. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. FASEB J. 2009;23:232–40.
Ford J, Hajibeigi A, Long M, Hahner L, Gore C, Hsieh JT, et al. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. J Bone Miner Res. 2011;26:298–307.
Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, et al. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol. 1999;145:527–38.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76.
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–602.
Zebaze RM, Libanati C, Austin M, Ghasem-Zadeh A, Hanley DA, Zanchetta JR, et al. Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone. 2014;59:173–79.
Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-pivotal fracture trial (PFT). J Bone Miner Res. 2012;27:243–54.
Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–23.
Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48.
Reid IR, Miller PD, Brown JP, Kendler DL, Fahrleitner-Pammer A, Valter I, et al. Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res. 2010;25:2256–65.
Brown JP, Reid IR, Wagman RB, Kendler D, Miller PD, Jensen LE, et al. Effects of up to 5 years of denosumab treatment on bone histology and histomorphometry: the FREEDOM study extension. J Bone Miner Res. 2014;29:2051–56.
Montagner F, Kaftandjian V, Farlay D, Brau D, Boivin G, Follet H. Validation of a novel microradiography device for characterization of bone mineralization. J X-ray Sci Technol. 2015;23:201–11.
Vittinghoff E, McCulloch CE, Woo C, Cummings SR. Estimating long-term effects of treatment from placebo-controlled trials with an extension period, using virtual twins. Stat Med. 2010;29:1127–36.
Ota S, Inoue R, Shiozaki T, Yamamoto Y, Hashimoto N, Takeda O, et al. Atypical femoral fracture after receiving antiresorptive drugs in breast cancer patients with bone metastasis. Breast Cancer. 2017;24:601–7.
Shabestari M, Eriksenb EF, Paschalis EP, Roschger P, Gamsjaeger S, Klaushofer K, et al. Presence of pyrophosphate in bone from an atypical femoral fracture site: a case report. Bone Rep. 2017;6:81–6.
Japanese Allied Committee on Osteonecrosis of the Jaw. Yoneda T, Hagino H, Sugimoto T, Ohta H, Takahashi S, et al. Antiresorptive agent-related osteonecrosis of the jaw: position paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw. J Bone Miner Metab 2017;35:6–19.
Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos Int. 2016;27:1917–21.
Lamy O, Gonzalez-Rodriguez E, Stoll D, Hans D, Aubry-Rozier B. Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab. 2017;102:354–8.
Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190–8.
Acknowledgements
This study was supported in part by Grants-in-Aid for Scientific Research from the Japanese Breast Cancer Society.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Dr Tetsuya Taguchi received Research grant from Chugai, Taiho and Daiichi Sankyo. Dr Shunji Takahashi received Research grant from Daiichi Sankyo. Other authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standard.
Informed consent
Informed consent was obtained from all individual participants included in the study.
About this article
Cite this article
Nakatsukasa, K., Koyama, H., Ouchi, Y. et al. Effect of denosumab on low bone mineral density in postmenopausal Japanese women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer: 24-month results. Breast Cancer 26, 106–112 (2019). https://doi.org/10.1007/s12282-018-0896-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-018-0896-y