Abstract
Although adjuvant aromatase inhibitor (AI) therapy is widely used in postmenopausal women with hormone receptor-positive breast cancer, it is known to be associated with bone loss and increased fracture risk. Denosumab, a fully human monoclonal antibody against the receptor activator of nuclear factor-κB ligand, has been shown to protect against AI-induced bone loss. However, the efficacy of denosumab in the treatment of AI-associated bone loss has not been prospectively evaluated in Japan. We prospectively monitored bone mineral density (BMD) of the lumbar spine and bilateral femoral necks in 100 postmenopausal women with hormone receptor-positive postoperative breast cancer of clinical stage I–IIIA in whom treatment with AI as adjuvant endocrine therapy was planned or had been ongoing. Study participants received supplemental calcium and vitamin D every day and denosumab (60 mg) subcutaneously every 6 months. At enrollment, patients were required to have evidence of low bone mass without meeting the criteria for osteoporosis. The primary endpoint was percentage change from baseline in lumbar spine BMD at month 12. At 6 and 12 months, lumbar spine BMD increased by 3.3 and 4.7%, respectively. BMD of the femoral necks also increased. Hypocalcemia of grade ≥2, osteonecrosis of the jaw, and non-traumatic clinical fracture did not occur. In conclusion, semi-annual treatment with denosumab was associated with increased BMD in Japanese women receiving adjuvant AI therapy, regardless of prior AI treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aromatase inhibitors (AIs) are widely accepted as the adjuvant treatment of choice in postmenopausal women with hormone receptor-positive breast cancer. The main adverse effect associated with the use of AI therapy is damage to bones. AIs have been shown to decrease bone mineral density (BMD) and increase bone fracture incidence compared with tamoxifen. In light of the known protective effect of tamoxifen on bones, however, it is possible that the negative effect of AIs has been exaggerated [1,2,3,4,5,6,7,8].
Bone loss is mediated by osteoclasts, whose formation, function, and survival depend on the receptor activator of nuclear factor-κ-B ligand (RANKL). RANKL binds to its receptor (RANK) and activates osteoclast-mediated bone resorption [9,10,11].
Denosumab is a fully human monoclonal anti-RANKL antibody that suppresses differentiation, activation, and survival of osteoclasts by inhibiting the binding of RANKL to its receptor [12].
Ellis et al. previously reported the results of a 2-year randomized, double-blind, placebo-controlled study, in which denosumab increased BMD of the lumbar spine and other skeletal sites in patients with hormone receptor-positive breast cancer who were receiving adjuvant AI therapy and had evidence of low BMD [13]. Gnant et al. demonstrated that adjuvant therapy with denosumab (60 mg twice per year) reduces the risk of clinical fractures in postmenopausal women with breast cancer receiving AI therapy, and that denosumab can be administered without added toxicity [14]. However, the efficacy of denosumab in the treatment of AI-associated bone loss in the Japanese population has not been evaluated in a prospective study. Therefore, we prospectively evaluated BMD of the lumbar spine and bilateral femoral necks in postmenopausal patients with hormone receptor-positive postoperative breast cancer of clinical stage I–IIIA for whom AI treatment as an adjuvant endocrine therapy had been planned or ongoing.
Materials and methods
Patients
Eligibility criteria were (1) adequately treated invasive breast cancer of clinical stage I, II, or IIIA; (2) tumor removed using an appropriate surgical procedure such as mastectomy or breast-conserving surgery; (3) estrogen receptor- and/or progesterone receptor-positive disease as determined by immunohistochemical staining; (4) postmenopausal status defined based on the presence of one of the following criteria—(a) age >54 years and absence of menstruation; (b) spontaneous cessation of menses within the past 12 months OR age <55 years and amenorrhea OR follicle-stimulating hormone and estradiol levels in the postmenopausal range; (5) lumbar spine or femoral neck BMD T-score between −1.0 and −2.5; (6) electrocorticography (ECOG) performance status between 0 and 2; (7) >4 weeks of chemotherapy completed prior to study entry; (8) the following drugs known to affect the skeleton discontinued more than 4 weeks prior to study entry—oral bisphosphonates, estrogen, raloxifene, calcitonin, vitamin K, and activated vitamin D; and (9) the provision of written informed consent. The main exclusion criteria were (1) diagnosis of clinical or radiological distant metastasis before inclusion in the study, (2) invasive bilateral breast cancer, (3) prior treatment with intravenous bisphosphonates within the previous 12 months, (4) presence of any disease that might interfere with dual-energy X-ray absorptiometry (DXA) measurements such as severe scoliosis or vertebral disease, (5) current dental problems including infection of the teeth or jawbone or recent (within 6 weeks) or planned dental or jaw surgery (e.g., extraction, implants), and (6) other conditions judged to be inappropriate for the study by the investigators. Patients who developed or were suspected to have osteonecrosis of the jaw (ONJ) were to be withdrawn from the study.
Study design
This non-randomized prospective study was conducted at three institutions in Japan. Patients were to receive 60 mg of denosumab subcutaneously every 6 months. Daily supplements containing 500 mg of elemental calcium and at least 400 international units of vitamin D were highly recommended throughout the study. No changes in AI therapy were mandated by the study protocol. Approval from the research ethics committees of each participating study center was obtained. All patients provided written informed consent. The study was approved by the Institutional Review Board of Kyoto Prefectural University of Medicine on August 2, 2013. This study was registered with the UMIN Clinical Trial Registry (UMIN-CTR, UMIN 000016173).
Assessment of outcomes
Denosumab was administered subcutaneously on day 1 of the study and then after 6 and 12 months. BMD was measured by dual-energy DXA using a Hologic (Hologic Inc., Bedford, MA, USA) or Lunar (General Electric Lunar Corp, Madison, WI, USA) densitometer. All DXA devices were standardized and cross-calibrated using four Bio-Imaging Bona Fide Phantoms. Lumbar spine and bilateral femoral neck BMDs were measured at baseline and after 6 and 12 months.
Levels of bone turnover markers, serum tartrate-resistant acid phosphatase isoform 5b (TRAP5b) and bone alkaline phosphatase (BAP), were determined at baseline and after 6 and 12 months. Albumin-corrected serum calcium concentration was measured at baseline and after 1, 6, and 12 months. Hypocalcemia was defined as corrected calcium level <8.0 mg/dL, which corresponds to grade 2 hypocalcemia according to the common terminology criteria for adverse events.
Endpoints
The primary endpoint was percentage change from baseline to month 12 in lumbar spine BMD. The secondary endpoints were (1) percentage change in lumbar spine (L1–L4) BMD from baseline to 6 months; (2) percentage change in bilateral femoral neck BMD from baseline to 6 months and 12 months; and (3) changes in serum markers of bone turnover, TRAP-5b and BAP, from baseline to 6 and 12 months.
Statistical analysis
According to preliminary calculations, a sample size of 74 patients was required to obtain a power of 80% and detect a 4% difference in percent change in lumbar spine (L1–L4) BMD from baseline to 12 months. To allow for a 20% dropout rate, at least 90 patients were required. Paired t tests were used to compare the two groups. P values reported are based on a two-sided comparison. A P value of ≤ 0.05 was considered to represent a statistically significant difference. All statistical analyses were performed using the JMP software, version 12.
Results
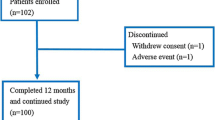
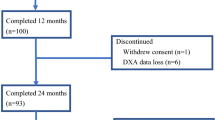
A total of 103 patients were enrolled (Fig. 1). Most patients (97.1%) completed the study. Three patients discontinued the study. One patient withdrew consent, one developed grade 2 arthralgia, and one was removed because of disease progression (bone metastasis). The baseline characteristics are shown in Table 1. The majority of patients (70%) had received AI therapy (mean period 24 months) before the initiation of denosumab treatment.
BMD
At 12 months, the lumbar spine BMD increased by 4.7% (95% CI 3.9–5.4) (Fig. 2). At 12 months, the right femoral neck and left femoral neck BMD increased by 2.4% (95% CI 1.5–3.3) and 1.4% (95% CI 0.5–2.2), respectively. The right femoral neck BMD increased more than the left femoral neck BMD, but the difference was not significant (1.9% mean difference, P = 0.0516) (Fig. 3). The percentage change from baseline in BMD of the lumbar spine over the 12 months of the study was 4.5% (95% CI 3.1–5.8) in patients who started to received AI therapy and denosumab simultaneously and 4.7% (95% CI, 3.8-5.7) in patients who had received AI therapy before the initiation of the denosumab therapy, with no significant difference (0.2% mean difference; P = 0.8385) (Fig. 4).
Fractures
At month 12, no non-traumatic clinical fractures occurred in patients receiving AI therapy and denosumab.
Safety
Safety analysis of the administered drugs was conducted in 100 patients. Adverse effects with an occurrence of ≥5% are shown in Table 2. Arthralgia occurred in approximately 30% of study participants, but almost all cases were grade 1 and controllable with nonsteroidal anti-inflammatory drugs. One patient withdrew from the study because of arthralgia. There were no cases of ONJ. The levels of serum calcium are shown in Fig. 5. Hypocalcemia of grade ≥2 did not occur.
Markers of bone remodeling
Levels of markers of bone remodeling (TRAP5b and BAP) were rapidly reduced by denosumab (Fig. 6). The mean percentage reductions in TRAP5b and BAP levels at 12 months were 59.2% and 52.2%, respectively. This extent of reduction remained constant between 6 and 12 months.
Discussion
Bone loss contributing to increased fracture risk is a predictable consequence of AI therapy [15, 16]. At present, AI therapy is the recommended standard of care for all postmenopausal women with hormone receptor-positive breast cancer [17], and the duration of adjuvant endocrine therapy is increasing beyond 5 years [18]. Because of the increasing use of AIs, accelerated bone loss represents a challenge in the management of these patients.
The International Osteoporosis Foundation recommends an algorithm for managing bone health in women receiving AI therapy for breast cancer. For example, any patient starting or receiving AI therapy who has any two of the following risk factors—T-score < −1.5, age >65 years, low BMI (<20 kg/m2), family history of hip fracture, personal history of a fragility fracture after the age of 50, oral corticosteroid use of >6 months, and a history of smoking, should receive denosumab or bisphosphonate therapy [19].
Denosumab is a fully human monoclonal antibody with a novel mechanism of action. It binds to RANKL with high specificity but does not bind to other tumor necrosis factor ligands. By neutralizing RANKL, denosumab inhibits osteoclast formation, function, and survival. Because of its narrow specificity and a high rate of compliance associated with semi-annual subcutaneous injections, denosumab is being investigated as an alternative to bisphosphonates for the long-term management of bone loss in women with breast cancer. In this regard, the American Society for Bone and Mineral Research Working Group suggests that injectable therapy should be considered when adherence to an oral agent is inadequate [20].
In this trial of patients with breast cancer and at risk of AI-induced bone loss, denosumab treatment resulted in rapid increases in lumbar spine BMD over 12 months. Consistent increases in BMD were also observed at all measured skeletal sites. In the anastrozole adjuvant trial of postmenopausal women with breast cancer, 5 years of anastrozole treatment was associated with a cumulative 6.1% decrease in lumbar spine BMD [9]. In this study, after 1 year, denosumab therapy resulted in a 4.7% gain in lumbar spine BMD. Significant gains in lumbar spine BMD were seen regardless of whether the patients had received AI therapy prior to denosumab treatment initiation. In our study, right femoral neck BMD increased more substantially than left femoral neck BMD. Marina et al. reported that the left–right femoral neck BMD asymmetry might be associated with asymmetrical gait and standing [21]. We speculate that the reason for this asymmetry in BMD increase is that the right foot pressure was higher than the left foot pressure.
Adverse effects observed in this trial were typical for AI therapy. Denosumab treatment was generally well tolerated in this trial population. The calcium level tended to decrease at 1 month, but hypocalcemia of grade ≥2 was not observed in these patients, who were receiving calcium and vitamin D supplementation. Both bisphosphonates and denosumab at higher doses cause higher rates of ONJ [22], which is a concern in the treatment of metastatic breast cancer and other cancers. Because ONJ negatively affects a cancer patient’s quality of life, it is especially important to determine the risk of denosumab-related ONJ. However, we did not detect any cases of ONJ in this trial.
Bone-modifying agents are promising therapeutic agents not only because of their action on bone but also because they improve prognosis. Adjuvant bisphosphonates have been shown to reduce breast cancer recurrence and improve outcomes in several adjuvant breast cancer trials [23]. A recent large meta-analysis provided convincing evidence that disease-free and overall survival are improved in postmenopausal patients who are treated with adjuvant bisphosphonates [24]. The D-CARE trial (ClinicalTrial.gov identifier NCT01077154), which will use a higher dose of denosumab, will provide information as to whether this is also true for the anti-RANK ligand antibody.
Although the findings of this study are important, there were several limitations. First, this was a non-randomized study, which limited data availability and did not allow collecting additional data to facilitate the investigation. However, a placebo-controlled study is impossible from the ethical standpoint because AI treatment clearly decreases BMD. Therefore, we felt that a non-randomized prospective study was the best option in this case. The second limitation is the small sample size of the clinical study on which this analysis was based. A larger sample size could have provided more reliable results. The final limitation of our study is that a centralized DXA data review was not performed, as we felt that this was beyond the scope of this investigation. A more extensive review of the literature could provide additional data to support our findings in the future.
In summary, semi-annual treatment with denosumab was associated with consistently increasing BMD in Japanese women receiving adjuvant AI therapy, regardless of prior AI therapy.
References
Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L et al (2006) 5 years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 25:486–492
Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, Cawthorn SJ, Patel A, Snowdon CF, Hall E, Bliss JM (2007) Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol 8:119–127
Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G (2008) Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol 26:1051–1057
Eastell R, Hannon R (2005) Long-term effects of aromatase inhibitors on bone. J Steroid Biochem Mol Biol 95:151–154
Baum M, Buzdar A, Cuzick J, Forbes J, Houghton A, Howell A, Sahmoud T, ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists Group (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 98:1802–1810
Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J et al (2004) A randomized trial of exemestane after 2–3 years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1092
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL (2003) A randomized trial of letrozole in postmenopausal women after 5 years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793–1802
Lonning PE, Geisler J, Krag LE, Erikstein B, Bremnes Y, Hagen AI, Schlichting E, Lien EA, Ofjord ES, Paolini J, Polli A, Massimini G (2005) Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. Clin Oncol 23:5126–5137
Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, Hu S, Lacey DL (1999) The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 145:527–538
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Delmas PD (2008) Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J Clin Densitom 11:325–338
Ellis GK, Bone HG, Chlebowski R, Paul D, Spadfora S, Smith J, Jun S (2008) Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 26:4875–4882
Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R et al (2015) Adjuvant denosumab in breast cancer (ABCSG-18): a multicenter, randomized, double-blind, placebo-controlled trial. Lancet 386:433–443
Brufsky A, Harker WG, Beck JT, Carroll R, Tan-Chiu E, Seidler C, Hohneker J, Lacerna L, Petrone S, Perez EA (2007) Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol 25:829–836
Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T, ATAC Trialists Group (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel Members (2015) Tailoring therapies–improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol 26:1533–1546
Blok EJ, Derks MG, van der Hoeven JJ, van de Velde CJ, Kroep JR (2015) Extended adjuvant endocrine therapy in hormone-receptor positive early breast cancer: current and future evidence. Cancer Treat Rev 41:271–275
Hadji P, Aapro MS, Body JJ, Gnant M, Brandi ML et al (2017) Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO, IMS, and SIOG. J Bone Oncol 7:1–12
Cummings SR, Cosman F, Lewiecki EM, Schousboe JT, Bauer DC et al (2017) Goal-Directed treatment for osteoporosis: a progress report from the ASBMR-NOF working group on goal-directed treatment for osteoporosis. J Bone Miner Res 32:3–10
Brozgol M, Arbiv M, Mirelman A, Herman T, Hausdorff JM, Vaisman N (2017) Vertical ground reaction force during standing and walking: are they related to bone mineral density left–right asymmetries? Gait Posture 54:174–177
Qi WX, Tang LN, He AN, Yao Y, Shen Z (2014) Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int J Clin Oncol 19:403–410
Gnant M, Clezardin P (2012) Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treat Rev 38:407–415
Early Breast Cancer Trialists Collaborative Group (EBCTCG), Coleman R, Powles T, Paterson A, Gnant M, Anderson S, Diel I, Gralow J, van Minckwitz G, Moebus V, Bergh J, Pritchard KI, Bliss J, Cameron D, Evans V, Pan H, Peto R, Bradley R, Gray R (2015) Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomized trials. Lancet 386:1353–1361
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
About this article
Cite this article
Nakatsukasa, K., Koyama, H., Ouchi, Y. et al. Effect of denosumab administration on low bone mineral density (T-score −1.0 to −2.5) in postmenopausal Japanese women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer . J Bone Miner Metab 36, 716–722 (2018). https://doi.org/10.1007/s00774-017-0884-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0884-x