Abstract
Background
The heterogeneous nature of breast cancer makes it one of the most challenging cancers to treat. Due to the stimulatory effect of estrogen in mammary cancer progression, anti-estrogenic agents like melatonin have found their way into breast cancer treatment. Further studies confirmed a reverse correlation between nocturnal melatonin levels and the development of mammary cancer. In this study we reviewed the molecular inhibitory effects of melatonin in breast cancer therapy.
Methods
To open access the articles, Google scholar and science direct were used as a motor search. We used from valid external and internal databases. To reach the search formula, we determined mean key words like breast cancer, melatonin, cell proliferation and death. To retrieval the related articles, we continuously search the articles from 1984 to 2015. The relevance and the quality of the 480 articles were screened; at least we selected 80 eligible articles about melatonin molecular mechanism in breast cancer.
Result
The results showed that melatonin not only inhibits breast cancer cell growth, but also is capable of inhibiting angiogenesis, cancer cell invasion, and telomerase activity. Interestingly this hormone is able to induce apoptosis through the suppression or induction of a wide range of signaling pathways. Moreover, it seems that the concomitant administration of melatonin with other conventional chemotherapy agents had beneficial effects for patients with breast cancer, by alleviating unfavorable effects of those agents and enhancing their efficacy.
Conclusion
The broad inhibitory effects of melatonin in breast cancer make it a promising agent and may add it to the list of potential drugs in treatment of this cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer, the second most prevalent cause of cancer death, is turned into a global public health problem due to its complex etiology and poor response to the treatment [1]. According to the report by Turkoz et al. in 2013, breast cancer incidence rates increase sharply with age, obesity, oral contraceptives, postmenopausal hormone use, genetic factors, breast feeding [2], and last but not least, ionizing radiation and other environmental factors like smoking [3]. Along with surgery as a first line treatment, anti-estrogen based therapy, classified as selective estrogen receptor modulators (SERM) and selective estrogen enzyme modulators (SEEM) is recommended as the cornerstone treatment for patients with breast cancer [4–6]. Despite the positive effects of adjuvant therapy on patient outcome, their abundant side effects may attenuate the treatment efficacy, pointing out that major efforts need to be done to identify new agents to raise the anti-neoplastic efficacy of the conventional therapies and reduce their unfavorable side effects [7].
The relationship between melatonin, as a major product of the pineal gland, and cancer has been investigated for the last 80 years. In 1978, looking into the physiological function of melatonin in inhibiting sex hormone secretion, Cohen and his coworkers outlined the theory that a reduction in the amount of melatonin may augment the development of breast cancer by inducing the state of relative hyperestrogenism [8]. Following this study, in 1997, Bartsch and his colleagues reported a reverse correlation between melatonin concentrations and the tumor progression rate, suggesting that the urine amount of 6-sulfatoxymelatonin (melatonin metabolite) is lower in women suffering from breast cancer compared to healthy volunteers [9]. Further clinical investigations pointed out a reverse correlation between decrease nocturnal melatonin plasma level and the incidence of estrogen receptor positive type breast cancer, suggesting the administration of melatonin may be particularly advantageous to these patients [10–12]. Moreover, it has been established that disrupt the circadian rhythm of the melatonin level by some factors, including light at night, sleep deprivation, shift work, chronic jet lag, mutations in melatonin genes and ageing may increase susceptibility of normal breast cells to oxidative damages, leading to an increased risk of breast cancer mostly through elevated secretion of the activated cytokines [13]. By and large, these studies highlight the potential role of melatonin in the prevention of breast cancer and urge further investigation to dissect the possible underlying molecular mechanisms of this hormone in breast cancer.
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine), a derivative of the amino acid tryptophan, is essentially produced by the pineal gland and other organs, including retina, bone marrow, thymus and airway epithelium. Apart from the dark-light cycle [14, 15], age, seasons, gender, physiological and pathophysiological conditions control the biosynthesis of this hormone [10, 12]. This lipophilic molecule rapidly binds to albumin, diffuses into the blood stream and spreads throughout the body [16]. According to its regulatory characteristic, this multi-functional indoleamine not only contributes in circadian rhythm monitoring, but is also involved in immune system modulation, prevention of inflammation, free radical scavenging, vasoregulation, and last but not least a final well-documented function of this molecule is its oncostatic property [17–21]. A fair number of invivo and invitro experiments have been reporting the inhibitory ability of melatonin on tumor growth, motility and proliferation, introducing melatonin as a promising anticancer agent.
Materials and methods
To open access the articles, Google scholar and Science direct were used as a motor search for this study. We searched from ISI, Pubmed, and Scopus as a valid external databases and ISC and Iran medex as internal databases. To attain the search formula with the maximum collectivity, we determined mean key words like breast cancer, estrogen, melatonin, cell death, cell proliferation, telomerase, and molecular mechanisms and then identified equivalent terms with use of Mesh database. To retrieve the last related articles, we continuously searched the articles from 1984 to 2015. The relevance and the quality of the articles were screened by a specialist group, with considering the elements like sample size, the presence of control group, type of study, inclusion and exclusion criteria, the statistical analysis, and research design. Accordingly we obtained 480 articles, we selected at least 80 eligible articles about breast cancer molecular biology and melatonin mechanisms and based on the mentioned procedure, the traditional review was done.
This paper covered the investigations on the inhibitory role of melatonin in different breast cancer cell lines, animal models, and patients. In the following parts the most important underlying mechanisms of melatonin in this cancer will be discussed.
Results
The number and the subject of the relevant articles which are used to explain the inhibitory effects of melatonin in breast cancer are summarized in Table 1.
Melatonin receptors
The global expression of two high-affinity G coupled receptor known as MT1 and MT2, plus other investigated receptors, including nuclear receptors ROR/RZR, quinone reductase 2 (MT3), and calmodulin [22–29], highlighting the regulatory role of melatonin in multitude cellular processes. By binding to either of these receptors, melatonin modulates a variety of G proteins (Gaie, Gai3, Gaq and Ga11) [30, 31], decrease AMP and cGMP formation, and regulates apoptosis and cell cycle by targeting curtail nodal points in signaling pathways [27, 29, 32–34].
Regarding to the co-localization of MT and caveolin-1 on the breast cancer cells membrane [35], other investigations had been studied a correlation between melatonin receptor expression and the progression of breast cancer, supporting this hypothesis that the overexpression of MT1 receptor may diminish ERα expression, relating to the lower stage and smaller size of the tumor at the time of diagnosis [35–38].
Melatonin modulates estrogen pathway
Early studies on the possible correlation and effects of estrogen and breast cancer can be traced back to the 1870, when Beatson revealed that estrogen has a stimulatory effect on breast cancer, and hyperestrogism has been recognized as a risk factor for breast cancer progression [6]. In the meantime, this hormone is capable of inducing oxidative damage [6], upregulating telomerase activity [6] and enhancing cancer cell proliferation and motility [39, 40]. Despite the important role of estrogen in breast cancer development, the role of its receptor (ER alpha) on the mammary carcinogenesis should not be ignored, as phosphoactivation of this receptor leads to cancer development through alteration of a wide variety of genes.
According to the role of melatonin in sex hormone production, recent studies proposed a correlation between this hormone and the incidence of breast cancer [8]. Experiments conducted on animal models confirmed that pinealectomized animals, or those with lower melatonin level, are more prone to develop breast cancer, while treating the animals with melatonin reduces the serum estradiols level, estrogen receptor expression, leading to lower rate of tumor growth; by and large indicating a promising role of melatonin in preventing hyperestrogenism and introducing this compound as an antiestrogenic agent [8, 41–44].
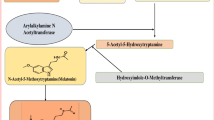
The anti-estrogenic property of melatonin could be explained in a variety of ways (Fig. 1). Acting as both SERM and SEEM, melatonin not only inhibits estrogen local synthesis by repressing its expression and transactivation, but also prevents its binding to ERα [43, 45].
Melatonin modulated estrogen/ERα signaling pathway. Melatonin inhibits estrogen response pathway through binding to MT1 receptor. This hormone inhibits both estrogen and ERα expression and meanwhile, inhibits their transcriptional activity. Inhibition of estrogen simultaneously suppresses telomerase and some adhesion molecules expression, leading to inhibition of cell proliferation and metastasis. Melatonin also blocks calmodulin and by this virtue prevents ERα phosphor-activation. E estrogen, ERα estrogen receptor alpha, ERE estrogen response element
Noteworthy, unlike tamoxifen (as a well-known SERM), melatonin down-regulates both ER-α mRNA and protein expression in receptor dependent manner by reducing the amount of cAMP [45, 43]. Otherwise, the ERα phosphoactivation may be suppressed, as melatonin counteracts calmodulin related action in ERα activation and translocation into nucleus [43, 44, 46–48].
Melatonin modulates aromatase synthesis and activation
Aromatase is one of the main enzymes in the conversion of androgens to estrogen [49–51]. In light of the role of estrogen in breast cancer, it is obvious that aromatase can play a crucial role in breast cancer progression [49–51]. Higher activity of aromatase in breast cancer tissue has prompted recent approaches to drugs such as aminoglutethimide that are capable of inhibiting aromatase [44, 52].
One of the other abilities of melatonin is the inhibition of aromatase expression and activity in both invivo and invitro [44, 49–51, 53–55]. With respect to this investigation, melatonin not only interacts with the estrogen-responsive pathway, but also inhibits local synthesis of estrogen through preventing the conversion of androgens to estrogen [44, 50–54]. In order to investigate whether melatonin could promote aminoglutethimide in the MCF-7 cell line, MCF-7 cells were pretreated with melatonin within 24 h and later treated with aminoglutethimide. It was shown that melatonin eminently augments aminoglutethimide inhibitory action on aromatase expression [51].
Melatonin suppresses cell proliferation in breast cancer
Based on the effects of melatonin in suppressing cell growth kinetics and metabolic activity in breast cancer, it is not surprising that increasing attentions have been devoted to uncover its underlying molecular mechanism [56]. A fair number of studies revealed that both ER positive and negative breast cancer cell lines were growth inhibited upon exposing to physiological concentration of melatonin [6, 23, 57]. Moreover, further experiments suggested that melatonin anti proliferative actions are associated with activation of MT1 receptor, as the MT1 transactivation potentiated the melatonin anti proliferative effects in MT1-transfected MCF-7 cell line. The melatonin MT1-dependent anti proliferative actions could be attributed to several mechanisms, including repression of estrogen and ERα transcription, Aromatase down regulation, disruption in internal ca2+ hemostasis [58], the ribosome biogenesis suppression [59], inhibition of cAMP formation, induction of p53, and p21 upregulation. Additionally it seems that the suppression of linoleic acid uptake and its conversion to 13-hydroxyoctadexadienoic acid (13-HODE), associated with the reduction of breast cancer cells metabolism through melatonin treatment may boost the melatonin anti proliferative effects [12, 29, 60].
Melatonin potentiates apoptosis in breast cancer cells
Despite much evidence that introduced melatonin only as an oncostatic agent, it was observed recently that this hormone could induce apoptosis in breast cancer cells regardless of the expression of the estrogen receptor [13, 61, 62]. The melatonin-induced apoptosis response is affected through both early and late apoptosis due to the incubating time [63]. Since the early apoptosis is a caspase independent response, it is triggered by an Apoptotic Induce Factor (AIF) and independent of BCL-2 protein family, it was shown that exposing breast cancer cells to 1 nM concentration of melatonin within 3 h induces apoptosis through downregulation of MDM2 (murine double minute 2) and sirt1 (silent mating type information regulation 1 homolog), leading to alteration of the p53-MDM2 balance. In contrast, after a 96 h incubation of the breast cancer cells, TGF-β, caspase7, caspase9 and PARP could be considered as the main contributors of the late apoptosis [63]. Notably the cytotoxic potency of melatonin had been observed in the triple negative breast cancer cell line MDA-MB361 as well. It was reported that the liberation of cytochrome C from mitochondria, the overexpression of apaf-1 and finally the cleavage and activation of caspase3, caspase9 and PARP are consequences of 72 h treatments of the cells with melatonin [55]. Along with this subject El-Aziz et al. suggested that the treatment of mammary bearing rats with melatonin retards breast cancer growth by decreasing the level of prolactine, estradiols, oxidative stress and, precisely through elevation of caspase3 activation, DNA fragmentation and TNF-α [64]. Some other studies documented that melatonin and retinoic acid are capable of inducing apoptosis in the MCF-7 cell line. Collectively, some possible mechanisms are involved in melatonin-mediated apoptosis; still, however, there is an inconsistency between the oncostatic and cytotoxic modes of action of this hormone in breast cancer cells [11].
Melatonin regulates Cyclooxygenase 2, NFkB and PI3k/Akt signaling pathways
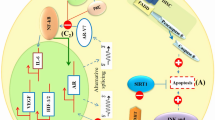
COX2, an important signaling mediator in cancer cells, has been shown to be involved in regulation of several essential cellular process in breast cancer, including cell proliferation, cell survival, angiogenesis, cAMP formation, and most notably aromatase expression [53]. It is worth to note that COX-2 PI3K/Akt signaling pathway and NFkB are tightly interconnected: PI3K/Akt activates NFkB through Akt-dependent phosphorylation, hence NFkB-p300 complex binds to COX-2 promotor and increases its expression [53, 55]. Some studies show that melatonin, either by abrogating the binding of NFkB/p300 to the COX-2 promoter or through binding to the active sites of this enzyme, may alter COX-2 activity and expression, consequently downregulates its downstream target genes (Fig. 2) [43, 50, 53, 55, 65].
Melatonin inhibitory action in breast cancer. Melatonin either by receptor dependent or independent mechanism involves in many cellular pathways. By binding to MT1 receptor, melatonin activates Giα, suppressing adenylate cyclase and cAMP production, leading to inactivation of NFkB pathway and c-Myc down-regulation. In addition melatonin interacts with PI3K/Akt pathway and via modulation of its downstream targets such as GSK-3β, MDM-2, NFkB, and Cox-2 retards breast cancer progression
Melatonin contributes to p53 activation and phosphorylation
Cell behavior toward proliferation and apoptosis is dependent on the balance between MDM-2 and P53 [65]. Several lines of evidence have shown that both melatonin cytostatic and cytotoxic actions are coupled with enhancing p53 pathway by targeting different molecules [63, 66, 67]. Noteworthy, the PI3 K/Akt signaling pathway is involve in DNA damage response and p53 inhibition through phosphoactivation of MDM2 (murine double minute 2), a p53 ubiquitination regulator. On this basis, it was shown that melatonin not only inhibits MDM-2 transcriptional activity, but also prevents MDM-2 phosorylation through reducing the pAkt/Akt ratio, leading to elevated transactivating and acetylation of p53 in melatonin-treated breast cancer cells [63, 66, 67]. Moreover, due to the fundamental role of sirt1(silent mating type information regulation 1 homolog) in p53 inactivation via downregulating p300, some studies found a simultaneous decrease in sirt1 expression and p300 upregulation in response to melatonin treatment, leading to p53 acetylation and activation [63]. By and large, an increase in the p53/MDM2 ratio guides the melatonin-treated breast cancer cells toward cell cycle arrest or apoptosis by altering the transcriptional activity of p53 downstream target genes [67].
Melatonin and angiogenesis
Given to the high expression of MT1/2 and nuclear receptors in human vein endothelial cells, Extensive evidence revealed that melatonin directly inhibits vascular endothelial factor, epidermal growth factor, endothelin-1, and insulin like growth factor-1 in both ER-positive and ER-negative cell lines [68–70]. Additionally, a significant reduction in VEGFR expression and micro vessel density in murine models followed by melatonin administration, confirming the melatonin anti angiogenesis action [69]. According to the pivotal role of both PKC and NFkB in tumor angiogenesis, it is assumed that interaction of melatonin with either of these molecules contributes in prevention of angiogenesis (Fig. 2) [70].
Melatonin and metastasis
The early studies on the correlation between the pineal gland and the spread of solid tumors have been traced back to 1976 when it was revealed that pinealoctomy in rodent models increases the risk of metastasis, and that the administration of melatonin may prevent this phenomenon [71]. This concept has also been confirmed by invivo studies on her2/neu transgenic mice which indicated that oral administration of this hormone may reduce the risk of metastasis through downregulation of her2/neu transcript [72].
An important mediation mechanism of melatonin on cancer cell invasion has been discussed in an article by Cos and his coworkers in 1998, which declared that melatonin anti-metastatic action is compromising with its anti-estrogenic effect. It should be noted that 17β estradiol significantly impacts the attachment of cancer cells to the basement membrane through suppressing β1 integrin and E-cadherin expression [39]. Apparently, the inhibition of estrogen, suppression of cAMP formation, and PKA activity through melatonin administration is along with overexpression of β1 integrin and E-cadherin, the regulation of P38/MAPK signaling pathway regulation, and inhibition of MMP2 and 9 (matrix metalloproteinase 2 and 9) [39].
Epithelial to mesenchymal transition (EMT) is a common process in breast cancer cells which gives the malignant cells the ability to migrate. E-cadherin maintains epithelial cell polarity and the reduction in this adhesion molecule expression signifies as an EMT hallmark [73]. GSK-3B is constantly activated in cells but in response to the stimuli and phosphoactivation of Akt, this molecule become inactivated which in turn activates WNT signaling and accumulates B-cathenin in the nucleus. Therefore by augmentation of Snail and vimentin (E-cadherin repressors), the amount of E-cadherin is reduced and eventually EMT triggers the process of metastasis. As it was mentioned earlier in this article, one of the distinguishing features of melatonin is it’s inhibitory effects on PI3K and Akt phosphorylation, which disrupts GSK-3β activation and ultimately upregulates E-cadherin and eventually prevents EMT [73].
Melatonin inhibits telomerase activity
Since telomerase (the main enzyme in telomer elongation) is activated in all cancers, it is considered as a potential target for cancer treatment [74]. Estrogen is one of the eminent activators for telomerase in breast cancer [75]. Indeed, the E-ER complex enhances the TERT expression by binding to its promoter [75]. Because of anti-estrogenic properties of melatonin in breast cancer, it was hypothesized that melatonin could also act as an anti-telomerase agent. An invivo and invitro study conducted by Leon-Blanco et al. revealed that melatonin has the ability to reduce both telomerase activity and the expression of TERT mRNA [74, 75]. It is suggested that the anti-telomerase activity of melatonin is coupled with its anti-estrogenic property [75].
The concomitant use of melatonin and chemotherapeutic agents in breast cancer: the entrance of melatonin in clinical trial
Although endocrine therapy and radiation are the cornerstone in breast cancer treatment, still some patients will not respond to the treatment due to the primary or secondary residence, including overexpression of ERα and receptor tyrosin kinase signaling activation. Moreover, the cytotoxic effects of conventional chemotherapies like chemotherapy-induced thrombocytopenia or adriamycin-dependent cardiotoxicity are considered as a major impediments to the successful treatment and restrict the application of these drugs for the patients [76, 77].
Based on the very early studies, the pineal gland has been considered as an oncostatic gland, as the pinealoctomy exacerbated the mammary tumor in murine models, while the administration of melatonin alleviated the character of the malignancy. On the basis of the marked anti-cancer characteristics of this hormone, a great deal of attraction has been devoted to the capability of melatonin in thwarting the detrimental side effects of conventional therapies and raising their antineoplastic efficacy. Several clinical experiments have evaluated the efficacy of melatonin administration along with some chemotherapy drugs. These investigations suggested that melatonin may ameliorate the adriamycin-induced cardiac dysfunction through free radical scavenging or abrogating lipid peroxidation, furthermore the adriamycin and melatonin co-treatment enhance the cell death in breast cancer cells and improves the patient outcome [76, 77]. Given to the similarity in melatonin and tamoxifen mechanism of action, several experiments have been designed to investigate the synergistic effects of melatonin and tamoxifen, these studies suggested that melatonin amplified tamoxifen cytotoxicity in metastatic breast cancer patients [5, 6, 43, 44]. In corroboration, another study showed that restoration of nocturnal melatonin levels in breast cancer patients back to normal by supplementing with melatonin reestablishes the sensitivity of breast tumor to conventional chemotherapies such as Tamoxifen [78]. It is ascertained that melatonin is able to prevent platelet decline and may normalize the platelets number in metastatic breast cancer patients which were treated with either epirubicin or adriamycin [76, 77].
As it was mentioned, resistance to chemo- and radiotherapy is a major reason for treatment failure. It has been demonstrated that breast cancer patient with RAD51, a key player in homologous recombination and DNA repair system overexpression are more prone to resist against radiotherapy. Noteworthy, it was observed that melatonin administration for these subset of patients sensitized them to radiotherapy by downregulation of RAD51 [79].
Noteworthy, introducing melatonin as a complementary therapy for the treatment of breast cancer, a systematic review of randomized controlled trials (RCTs) and meta-analysis conducted in 2005 suggested that administrating melatonin in breast cancer patients resulted in the reduction of cancer mortality at 1 year. This considerable reduction in cancer mortality, along with low side effects and low costs related to this hormone suggested a great potential for melatonin in treatment of breast cancer [78].
Conclusion
Melatonin is a chief product of the pineal gland. Because melatonin is produced in the darkness, for a long time this hormone was considered only as a circadian rhythm regulator until, further studies investigated other functions for this indoleamine. The studies on anti-tumoral effects of melatonin has spanned nearly five decades and tremendous efforts have been devoted up to now to determine its extend roles in breast tumor repression. As documented in majority of experiments, melatonin exerts its inhibitory actions through interacting with either MT1 or MT2 or receptor independent mechanism. In general, melatonin anti-cancer effects in breast cancer could be summarized into four group; the pro-apoptotic, anti-proliferative, anti-metastatic, and anti angiogenesis actions (Fig. 3). These mechanisms are clearly complex and insofar, they influence many cellular pathways. The majority of studies suggested that melatonin retards breast cancer progression by halting cell growth and proliferation. As estrogen and its receptor ERα are considered as stimulatory factors in breast cancer pathogenesis and due to the inhibitory physiological function of melatonin in estrogen synthesis, these studies concluded that the oncostatic action of melatonin is heavily mediated through interaction with estrogen response pathway. It seems that melatonin not only suppresses estrogen and ERα expression, but also inhibits their transcriptional activity. Otherwise, recent investigations identified other molecular mechanisms in this manner and cytostatic action of melatonin extended into the inhibition of telomerase, interaction with nuclear receptor RARα, regulation of aromatase expression, and modulation of PI3k signaling pathway. Although most of the studies highlighted anti-proliferative effects, more recent studies open a new avenue in anti-cancer effects of melatonin and introduced it as a potent cytotoxic agent. Melatonin alters Bax/Bcl2 ratio, accelerates cytochrome c release into cytosol, activates caspases, and finally induces apoptosis in breast cancer cell lines. Induction of programmed cell death could be related to the inhibition of PI3K, NfkB, and the induction of p53 pathway.
Since estrogen and PI3K pathways have been recognized as a major and, to date phenotypic hallmark of metastasis, it is reasonable to suggest that down-regulation of estrogen and PI3k signaling by melatonin alters the expression of some adhesion molecules such as B1Intergrin and E-cadherin, leading to suppression of metastasis. Experiments conducted on breast cancer cell lines also confirmed that melatonin anti-angiogenesis characteristic is mediated through inhibition of VEGF.
The broad inhibitory effects of melatonin in breast cancer make it a potent candidate in mammary cancer treatment research and put it in a priority for clinical trials. Still, further investigations need to be done to uncover underlying melatonin molecular mechanism.
References
Movahedi M, et al. Survival rate of breast cancer based on geographical variation in Iran, a national study. Iran Red Crescent Med J. 2012;14(12):798.
Turkoz FP, et al. Association between common risk factors and molecular subtypes in breast cancer patients. Breast. 2013;22(3):344–50.
Luo J et al. Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. BMJ. 2011;342:d1016.
Ramezanpour H, Setayeshi S, Akbari M. A novel scheme for optimal control of a nonlinear delay differential equations model to determine effective and optimal administrating chemotherapy agents in breast cancer. Iran J Cancer Prev. 2012;4(4):154–62.
Lissoni P, et al. Modulation of cancer endocrine therapy by melatonin: a phase II study of tamoxifen plus melatonin in metastatic breast cancer patients progressing under tamoxifen alone. Br J Cancer. 1995;71(4):854–6.
Sánchez-Barceló EJ, et al. Melatonin–estrogen interactions in breast cancer. J Pineal Res. 2005;38(4):217–22.
Ozer H, et al. 2000 Update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol. 2000;18(20):3558–85.
Cohen M, Lippman M, Chabner B. Role of pineal gland in aetiology and treatment of breast cancer. Lancet. 1978;312(8094):814–6.
Bartsch C, et al. Nocturnal urinary 6-sulphatoxymelatonin excretion is decreased in primary breast cancer patients compared to age-matched controls and shows negative correlation with tumor-size. J Pineal Res. 1997;23(2):53–8.
Rondanelli M, et al. Update on the role of melatonin in the prevention of cancer tumorigenesis and in the management of cancer correlates, such as sleep-wake and mood disturbances: review and remarks. Aging Clin Exp Res. 2013;25(5):499–510.
Dauchy RT, et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014;74(15):4099–110.
Jung B, Ahmad N. Melatonin in cancer management: progress and promise. Cancer Res. 2006;66(20):9789–93.
Grant SG, et al. Melatonin and breast cancer: cellular mechanisms, clinical studies and future perspectives. Expert Rev Mol Med. 2009;11:e5.
Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9(1):11–24.
Srinivasan V, et al. Therapeutic actions of melatonin in cancer: possible mechanisms. Integr Cancer Ther. 2008;7(3):189–203.
Tricoire H, et al. Origin of cerebrospinal fluid melatonin and possible function in the integration of photoperiod. Reprod (Cambridge, England) Suppl. 2002;61:311–21.
Bonnefont-Rousselot D, Collin F. Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology. 2010;278(1):55–67.
Fernández-Mar M, et al. Bioactive compounds in wine: resveratrol, hydroxytyrosol and melatonin: a review. Food Chem. 2012;130(4):797–813.
Reiter RJ, Tan D-X, Fuentes-Broto L. Melatonin: a multitasking molecule. Prog Brain Res. 2010;181:127–51.
Miller SC, et al. The role of melatonin in immuno-enhancement: potential application in cancer. Int J Exp Pathol. 2006;87(2):81–7.
Montilla P, et al. Melatonin versus vitamin E as protective treatment against oxidative stress after extra-hepatic bile duct ligation in rats. J Pineal Res. 2001;31(2):138–44.
Morgan PJ, et al. Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int. 1994;24(2):101–46.
Mao L, et al. Molecular deficiency (ies) in MT1 melatonin signaling pathway underlies the melatonin-unresponsive phenotype in MDA-MB-231 human breast cancer cells. J Pineal Res. 2014;56(3):246–53.
Ebisawa T, et al. Expression cloning of a high-affinity melatonin receptor from Xenopus dermal melanophores. Proc Natl Acad Sci. 1994;91(13):6133–7.
Reppert SM, et al. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci. 1995;92(19):8734–8.
Nosjean O, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275(40):31311–7.
Becker-André M, et al. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J Biol Chem. 1994;269(46):28531–4.
Benitez-King G, Anton-Tay F. Calmodulin mediates melatonin cytoskeletal effects. Experientia. 1993;49(8):635–41.
Cutando A, et al. Role of melatonin in cancer treatment. Anticancer Res. 2012;32(7):2747–53.
Kiefer T, et al. Melatonin inhibits estrogen receptor transactivation and cAMP levels in breast cancer cells. Breast Cancer Res Treat. 2002;71(1):37–45.
Lai L, et al. The Gαi and Gαq proteins mediate the effects of melatonin on steroid/thyroid hormone receptor transcriptional activity and breast cancer cell proliferation. J Pineal Res. 2008;45(4):476–88.
Witt-Enderby PA, et al. Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sci. 2003;72(20):2183–98.
Beckerandre M, Andre E, DeLamarter J. Identification of nuclear receptor mRNAs by RT-PCR amplification of conserved zinc-finger motif sequences. Biochem Biophys Res Commun. 1993;194(3):1371–9.
Dong C, et al. Melatonin inhibits mitogenic cross-talk between retinoic acid-related orphan receptor alpha (RORα) and ERα in MCF-7 human breast cancer cells. Steroids. 2010;75(12):944–51.
Lai L, et al. Alteration of the MT1 melatonin receptor gene and its expression in primary human breast tumors and breast cancer cell lines. Breast Cancer Res Treat. 2009;118(2):293–305.
Jablonska K, et al. Expression of melatonin receptor MT1 in cells of human invasive ductal breast carcinoma. J Pineal Res. 2013;54(3):334–45.
Rögelsperger O, et al. Coexpression of the melatonin receptor 1 and nestin in human breast cancer specimens. J Pineal Res. 2009;46(4):422–32.
Oprea-Ilies G, et al. Expression of melatonin receptors in triple negative breast cancer (TNBC) in African American and Caucasian women: relation to survival. Breast Cancer Res Treat. 2013;137(3):677–87.
Mao L, et al. Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res. 2010;12(6):R107.
Cos S, et al. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998;58(19):4383–90.
Cos S, Sánchez-Barceló EJ. Melatonin and mammary pathological growth. Front Neuroendocrinol. 2000;21(2):133–70.
Barceló EJS, Cos S. Melatonin, experimental basis for a possible application in breast cancer prevention and treatment. Histol Histopathol. 2000;15(2):637–47.
Sánchez-Barceló E et al. Melatonin: an endogenous antiestrogen with oncostatic properties. Melatonin From Molecules to Therapy. 2007;261–72.
Hill SM, et al. The growth inhibitory action of melatonin on human breast cancer cells is linked to the estrogen response system. Cancer Lett. 1992;64(3):249–56.
Pandi-Perumal SR, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85(3):335–53.
García Pedrero JM, et al. Calmodulin is a selective modulator of estrogen receptors. Mol Endocrinol. 2002;16(5):947–60.
Dai J, et al. Modulation of intracellular calcium and calmodulin by melatonin in MCF-7 human breast cancer cells. J Pineal Res. 2002;32(2):112–9.
del Río B, et al. Melatonin, an endogenous-specific inhibitor of estrogen receptor α via calmodulin. J Biol Chem. 2004;279(37):38294–302.
Conley A, Hinshelwood M. Mammalian aromatases. Reproduction. 2001;121(5):685–95.
Cos S, et al. Melatonin inhibits the growth of DMBA-induced mammary tumors by decreasing the local biosynthesis of estrogens through the modulation of aromatase activity. Int J Cancer. 2006;118(2):274–8.
Knower KC, et al. Melatonin suppresses aromatase expression and activity in breast cancer associated fibroblasts. Breast Cancer Res Treat. 2012;132(2):765–71.
Martínez-Campa C, et al. Melatonin enhances the inhibitory effect of aminoglutethimide on aromatase activity in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 2005;94(3):249–54.
Martínez-Campa C, et al. Melatonin inhibits aromatase promoter expression by regulating cyclooxygenases expression and activity in breast cancer cells. Br J Cancer. 2009;101(9):1613–9.
Cos S, et al. Melatonin modulates aromatase activity in MCF-7 human breast cancer cells. J Pineal Res. 2005;38(2):136–42.
Wang J, et al. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res. 2012;53(1):77–90.
Blask DE, et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65(23):11174–84.
Collins A, et al. Overexpression of the MT1 melatonin receptor in MCF-7 human breast cancer cells inhibits mammary tumor formation in nude mice. Cancer Lett. 2003;189(1):49–57.
Squecco R, et al. Melatonin affects voltage-dependent calcium and potassium currents in MCF-7 cell line cultured either in growth or differentiation medium. Eur J Pharmacol. 2015;758:40–52.
Jung JH et al. Melatonin suppresses the expression of 45S preribosomal RNA and upstream binding factor and enhances the antitumor activity of puromycin in MDA-MB-231 breast cancer cells. Evidence-Based Complementary and Alternative Medicine. 2013;2013:879746.
Blask DE et al. Light exposure at night disrupts host/cancer circadian regulatory dynamics: impact on the Warburg effect, lipid signaling and tumor growth prevention. 2014;9(8):e102776.
Hill SM, et al. Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J Mammary Gland Biol Neoplasia. 2011;16(3):235–45.
Proietti S, et al. Molecular mechanisms of melatonin’s inhibitory actions on breast cancers. Cell Mol Life Sci. 2013;70(12):2139–57.
Proietti S, et al. Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells. J Pineal Res. 2014;57(1):120–9.
El-Aziz MAA, et al. The biochemical and morphological alterations following administration of melatonin, retinoic acid and Nigella sativa in mammary carcinoma: an animal model. Int J Exp Pathol. 2005;86(6):383–96.
Vriend J, Reiter RJ. Melatonin as a proteasome inhibitor. Is there any clinical evidence? Life Sci. 2014;115(1):8–14.
Proietti S, et al. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. J Pineal Res. 2011;50(2):150–8.
Santoro R, et al. Melatonin triggers p53Ser phosphorylation and prevents DNA damage accumulation. Oncogene. 2012;31(24):2931–42.
Jardim-Perassi BV, et al. Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLoS ONE. 2014;9(1):e85311.
Alvarez-García V, et al. Antiangiogenic effects of melatonin in endothelial cell cultures. Microvasc Res. 2013;87:25–33.
Romon R, et al. Research Nerve growth factor promotes breast cancer angiogenesis by activating multiple pathways. Breast Cancer. 2010;9:11.
Blask D. The pineal: an oncostatic gland, in the pineal gland. New York: Raven Press; 1984. p. 253–84.
Anisimov VN, et al. The effect of melatonin treatment regimen on mammary adenocarcinoma development in HER-2/neu transgenic mice. Int J Cancer. 2003;103(3):300–5.
Mao L, et al. Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin-regulation of GSK3β. Mol Endocrinol. 2012;26(11):1808–20.
Leon-Blanco MM, et al. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. J Pineal Res. 2003;35(3):204–11.
Martínez-Campa CM, et al. Melatonin down-regulates hTERT expression induced by either natural estrogens (17β-estradiol) or metalloestrogens (cadmium) in MCF-7 human breast cancer cells. Cancer Lett. 2008;268(2):272–7.
Arif I, et al. Increasing doxorubicin activity against breast cancer cells using PPARγ-ligands and by exploiting circadian rhythms. Br J Pharmacol. 2013;169(5):1178–88.
Ma C, et al. Protective and sensitive effects of melatonin combined with adriamycin on ER + (estrogen receptor) breast cancer. Eur J Gynaecol Oncol. 2014;36(2):197–202.
Mills E, et al. Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis. J Pineal Res. 2005;39(4):360–6.
Alonso-González C, et al. Melatonin sensitizes human breast cancer cells to ionizing radiation by downregulating proteins involved in double-strand DNA break repair. J Pineal Res. 2015;58(2):189–97.
Acknowledgment
This work was supported by cancer research center, shahidbeheshti university of medical sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
About this article
Cite this article
Nooshinfar, E., Safaroghli-Azar, A., Bashash, D. et al. Melatonin, an inhibitory agent in breast cancer. Breast Cancer 24, 42–51 (2017). https://doi.org/10.1007/s12282-016-0690-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-016-0690-7