Abstract
Metformin has been used clinically for more than 60 years. As time goes by, more and more miraculous effects of metformin beyond the clinic have been discovered and discussed. In addition to the clinically approved hypoglycemic effect, it also has a positive metabolic regulation effect on the human body that cannot be ignored. Such as anti-cancer, anti-aging, brain repair, cardiovascular protection, gastrointestinal regulation, hair growth and inhibition of thyroid nodules, and other nonclinical effects. Metformin affects almost the entire body in the situation taking it over a long period, and the preventive effects of metformin in addition to treating diabetes are also beginning to be recommended in some guidelines. This review is mainly composed of four parts: the development history of metformin, the progress of clinical efficacy, the nonclinical efficacy of metformin, and the consideration and prospect of its application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the rapid development of society, a high-pressure and rapid-paced life has become the norm. Many people would like to eat sweets to deal with the pressure they have. However, eating too much sugar can easily lead to some chronic diseases. For example, liver disease, cardio-cerebrovascular disease (hypertension, coronary heart disease, stroke, etc.), diabetes, malignant tumors, chronic obstructive pulmonary disease (chronic bronchitis, emphysema, etc.), mental abnormalities, psychosis, and so on (Lustig et al. 2012; Rippe and Angelopoulos 2016; Stanhope 2016; Sigala et al. 2021). They all have the characteristics of a long course, complex etiology, health damage, and serious social harm. Studies show that the number of adults with diabetes in China ranks first in the world, and it is even estimated that the number of adults with diabetes may reach 642 million in 2040. At the same time, diabetes tends to induce tumors and other diseases (Zhang et al. 2020b; Saleh et al. 2021), which means that diabetic patients often have more than one disease. Therefore, drugs that can simultaneously prevent or treat multiple concurrent diseases in diabetic patients are worthy of looking forward to (Zajda et al. 2020).
Years of research have shown that metformin, a first-line treatment for type 2 diabetes mellitus (T2DM), may be able to treat or prevent other complications in people with diabetes (Griffin et al. 2017; Jia et al. 2021; Schernthaner et al. 2022). Perhaps metformin could be the expected savior. Common chronic diseases such as cancer or tumor, cardiovascular disease, and organ disease can make diabetic patients uncomfortable, and the clinical efficacy of metformin found in clinical trials just corresponds to them (Fig. 1) (Kalmykova et al. 2019a; Kalmykova et al. 2019b; Pugliese et al. 2019; Munoz et al. 2021; Zaccardi et al. 2021). To some extent, it is not too much to say that metformin is a lifesaver for people with diabetes or chronic diseases. However, most of the studies on the efficacy of metformin have not been validated by clinical trials. Most of the effects of metformin are expected, and the various magical therapeutic and preventive effects of metformin in clinical application still need further research and clinical validation.
Through data retrieval, the literature on metformin in anticancer, tumor inhibition, anti-aging, brain repair, cardiovascular protection, pulmonary inflammation inhibition, anxiety relief, anti-inflammatory, adjustment of intestinal flora, hair growth, and inhibition of thyroid nodules, prevention of stroke and other relevant studies was collected. According to the research results, the mechanism of action of the relevant efficacy was summarized, and the clinical application of metformin was predicted. This review is mainly composed of the historical development of metformin, its clinical curative effect, and its mechanisms and thinking, and looking forward to this review will focus on three parts around the various clinical efficacy of metformin and prevention mechanisms to carry on the summary. We would like to describe the current progress in investigating various clinical efficacy and preventive mechanisms of metformin in detail.
The history of metformin
Metformin is a precursor to the French Lilac, a medieval drug used to reduce polyuria and sugar in diabetics. During 1920–1950, due to the discovery of insulin and the toxic side effects of guanidine and its derivatives, guanidine glucose-lowering drugs (including metformin and metformin) temporarily faded out of people's sight. Metformin was first used in the clinical treatment of diabetes in 1957. Later, metformin was withdrawn from the market due to the side effects of lactic acidosis, thus confirming metformin as the only hypoglycemic drug that can reduce the complications of macrovascular and reduce the complications and mortality of T2DM. In 2000, metformin sustained-release tablets (Gvachin) were approved for sale in the United States, and some metformin drugs were developed in combination with other drugs. After that, metformin has been widely used and researched and has acquired a certain position in hypoglycemic drugs. In 2006, the American Diabetes Federation (ADA) and the European Diabetes Research Association (EASD) jointly issued a new consensus for the treatment of T2DM: newly diagnosed patients with T2DM should be treated with metformin—a first-line drug through the treatment process—along with lifestyle interventions (Fig. 2). Up to now, many of metformin's special effects have yet to be clinically proven to play a role in disease treatment (Bailey 2017).
Clinical efficacy
Effect of lowering blood sugar and reducing weight
Metformin, as a drug to lower blood sugar (Moses 2010), can reduce the production of liver glycogen without increasing insulin levels in the body, inhibit the absorption of glucose in the intestinal tract and increase the uptake and utilization of glucose in the peripheral tissues, thereby increasing insulin sensitivity (Hausmann and Schubotz 1975; van Bommel et al. 2020). Metformin also can reduce the synthesis and storage of cholesterol and the level of blood triglyceride (TG) and total cholesterol. It does not cause hypoglycemia when taken alone, but should be treated with caution in combination with insulin or other oral hypoglycemic agents such as sulfonylureas and nateglinide (Flory and Lipska 2019). Studies have shown that long-term use of metformin can not only improve symptoms of hyperglycemia (Xu et al. 2019) but also contribute to weight loss in obese T2DM patients with a proper diet (low-glycemic diet or intermittent fasting) (Lee and Morley 1998; Masarwa et al. 2021). It can significantly reduce hyperglycemia and hyperlipidemia in patients with T2DM, and help to reduce the risk of some tumors. However, metformin cannot be taken as a weight loss drug alone, and so far no guidelines have been found to list it as a weight loss drug and recommend it.
How to inhibit glycogenosis in liver
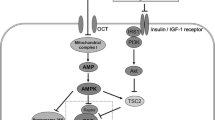
Metformin can inhibit liver glycogenosis and reduce insulin resistance through the adenosine monophosphate-activated protein kinase (AMPK) signal transduction pathway (Musi et al. 2002; Wen et al. 2021) and inhibition of acetyl-CoA carboxylase (ACC), thus improving the sensitivity of surrounding tissues to insulin (Fig. 3A).
Metformin works through the AMPK pathway. A. Metformin can increase peripheral tissue sensitivity to insulin via inhibiting ACC through the AMPK signal transduction pathway. B. Metformin inhibits tumor angiogenesis under hypoxia via down-regulating HIF-1 α expression through the AMPK/mTOR signal pathway
AMPK plays a key role in regulating cellular energy homeostasis (Zang et al. 2004; Banerjee et al. 2016). The liver metabolizes sugars mainly through the synthesis and decomposition of liver glycogen, as well as gluconeogenesis, to maintain the relative stability of blood glucose concentration. Metformin promotes the transfer of glucose transporters to the cell membranes of hepatocytes and increases the activity of insulin receptors in hepatocytes. Inhibition of fatty decomposition and TG degradation by brown fat cells reduced the level of free fatty acid (FFA) in blood. Experiments in mice showed that metformin inhibited inflammation caused by a high-fat diet (Coll et al. 2020). Metformin inhibits cellular fat accumulation in the liver and bone by activating the AMPK phosphorylation of ACC. Studies have confirmed that metformin can reduce the glucose output of the liver by 20–30% (Foretz et al. 2019). In the human body, brain blood cells, kidney medullary, intestinal, skin, and other tissues can use glucose without the help of insulin. Therefore, metformin can reduce the burden of insulin and the effect of insulin resistance by increasing glucose utilization in insulin-independent tissues.
The mechanism of inhibition of gluconeogenesis independent of AMPK is mainly through the influence of energy, redox, cell membrane potential, and so on. Metformin can inhibit mitochondrial complex 1, reduce the production of ATP, and reduce the allosteric inhibition of ATP on key enzymes involved in glycolysis, therefore, increasing intrahepatic glycolysis. The decrease of ATP level in vivo will increase AMP's inhibition of the glucagon signaling pathway and inhibit gluconeogenesis induced by PKA activation. AMP can allosterically inhibit the key enzyme of gluconeogenesis, fructose-1, 6-bisphosphatase, and allosterically activate the key enzyme of glycolysis, phosphofructokinase, thereby inhibiting the process of glycerol and lactic acid to blood sugar. Metformin can promote the chloride outflow of hepatocytes and lead to the depolarization of cell membranes, thus reducing the uptake of gluconeogenic substrates (Foretz et al. 2019).
How to affect the absorption of sugar from the intestine
Studies have shown that metformin has a high concentration in the intestinal tract (Coll et al. 2020). By changing the structure and diversity of intestinal microbiota, metformin can restore the proportion of intestinal microbiota, increase the probiotics of intestinal microbiota (such as Blautia SPP and Faecali bacterium SPP.), enhance the ability of bacteria to produce special types of short-chain fatty acids, inhibit glucose absorption (Bybel et al. 2011; de la Cuesta-Zuluaga et al. 2017; Zhang et al. 2020a), thus playing a role of lowering blood sugar and positively regulating the immune system.
How does metformin reduce appetite and weight loss?
Gregory R. Steinberg's team found that metformin upraised the growth differentiation factor (GDF15) in the body without using the AMPK pathway. Metformin can upregulate GDF15 in vivo without the AMPK pathway. Metformin over-regulates the transcription factors activating transcription factor 4 (ATF4) and C/EBP-homologous protein (CHOP) upstream of GDF15 to increase the secretion of GDF15 in hepatocytes, achieve weight loss by suppressing appetite, increase satiety and reduce hunger (Duan et al. 2013; Kim et al. 2013; Calco et al. 2021). The highly expressed GDF15 in the liver interacts with the glial cell-derived neurotrophic factor (GDNF) family receptor-like (GFRAL) receptor in the posterior brain to inhibit appetite for high-fat foods and regulate body weight and energy balance. This study demonstrates the potential value of GDF-15 in the study of metformin for weight loss (Fig. 4).
Anticancer/tumor effects
Metformin has several anticancer mechanisms and may be used to prevent many types of cancer, while combination drugs may enhance its anticancer effect (Chen et al. 2021c; Misirkic Marjanovic et al. 2021; Xia et al. 2021). It may have a direct inhibitory effect on individual cancers and this anticancer therapeutic effect may have a significant impact on the quality of human life. In summary, metformin can reduce the risk of tumors in patients with T2DM. However, the specific mechanism of metformin's tumor inhibition is not yet clear, and most of the studies are only at the experimental stage. Metformin has great potential in anticancer treatment.
Intermittent fasting combined with oral administration of metformin inhibits tumor growth and reduces the risk of developing tumors in patients with T2DM because tumors are sensitive to metformin in low-glucose conditions. Metformin can reduce the level of myeloid cell leukemia-1 (MCL-1) by activating protein phosphatase 2A (PP2A) and glycogen synthase kinase 3β (GSK3β) and cancerous inhibitor of PP2A (CIP2A) [CIP2A are highly expressed in a variety of tumors, and inhibit the activity level of PP2A] to inhibit tumor growth (Elgendy et al. 2019). In addition, hypoxia-inducible factor-1 α (HIF-1α), which controls oxygen change and regulates cell function, and is an important factor for tumor cells to adapt to the hypoxia environment, is expected to be a target for tumor therapy. Finally, the expression of HIF-1α is inhibited through the AMPK/mTOR signal transduction pathway, and the formation of tumor neovascularization under hypoxia is inhibited, thus inhibiting tumorigenesis (Fig. 3B) (Palazon et al. 2017).
Meta-analysis showed that metformin can reduce the incidence of cancers and increase the survival time of T2DM patients with cancers (Coyle et al. 2016). The specific mechanism has not been determined, but there are many speculations and possible tumor inhibition pathways.
Metformin may inhibit the reduction of CD8+tumor-infiltrating lymphocytes (TILs) due to apoptosis by increasing the number of CD8+TILs in tumors, thereby taking an anticancer effect (Fig. 5A) (Kim et al. 2020). Metformin may indirectly play an anti-cancer role in reducing the growth stimulation effect on tumor cells via lowering the levels of insulin/insulin-like growth factor-1 (IGF-1) and serum insulin (Fig. 5B) (Sarmento-Cabral et al. 2017; Chen et al. 2021b). It is possible to induce the inhibition of mammalian target rapamycin complex 1 (mTORC1), thereby inhibiting the drug resistance and proliferation of tumor cells and activating autophagy to promote the apoptosis of tumor cells (Fig. 5C). This process can be achieved by either AMPK or non-AMPK—dependent pathways. It may also increase the number of memory T cells, promote the apoptosis of cancer cells, and enhance the antitumor function of T cells by inhibiting the signal transmission of mTORC1 (Mossmann et al. 2018). It can inhibit tumor micro-environmental immunosuppression, and inhibit the differentiation of macrophages to prevent tumor metastasis. Programmed cell Death 1 Ligand 1 (PD-L1) inhibits the expression of PD-L1 in the cancer cell. Metformin can reduce the stability and membrane localization of PD-L1 through the degradation of related proteins in cancer cells, and prevent the expression of PD-L1 in cancer cells (Fig. 5D) (Soliman et al. 2020). It is also possible to inhibit the activity of the transcription factor NF-KB signaling pathway by activating AMPK (killing liver cancer cells and inhibiting tumor growth) (Fig. 5E) (Xu et al. 2017). The expression of Kruppel Like Factor 5 (KLF5) can also be down-regulated by inhibiting PKA, and the proportion of rods in HCC1806 and HCC1937 cell lines and the number of microspheres formed by cell lines can also be reduced to inhibit the stem cells of cancer cells (Fig. 5F).
Antitumor mechanisms of metformin. A. Metformin may increase the number of CD8 + infiltrating lymphocytes (TILs) in tumors. B. Metformin lowers the levels of IGF 1 and serum insulin to reduce their stimulatory effects on the growth of tumor cells. C. Metformin inhibits tumor microenvironment immunosuppression and prevents tumor metastasis via inhibiting the mTORC1 signal pathway. D. Metformin reduces the stability and membrane localization of PD-L1 by degrading related proteins in cancer cells, thereby preventing PD-L1 expression in cancer cells. E. Metformin inhibits the activity of the NF-KB signaling pathway via activating AMPK. F. Metformin inhibits PKA to down-regulate the expression of KLF5
Triple-negative breast cancer (TNBC)
TNBC is the most difficult of the four types of breast cancer to cure and the one with the worst prognosis. It is most common in young women. Although TNBC has a poor prognosis and a higher risk of death, most TNBCs are treated with chemotherapy. The therapeutic targets of TNBC have always been a difficult problem in clinical treatment (Li et al. 2021a). Triple-negative refers to the three targets mainly targeted in treatment: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor (HER).
The scientists were surprised to find that the combination of metformin and heme significantly inhibited TNBC tumor growth. BTB and CNC Homology 1 (BACH1), an anti-cancer target transcription factor of heme, is a key factor regulating mitochondrial metabolism and is essential for cancer metastasis. However, inhibition of BACH1 expression has no significant side effects on normal cells, so BACH1 can be used as a therapeutic target for cancer. Heme can degrade the BACH1 protein in cancer cells, forcing them to change their metabolic pathways (from anaerobic respiration to aerobic respiration). BACH1 inhibits the transcription of mitochondrial electron transport chain (ETC) genes directly and regulates pyruvate dehydrogenase kinase (PDK) directly. BACH1 phosphorylates PDH Ser293 to inhibit its activity and enhance the tricarboxylic acid cycle metabolism, which is a key regulator of glycolysis and aerobic respiration metabolism. Metformin, as a low-toxicity ETC inhibitor, can inhibit the aerobic metabolism of cancer cell mitochondria, and the combination of the two drugs can more significantly inhibit the growth of TNBC tumor cells than the two alone (Fig. 6) (Wahdan-Alaswad et al. 2014; Shi et al. 2017; Strekalova et al. 2017; Liu et al. 2021).
Effects of metformin on mitochondrial metabolism. Metformin can inhibit the aerobic metabolism of mitochondria of cancer cells and heme can inhibit the regulation of BACH1 on aerobic respiration, which directly inhibits the transcription of the mitochondrial ETC (electron transport chain) gene. The combination of heme and metformin can significantly inhibit the growth of TNBC tumor cells
In addition, metformin combination therapy is promising for breast cancer – a triple therapy of metformin + Venetoclax + PD-1 antibody may be available for breast cancer (Bayraktar et al. 2012; Lee et al. 2019; Varghese et al. 2019). The treatment has been approved by the Food and Drug Administration as a first-line treatment for TNBC. Venetoclax is the world's first Bcl-2 inhibitor, primarily used to treat leukemia. It can inactivate the Bcl-2 protein, which can inhibit the apoptosis of cancer cells, restart the suicide program of cancer cells, and destroy “the immortal legend of cancer cells” (Wahdan-Alaswad et al. 2016; Wang et al. 2020a). Metformin combined with Venetoclax activates MCL cells, while PD-1 antibody (Tecentriq) and Abraxane (albumin paclitaxel) maintain MCL cells for longer periods, perhaps prolonging survival in cancer patients. However, it has been found that adding metformin to standardized breast cancer treatment does not significantly improve survival in patients without diabetes (Goodwin et al. 2022). Therefore, whether metformin should be used in all breast cancer patients is controversial and needs further validation.
Cancers that are nischarin deficient
Protein Nischarin participates in a variety of biological reactions in vivo and plays an important role in inhibiting tumor migration and tumor cell invasion. Research by Dr. Suresh Alahari et al. has found that interfering with Nischarin may promote breast tumor growth and inhibit AMPK activation (Gollavilli et al. 2015). However, with metformin treatment, the above conditions can be reversed under Nischarin’s absence. Therefore, for cancers lacking the Nischarin protein, it is possible to inhibit the occurrence of cancer by activating the AMPK function. There is still much room for research on the specific mechanism of metformin acting on breast cancer cells (Dong et al. 2020). Activation of AMPK prevents the Nischarin gene from enhancing tumor growth and metastasis, which can be used to treat malignant diseases.
Effect on hepatobiliary tumor/hepatobiliary cancer
Surgical resection is the main treatment for intrahepatic cholangiocarcinoma (ICC). Studies have found that in hepatobiliary cancer/hepatobiliary tumors, the abnormal FGF19-FGFR4 signaling pathway is an important factor causing cancer, and fibroblast growth factor receptor (FGFR) inhibitory therapy may be a new target for cancer treatment to resist HCC/ICC (Chen et al. 2021a).
Metformin combined with arsenic trioxide (ATO) may inhibit ICC through conventional chemotherapy (Ling et al. 2017). By promoting apoptosis, inducing G0/G1 cell cycle arrest, increasing intracellular reactive oxygen species (ROS) synergistic inhibition of ICC cell proliferation, and so on, they effectively reduced ICC growth in the ICC xenograft model but failed to increase the survival rate (Chaiteerakij et al. 2013; Kaewpitoon et al. 2015; Yang et al. 2016; Trinh et al. 2017; Di Matteo et al. 2021). In diabetics, metformin reduced the risk of ICC by 60 percent. In addition, metformin can effectively enhance the sensitivity of ICC cells to chemotherapy drugs such as sorafenib 5-fluorouracil ATO. Metformin combined with conventional chemotherapy can improve efficacy. It may be that metformin inhibits ICC by regulating the AMPK/ P38 MAPK ERK3/mTORC1 pathway and enhancing ATO sensitivity (Ling et al. 2017).
In the liver, adiponectin secreted by fat cells is involved in the control of body metabolism. Perhaps adiponectin combined with metformin can be used as a new treatment for liver cancer, whose efficacy and mechanism remain to be verified. Murff et al. found that T2DM patients taking metformin were less likely to develop liver cancer (Murff et al. 2018). Metformin inhibits the activity of the transcription factor NF-KB signaling pathway by activating AMPK. This study revealed that metformin has great potential in the treatment of hepatocellular carcinoma.
Esophageal cancer
Esophageal cancer is the second largest gastrointestinal tumor after gastric cancer (Siegel et al. 2022). Studies have shown that metformin at a normal dose can selectively inhibit the growth of esophageal squamous cell cancer cells, induce apoptotic cell death, inhibit cell proliferation and induce autophagy (Wang et al. 2020b). The induction of autophagy was discovered by scientists through the establishment of a 4-Nitroquinoline N-oxide (4NQO) -induced mouse model of esophageal squamous cell carcinoma. Metformin can enhance the phagocytic function of esophageal squamous cell carcinoma at a low dose. Metformin changes the tumor microenvironment of esophageal squamous cell carcinoma cells and thus has the effect of inhibiting tumor growth. Metformin may activate STAT3 and increase AMPK content in immune cells by changing the cytokine secretion profile of immune cells, thus inactivating the STAT3 network signaling pathway (especially the STAT3-BCL2-Beclin1 network signaling pathway) to promote cross-talk between apoptosis and autophagy and achieve the purpose of inhibiting tumor growth. Studies have shown that metformin can not only accelerate the differentiation of gastric stem cells into gastric acid-producing gastric wall cells, but also increase the number to regulate the secretion of gastric acid in the stomach and greatly reduce the risk of gastric cancer in people infected with Helicobacter pylori, but also reduce the risk of rectal cancer (Zell et al. 2020; Lu et al. 2021a).
Head and neck cancer
Some studies have shown that metformin may eliminate the stem cell characteristics of head and neck cancer cells and prevent cancer cells from becoming malignant (Crist et al. 2022). It can be used to prevent high-risk cancer populations from cancer. Metformin blocks cancer cell metabolism by inhibiting the mitochondrial complex of cancer cells to reduce the expression of cancer stem cell programs. And metformin triggers the differentiation of cancer cells, directly acting on the head and neck cancer initiation cells.
Pancreatic cancer
Diabetes mellitus can increase the risk of pancreatic cancer and is a high-risk factor for pancreatic cancer. Some research suggests that metformin may reduce the risk of pancreatic cancer in diabetics. Bodmer et al. found that the odds ratio of pancreatic cancer was 0.43 (95%CI 0.23–0.80) in women with diabetes who took metformin for a long period compared with those who did not take the drug. However, long-term insulin or sulfonylureas use had a ratio of 1.90 for pancreatic cancer compared with non-users (Bodmer et al. 2012). Inhibition of the signaling pathway of the pancreatic stellate cell (PSC) used to synthesize hyaluronic acid and type 1 collagen also prevents the recruitment of tumor-related macrophages. Chen et al. showed reduced pancreatic acinar-ductal metaplasia and pancreatic intraepithelial neoplasia in mice treated with metformin. Metformin can inhibit tumor proliferation and metastasis and inhibit the transformation from chronic pancreatitis to pancreatic cancer (Chen et al. 2017).
Lung cancer
Previous studies have suggested that metformin plays an anticancer role primarily by AMPK and inhibiting protein synthesis (Wang et al. 2013; Lu et al. 2021b). Meta-analysis showed that metformin could reduce the risk of lung cancer in patients with T2DM and improve the survival rate, and its protective effect on patients in Asia was clear, so it was promising to be used in the treatment of patients with T2DM complicated with lung cancer (Gupta et al. 2018). The roles of metformin in prolonging the life cycle of patients with advanced lung cancer with epidermal growth factor receptor (EGFR) mutations and benefiting non-small cell cancer patients remain controversial (Salani et al. 2012; Ko et al. 2020).
Effects on the brain
Repair the brain
Metformin has great potential in promoting brain-damaged and episodic cognitive recovery and brain repair and growth. A related study conducted at the University of Toronto involved both human patients and animal models. Metformin promoted the formation of new neurons in the forebrain and hippocampus dentate Gyrus in animals after brain injury, which promoted brain nerve growth and enhanced cognition and memory (Hwang et al. 2010; Ng et al. 2014; Zhu et al. 2020). Previous animal studies have shown that metformin selectively activates neural stem cells in adult female mice to repair the brain and restore cognitive function (Kuan et al. 2017; Mandwie et al. 2021). Metformin, on the other hand, has been shown in clinical trials to help repair the brains of patients with neurological disease, saving a radiation-induced decline in the neural stem cell pool (no gender difference, but more significant in women) (Ayoub et al. 2020; Yuen et al. 2021). This study provides a credible basis for the treatment of metformin to repair the brain. Metformin, in the presence of estrogen (mainly estrogen estradiol), activates neural stem cells in the brain and improves brain function. The aPKC (atypical protein kinase C)-CBP (cAMP-response element binding protein) pathway is an important pathway mediating the directional differentiation of neural stem cells into mature neurons. Metformin may activate the aPKC-CBP pathways in hepatocytes and promote neuron regeneration in rodents and humans (Wang et al. 2012).
Reduce the risk of dementia
Metformin has the potential to reduce the risk of vascular events and vascular dementia (Lin et al. 2018b; Ma et al. 2021). It may be related to metformin-reducing systemic inflammation (Lin et al. 2018a; Li et al. 2021b; Xiong et al. 2021). This may be good news for many middle-aged and elderly patients with T2DM. Using metformin for a long time may help them avoid the trouble of Alzheimer's disease and save a lot of burden for their families.
Relieve anxiety
Anxiety disorder is one of the greatest psychological problems in today's society. Proper anxiety might make people work more efficiently, while excessive anxiety tends to make people sick, not only physically unhappy, but also makes them less happy in life, negatively affects the people around them, and so on. It has been found that metformin may be used to treat some forms of autism, such as fragile X syndrome, metabolic or psychiatric disorders, or depression. By reducing the content of branched-chain amino acids in the diet, it was speculated that the experiment also achieved the effect of reducing the anxious behavior of male mice (Fan et al. 2019). Metformin may reduce anxiety-like behavior in male mice by lowering the levels of circulating branched-chain amino acids, increasing the availability of serotonin in the brain, and improving neurotransmission in the hippocampus (Zemdegs et al. 2019).
Cardiovascular diseases
Preclinical and clinical studies have shown that metformin is directly anti-inflammatory through AMPK-dependent or independent inhibition of nuclear transcription factor B (NFκB) (Ba et al. 2019). Perhaps metformin can improve chronic inflammation by improving metabolic parameters such as hyperglycemia, insulin resistance, and dyslipidemia, which leads to atherosclerosis (Li et al. 2009; Feng et al. 2021; Seneviratne et al. 2021). Consequently, it has a direct anti-inflammatory effect.
Reduce the risk of cardiovascular diseases
Studies have shown that taking metformin can reverse heart damage in people without diabetes (Mohan et al. 2019). T2DM patients taking metformin had a relatively low risk of cardiovascular disease, mortality from varicose veins, T2DM venous thromboembolism (VTE), and hospitalization for heart failure. Therefore, we inferred that metformin has a cardiovascular protective effect. Metformin reduces the risk of heart attacks and stroke by reducing inflammation in the lungs that cause clotting caused by air pollution. Metformin can also prevent inflammation caused by smog, prevent immune cells from releasing a dangerous molecule into the blood, inhibit the formation of arterial thrombosis, and thus reduce the risk of cardiovascular disease by reducing the excessive production of dicarbonyl (Beisswenger et al. 1999), which may improve cardiac autonomic nerve function in this population.
Metformin can also intervene in all stages of the chain of cardiovascular events: Reduce the risk factors of cardiovascular disease (weight loss, insulin resistance, blood pressure reduction, blood lipid improvement, anticoagulation, etc.) and exert cardiovascular protective effects (He et al. 2021). It can directly improve vascular endothelial function. It can reduce the formation of atherosclerosis, reduce myocardial hypertrophy myocardial infarction, and heart failure; Long-term treatment can improve cardiovascular outcomes in patients with T2DM, metformin can improve endothelial function, and reduce the level of endothelial function markers such as von Willebrand factor (vWF) soluble vascular adhesion molecule-1 (SVCAM-1) and plasminogen activator inhibitor-1 (PAI-1) (Fidan et al. 2011; Ding et al. 2021). Metformin can reduce the size of acute myocardial infarction and improve the absence of reflow after angioplasty of acute myocardial infarction. A retrospective study has shown that long-term application of metformin can significantly reduce the level of creatine kinase (CK) creatine kinase isoenzyme (CK-MB) troponin T in patients and reduce myocardial infarction area (Eppinga et al. 2017; Thein et al. 2020; Sardu et al. 2021). The cardiovascular effects of metformin also reduced the risk of stroke in patients with diabetes who received metformin compared to those who did not (Westphal et al. 2020; Zemgulyte et al. 2021).
Aortic aneurysm
Studies have shown that metformin is negatively correlated with aortic aneurysm development, and a randomized double-blind trial is still needed to verify that metformin may also reduce the probability of aortic disease and related events (Han et al. 2019; Thanigaimani et al. 2021) which effectively reduce the risk of atherosclerosis (ASCVD) in T2DM patients.
Type 1 diabetes (T1DM)
Studies have shown that metformin can also improve endothelial function, insulin sensitivity, and vascular health, and improve cardiovascular risk in patients with T1DM, which is expected to achieve cardiovascular benefits (Liu et al. 2020). Metformin treatment independently increased levels of the oxidative stress marker prostaglandin F2 (PGF2) and improved flow-mediated dilation (FMD) in patients with type 1 diabetes mellitus. Metformin may improve atherosclerosis and reduce myocardial injury by improving cardiovascular risk factors, and other mechanisms have cardiovascular protective effects (Snell-Bergeon 2017).
Anti-aging
Clinical trials in the United States have found that metformin may increase health and prolong life (Podhorecka et al. 2017; Kumari et al. 2021). Studies have found that the anti-aging effect of metformin is related to the effect of its metabolites, guanidine, and dietary intake on the intestinal microbiome through a high-throughput quadrate screening method that integrates the four major elements of the host microbiome, drug-nutrition (Piskovatska et al. 2019). There have also been studies using cocktails of growth hormones and dehydroepiandrosterone (DHEA) and metformin to regenerate the thymus in users, reducing biological age (Torres et al. 2020). Metformin can directly inhibit the activity of many enzymes involved in ATP synthesis and decomposition by activating AMPK, thus reducing energy consumption. It is also possible to increase mitochondrial biosynthesis by modulating the peroxisome proliferator-activated receptor (PPAR) co-activator. Activation of AMPK also increases autophagy in the body (Ma et al. 2022). Metformin may inhibit mTOR. Inhibited mTORC1 increases ACAD10 transcription through SKN-1/Nrf2. ACAD10 may be related to some proteins that affect the growth and development of the body and the functions of ribosomes and mitochondria. Metformin may regulate the IGF-1 signaling pathway to reduce the patient's blood glucose levels, slow down body aging, and extend life. Metformin may inhibit electron transport chain complex 1 by reducing ROS production to reduce the number of electron transfers and prevent electron transfer, reduce the production of ROS, and reduce the cumulative damage of DNA, to achieve the anti-aging effect. Metformin may regulate sirtuin (SIRT) expression to prolong life: a variety of SIRT may regulate mitochondrial function and affect lifespan (Glossmann and Lutz 2019).
The lungs
Metformin has been shown to protect against lung cancer (Xiao et al. 2020; Lu et al. 2021b), improve the prognosis of lung cancer patients, and prevent pulmonary inflammation caused by haze. It can relieve allergic eosinophilic airway inflammation in obese mice. It reduces the risk of lung disease in non-smokers and increases the risk of lung disease in smokers. A team led by Professor Scott Budinger found metformin prevented inflammation caused by smog in mice (Soberanes et al. 2019). It prevents immune cells from releasing a dangerous molecule into the bloodstream, which inhibits the formation of arterial clots and thus reduces the risk of cardiovascular disease.
Pulmonary idiopathic pulmonary fibrosis
Studies have shown that metformin can reverse pulmonary fibrosis in mice. Metformin showed significant anti-fibrosis effects (Kheirollahi et al. 2019; Gu et al. 2021). Metformin plays an effective anti-fibrotic role by regulating metabolic pathways and inhibiting the effect of transforming growth factor 1 (TGF 1) on collagen formation, activating PPARγ signaling, and inducing adipogenic differentiation in lung fibroblasts.
Asthma
The study has shown that metformin can relieve allergic eosinophilic airway inflammation in obese mice (Guo et al. 2021). The study also found that metformin significantly reduced the incidence of asthma attacks and hospitalizations in patients with asthma, and was closely associated with survival benefits in patients with COPD and diabetes. Metformin may relieve allergic eosinophilic airway inflammation and restore normal eosinophilic and tumor necrosis factor (TNF-A) levels in bronchoalveolar lavage and lung AMPK levels. Asthma can be alleviated by inhibiting airway smooth muscle cell proliferation through AMPK-dependent channels.
Assist in smoking cessation
The study found that long-term smoking in patients led to the activation of the AMPK signaling pathway, which was inhibited when nicotine withdrawal occurred. Therefore, it is speculated that activating the AMPK signaling pathway with drugs may alleviate the withdrawal response. Tests on mice have found that metformin relieves nicotine withdrawal symptoms in mice and may be used to quit smoking (Kaisar et al. 2017).
Ovary and uterus
Polycystic ovary syndrome (PCOS)
Tests in the 1990s showed that metformin restored ovulation function and improved PCOS (hyperandrogenism) by reducing insulin resistance (Tariq et al. 2007). AlHussain et al. found that metformin increases the live birth rate of clinical pregnancy and reduces anxiety in patients with PCOS (AlHussain et al. 2020). Another meta-analysis suggests that metformin may be considered in combination therapy for women with PCOS who have undergone in vitro fertilization or intracytoplasmic sperm injection and embryo transfer (IVF/ICSI-ET) cycles (Wu et al. 2020).
Gestational diabetes mellitus (GDM)
Data have shown that metformin has the same hypoglycemic effect on GDM as glibenzoide, and short-term use of metformin is safer, while long-term use of metformin has an impact on offspring (Bashir et al. 2020). This may increase the risk of obesity in offspring. Compared with glibenzoide, metformin was more inefficient in the treatment of GDM patients, and it could not reduce maternal triglyceride levels (Balsells et al. 2015).
Hair
Some studies have shown that metformin topical application may be used to treat hair loss during remanence, which stimulates the hair follicles of mice from remanence to growth and promotes hair growth (Araoye et al. 2020; Sun et al. 2021). However, there has been a reported case of acute hair loss in a woman with PCOS who took metformin (Rezvanian et al. 2009).
Hepatorenal
Metformin has been shown to reduce the risk of death (Crowley et al. 2017) and end-stage kidney disease (ESRD) in patients with T2DM who have developed cirrhosis of the liver or chronic kidney disease (CKD) with impaired renal function. Improve the risk of death from renal failure and kidney disease but be alert to the risk of lactic acidosis (Borbely 2016). Large studies have shown that metformin in patients with T2DM with CKD is associated with a reduced risk of death and ESRD without an increased risk of lactic acidosis, suggesting that metformin may reduce the risk of death from renal failure and kidney disease (Bakris and Molitch 2016; Gosmanov et al. 2021).
Thyroid
It has been found that metformin reduces thyroid volume and reduces the occurrence of goiter and thyroid nodules. In addition, studies have shown that metformin can treat thyroid nodules (Krysiak et al. 2015; He et al. 2019). Metformin can inhibit the growth of thyroid cells and different types of thyroid cancer cells by affecting the insulin/IGF1 and mTOR pathways. Furthermore, it is possible to enhance the role of thyroid hormone in the pituitary and adenosine by activating AMPK, thereby reducing fluctuations in thyroid stimulating hormone (TSH) levels in diabetic patients. Thyroid nodules can be treated by regulating the signal transduction pathway between TSH and IGF-1.
Prevention of stroke
Long-term studies have shown a sustained reduction in diabetes risk with metformin, showing the potential to prevent trends in cancer and stroke, and have expanded and updated the results including microvascular complications, cardiovascular events, cancer incidence, and the influence of age (Westphal et al. 2020). Age had a greater effect on the risk of kidney disease among all interventions. Metformin's ability to prevent stroke was more significant in people with higher baseline blood glucose levels and women with a history of GDM.
Thinking and perspectives
The clinical application prospect of metformin is infinite. Here, we summarize clinical trials involving metformin (Table 1). If we can prove the mechanism of its various curative effects, it will achieve certain breakthroughs in cancer treatment, anti-aging treatment, brain repair treatment, and other aspects, and improve the quality of human life. Due to the numerous clinical effects of metformin, the mechanism of action of each of its therapeutic effects may be related. If we want to investigate the mechanism of action of metformin, maybe we can start from the whole, link up the mechanism of action of each therapeutic effect, and study a series of influencing factors.
The clinical efficacy of metformin in various treatments is still controversial and needs more clinical and experimental support. Starting from the therapeutic effect of diabetes, exploring the mechanism of metformin's other effects may be able to develop into another new field. Combined treatment with metformin may be able to enhance the efficacy and achieve the multiplying effect. To sum up, there is a lot of room for research on cancer and anti-aging.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Alhussain F, Alruthia Y, Al-Mandeel H, Bellahwal A, Alharbi F, Almogbel Y, Awwad O, Dala’een R, Alharbi FA (2020) Metformin improves the depression symptoms of women with polycystic ovary syndrome in a lifestyle modification progrAm. Patient Prefer Adherence 14:737–746. https://doi.org/10.2147/PPA.S244273
Araoye EF, Thomas JA, Aguh CU (2020) Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep 6:106–108. https://doi.org/10.1016/j.jdcr.2019.12.008
Ayoub R, Ruddy RM, Cox E, Oyefiade A, Derkach D, Laughlin S, Ades-Aron B, Shirzadi Z, Fieremans E, Macintosh BJ, De Medeiros CB, Skocic J, Bouffet E, Miller FD, Morshead CM, Mabbott DJ (2020) Assessment of cognitive and neural recovery in survivors of pediatric brain tumors in a pilot clinical trial using metformin. Nat Med 26:1285–1294. https://doi.org/10.1038/s41591-020-0985-2
Ba W, Xu Y, Yin G, Yang J, Wang R, Chi S, Wang Y, Li C (2019) Metformin inhibits pro-inflammatory responses via targeting nuclear factor-kappaB in HaCaT cells. Cell Biochem Funct 37:4–10. https://doi.org/10.1002/cbf.3367
Bailey CJ (2017) Metformin: historical overview. Diabetologia 60:1566–1576. https://doi.org/10.1007/s00125-017-4318-z
Bakris GL, Molitch ME (2016) Should restrictions be relaxed for metformin use in chronic kidney disease? Yes, they should be relaxed! what’s the fuss? Diabetes Care 39:1287–1291. https://doi.org/10.2337/dc15-2534
Balsells M, Garcia-Patterson A, Sola I, Roque M, Gich I, Corcoy R (2015) Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 350:h102. https://doi.org/10.1136/bmj.h102
Banerjee J, Bruckbauer A, Zemel MB (2016) Activation of the AMPK/Sirt1 pathway by a leucine-metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism 65:1679–1691. https://doi.org/10.1016/j.metabol.2016.06.011
Bashir M, Aboulfotouh M, Dabbous Z, Mokhtar M, Siddique M, Wahba R, Ibrahim A, Brich SA, Konje JC, Abou-Samra AB (2020) Metformin-treated-GDM has lower risk of macrosomia compared to diet-treated GDM- a retrospective cohort study. J Matern Fetal Neonatal Med 33:2366–2371. https://doi.org/10.1080/14767058.2018.1550480
Bayraktar S, Hernadez-Aya LF, Lei X, Meric-Bernstam F, Litton JK, Hsu L, Hortobagyi GN, Gonzalez-Angulo AM (2012) Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer 118:1202–1211. https://doi.org/10.1002/cncr.26439
Beisswenger PJ, Howell SK, Touchette AD, Lal S, Szwergold BS (1999) Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 48:198–202. https://doi.org/10.2337/diabetes.48.1.198
Bodmer M, Becker C, Meier C, Jick SS, Meier CR (2012) Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol 107:620–626. https://doi.org/10.1038/ajg.2011.483
Borbely Z (2016) Chronic kidney diseases, metformin and lactic acidosis. Vnitr Lek 62:299–303
Bybel B, Greenberg ID, Paterson J, Ducharme J, Leslie WD (2011) Increased F-18 FDG intestinal uptake in diabetic patients on metformin: a matched case-control analysis. Clin Nucl Med 36:452–456. https://doi.org/10.1097/RLU.0b013e318217399e
Calco GN, Proskocil BJ, Jacoby DB, Fryer AD, Nie Z (2021) Metformin prevents airway hyperreactivity in rats with dietary obesity. Am J Physiol Lung Cell Mol Physiol 321:L1105–L1118. https://doi.org/10.1152/ajplung.00202.2021
Chaiteerakij R, Yang JD, Harmsen WS, Slettedahl SW, Mettler TA, Fredericksen ZS, Kim WR, Gores GJ, Roberts RO, Olson JE, Therneau TM, Roberts LR (2013) Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology 57:648–655. https://doi.org/10.1002/hep.26092
Chen K, Qian W, Jiang Z, Cheng L, Li J, Sun L, Zhou C, Gao L, Lei M, Yan B, Cao J, Duan W, Ma Q (2017) Metformin suppresses cancer initiation and progression in genetic mouse models of pancreatic cancer. Mol Cancer 16:131. https://doi.org/10.1186/s12943-017-0701-0
Chen L, Zhang Y, Yin L, Cai B, Huang P, Li X, Liang G (2021a) Fibroblast growth factor receptor fusions in cancer: opportunities and challenges. J Exp Clin Cancer Res 40:345. https://doi.org/10.1186/s13046-021-02156-6
Chen YH, Wang PH, Chen PN, Yang SF, Hsiao YH (2021b) Molecular and cellular mechanisms of metformin in cervical cancer. Cancers (Basel). https://doi.org/10.3390/cancers13112545
Chen YH, Wu JX, Yang SF, Chen ML, Chen TH, Hsiao YH (2021c) Metformin potentiates the anticancer effect of everolimus on cervical cancer in vitro and in vivo. Cancers (Basel). https://doi.org/10.3390/cancers13184612
Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, Cimino I, Yang M, Welsh P, Virtue S, Goldspink DA, Miedzybrodzka EL, Konopka AR, Esponda RR, Huang JT, Tung YCL, Rodriguez-Cuenca S, Tomaz RA, Harding HP, Melvin A, Yeo GSH, Preiss D, Vidal-Puig A, Vallier L, Nair KS, Wareham NJ, Ron D, Gribble FM, Reimann F, Sattar N, Savage DB, Allan BB, O’rahilly S (2020) GDF15 mediates the effects of metformin on body weight and energy balance. Nature 578:444–448. https://doi.org/10.1038/s41586-019-1911-y
Coyle C, Cafferty FH, Vale C, Langley RE (2016) Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol 27:2184–2195. https://doi.org/10.1093/annonc/mdw410
Crist M, Yaniv B, Palackdharry S, Lehn MA, Medvedovic M, Stone T, Gulati S, Karivedu V, Borchers M, Fuhrman B, Crago A, Curry J, Martinez-Outschoorn U, Takiar V, Wise-Draper TM (2022) Metformin increases natural killer cell functions in head and neck squamous cell carcinoma through CXCL1 inhibition. J Immunother Cancer. https://doi.org/10.1136/jitc-2022-005632
Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, Wang X, Tang S, Nagi A, Kosinski AS, Williams JW Jr (2017) Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med 166:191–200. https://doi.org/10.7326/M16-1901
De La Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velasquez-Mejia EP, Carmona JA, Abad JM, Escobar JS (2017) Metformin Is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care 40:54–62. https://doi.org/10.2337/dc16-1324
Di Matteo S, Nevi L, Overi D, Landolina N, Faccioli J, Giulitti F, Napoletano C, Oddi A, Marziani AM, Costantini D, De Rose AM, Melandro F, Bragazzi MC, Grazi GL, Berloco PB, Giuliante F, Donato G, Moretta L, Carpino G, Cardinale V, Gaudio E, Alvaro D (2021) Metformin exerts anti-cancerogenic effects and reverses epithelial-to-mesenchymal transition trait in primary human intrahepatic cholangiocarcinoma cells. Sci Rep 11:2557. https://doi.org/10.1038/s41598-021-81172-0
Ding Y, Zhou Y, Ling P, Feng X, Luo S, Zheng X, Little PJ, Xu S, Weng J (2021) Metformin in cardiovascular diabetology: a focused review of its impact on endothelial function. Theranostics 11:9376–9396. https://doi.org/10.7150/thno.64706
Dong S, Ruiz-Calderon B, Rathinam R, Eastlack S, Maziveyi M, Alahari SK (2020) Knockout model reveals the role of Nischarin in mammary gland development, breast tumorigenesis and response to metformin treatment. Int J Cancer 146:2576–2587. https://doi.org/10.1002/ijc.32690
Duan Y, Zhang R, Zhang M, Sun L, Dong S, Wang G, Zhang J, Zhao Z (2013) Metformin inhibits food intake and neuropeptide Y gene expression in the hypothalamus. Neural Regen Res 8:2379–2388. https://doi.org/10.3969/j.issn.1673-5374.2013.25.009
Elgendy M, Ciro M, Hosseini A, Weiszmann J, Mazzarella L, Ferrari E, Cazzoli R, Curigliano G, Decensi A, Bonanni B, Budillon A, Pelicci PG, Janssens V, Ogris M, Baccarini M, Lanfrancone L, Weckwerth W, Foiani M, Minucci S (2019) Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A-GSK3beta-MCL-1 axis. Cancer Cell 35:798–815. https://doi.org/10.1016/j.ccell.2019.03.007
Eppinga RN, Kofink D, Dullaart RP, Dalmeijer GW, Lipsic E, Van Veldhuisen DJ, Van Der Horst IC, Asselbergs FW, Van Der Harst P (2017) Effect of metformin on metabolites and relation with myocardial infarct size and left ventricular ejection fraction after myocardial infarction. Circ Cardiovasc Genet. https://doi.org/10.1161/CIRCGENETICS.116.001564
Fahy GM, Brooke RT, Watson JP, Good Z, Vasanawala SS, Maecker H, Leipold MD, Lin DT, Kobor MS, Horvath S (2019) Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 18:e13028
Fan J, Li D, Chen HS, Huang JG, Xu JF, Zhu WW, Chen JG, Wang F (2019) Metformin produces anxiolytic-like effects in rats by facilitating GABAA receptor trafficking to membrane. Br J Pharmacol 176:297–316. https://doi.org/10.1111/bph.14519
Feng X, Chen W, Ni X, Little PJ, Xu S, Tang L, Weng J (2021) Metformin macrophage dysfunction and atherosclerosis. Front Immunol 12:682853. https://doi.org/10.3389/fimmu.2021.682853
Fidan E, Onder Ersoz H, Yilmaz M, Yilmaz H, Kocak M, Karahan C, Erem C (2011) The effects of rosiglitazone and metformin on inflammation and endothelial dysfunction in patients with type 2 diabetes mellitus. Acta Diabetol 48:297–302. https://doi.org/10.1007/s00592-011-0276-y
Flory J, Lipska K (2019) Metformin in 2019. JAMA 321:1926–1927. https://doi.org/10.1001/jama.2019.3805
Foretz M, Guigas B, Viollet B (2019) Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol 15:569–589. https://doi.org/10.1038/s41574-019-0242-2
Glossmann HH, Lutz OMD (2019) Metformin and aging: a review. Gerontology 65:581–590. https://doi.org/10.1159/000502257
Gollavilli PN, Kanugula AK, Koyyada R, Karnewar S, Neeli PK, Kotamraju S (2015) AMPK inhibits MTDH expression via GSK3beta and SIRT1 activation: potential role in triple negative breast cancer cell proliferation. FEBS J 282:3971–3985. https://doi.org/10.1111/febs.13391
Goodwin PJ, Chen BE, Gelmon KA, Whelan TJ, Ennis M, Lemieux J, Ligibel JA, Hershman DL, Mayer IA, Hobday TJ, Bliss JM, Rastogi P, Rabaglio-Poretti M, Mukherjee SD, Mackey JR, Abramson VG, Oja C, Wesolowski R, Thompson AM, Rea DW, Stos PM, Shepherd LE, Stambolic V, Parulekar WR (2022) Effect of metformin vs placebo on invasive disease-free survival in patients with breast cancer: the MA.32 randomized clinical trial. JAMA 327:1963–1973. https://doi.org/10.1001/jama.2022.6147
Gosmanov AR, Gemoets DE, Kaminsky LS, Kovesdy CP, Gosmanova EO (2021) Efficacy of metformin monotherapy in US veterans with type 2 diabetes and preexisting chronic kidney disease stage 3. Diabetes Obes Metab 23:1879–1885. https://doi.org/10.1111/dom.14414
Griffin SJ, Leaver JK, Irving GJ (2017) Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 60:1620–1629. https://doi.org/10.1007/s00125-017-4337-9
Gu X, Han YY, Yang CY, Ji HM, Lan YJ, Bi YQ, Zheng C, Qu J, Cheng MH, Gao J (2021) Activated AMPK by metformin protects against fibroblast proliferation during pulmonary fibrosis by suppressing FOXM1. Pharmacol Res 173:105844. https://doi.org/10.1016/j.phrs.2021.105844
Guo Y, Shi J, Wang Q, Hong L, Chen M, Liu S, Yuan X, Jiang S (2021) Metformin alleviates allergic airway inflammation and increases Treg cells in obese asthma. J Cell Mol Med 25:2279–2284. https://doi.org/10.1111/jcmm.16269
Gupta G, Pinto DJA, T, Chellappan D K, Mishra A, Malipeddi H, and Dua K, (2018) A clinical update on metformin and lung cancer in diabetic patients. Panminerva Med 60:70–75. https://doi.org/10.23736/S0031-0808.18.03394-3
Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z (2019) Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol 18:96. https://doi.org/10.1186/s12933-019-0900-7
Hausmann VL, Schubotz R (1975) Proinsulin and insulin secretion in obese females before and after administration of metformin. Arzneimittelforschung 25:668–675
He X, Wu D, Hu C, Xu T, Liu Y, Liu C, Xu B, Tang W (2019) Role of metformin in the treatment of patients with thyroid nodules and insulin resistance: a systematic review and meta-analysis. Thyroid 29:359–367. https://doi.org/10.1089/thy.2017.0707
He S, Qian X, Chen Y, Shen X, Zhang B, Chen X, Xu X, Li G (2021) Risk of death and heart failure among patients with Type 2 diabetes treated by metformin and nonmetformin monotherapy: a real-world study. j Diabetes Res 2021:5534387. https://doi.org/10.1155/2021/5534387
Hwang IK, Kim IY, Joo EJ, Shin JH, Choi JW, Won MH, Yoon YS, Seong JK (2010) Metformin normalizes type 2 diabetes-induced decrease in cell proliferation and neuroblast differentiation in the rat dentate gyrus. Neurochem Res 35:645–650. https://doi.org/10.1007/s11064-009-0115-5
Jia W, Bai T, Zeng J, Niu Z, Fan D, Xu X, Luo M, Wang P, Zou Q, Dai X (2021) Combined administration of metformin and atorvastatin attenuates diabetic cardiomyopathy by inhibiting inflammation, apoptosis, and oxidative stress in Type 2 diabetic mice. Front Cell Dev Biol 9:634900. https://doi.org/10.3389/fcell.2021.634900
Kaewpitoon SJ, Loyd RA, Rujirakul R, Panpimanmas S, Matrakool L, Tongtawee T, Kootanavanichpong N, Kompor P, Chavengkun W, Kujapun J, Norkaew J, Ponphimai S, Padchasuwan N, Pholsripradit P, Eksanti T, Phatisena T, Kaewpitoon N (2015) Benefits of metformin use for cholangiocarcinoma. Asian Pac J Cancer Prev 16:8079–8083. https://doi.org/10.7314/apjcp.2015.16.18.8079
Kaisar MA, Villalba H, Prasad S, Liles T, Sifat AE, Sajja RK, Abbruscato TJ, Cucullo L (2017) Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: is Metformin a viable countermeasure? Redox Biol 13:353–362. https://doi.org/10.1016/j.redox.2017.06.006
Kalmykova ZA, Kononenko IV, Mayorov AY (2019a) Diabetes mellitus and chronic liver diseases. Literature review (part 2): treatment features. Ter Arkh 91:115–121. https://doi.org/10.26442/00403660.2019.12.000166
Kalmykova ZA, Kononenko IV, Mayorov AY (2019b) Diabetes mellitus and chronic liver diseases. Part 1: general mechanisms of etiology and pathogenesis. Ter Arkh 91:106–111. https://doi.org/10.26442/00403660.2019.10.000165
Khan MS, Solomon N, DeVore AD, Abhinav Sharma G, Felker M, Hernandez AF, Heidenreich PA, Matsouaka RA, Green JB, Butler J, Yancy CW, Peterson PN, Fonarow GC, Greene SJ (2022) Clinical outcomes with metformin and sulfonylurea therapies among patients with heart failure and diabetes. JACC Heart Fail 10:198–210
Kheirollahi V, Wasnick RM, Biasin V, Vazquez-Armendariz AI, Chu X, Moiseenko A, Weiss A, Wilhelm J, Zhang JS, Kwapiszewska G, Herold S, Schermuly RT, Mari B, Li X, Seeger W, Gunther A, Bellusci S, El Agha E (2019) Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun 10:2987. https://doi.org/10.1038/s41467-019-10839-0
Kim HJ, Park EY, Oh MJ, Park SS, Shin KH, Choi SH, Chun BG, Kim DH (2013) Central administration of metformin into the third ventricle of C57BL/6 mice decreases meal size and number and activates hypothalamic S6 kinase. Am J Physiol Regul Integr Comp Physiol 305:R499-505. https://doi.org/10.1152/ajpregu.00099.2013
Kim K, Yang WH, Jung YS, Cha JH (2020) A new aspect of an old friend: the beneficial effect of metformin on anti-tumor immunity. BMB Rep 53(10):512
Ko E, Baek S, Kim J, Park D, Lee Y (2020) Antitumor activity of combination therapy with metformin and trametinib in non-small cell lung cancer cells. Dev Reprod 24:113–123. https://doi.org/10.12717/DR.2020.24.2.113
Krysiak R, Szkrobka W, Okopien B (2015) The effect of metformin on the hypothalamic-pituitary-thyroid axis in patients with type 2 diabetes and subclinical hyperthyroidism. Exp Clin Endocrinol Diabetes 123:205–208. https://doi.org/10.1055/s-0034-1398621
Kuan YC, Huang KW, Lin CL, Hu CJ, Kao CH (2017) Effects of metformin exposure on neurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Prog Neuropsychopharmacol Biol Psychiatry 79:77–83. https://doi.org/10.1016/j.pnpbp.2017.06.002
Kumari S, Bubak M, Schoenberg HM, Davidyan A, Elliehausen CJ, Kuhn KG, Vanwagoner TM, Karaman R, Scofield RH, Miller BF, Konopka AR (2021) Antecedent metabolic health and metformin (ANTHEM) aging study: rationale and study design for a randomized controlled trial. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/glab358
Kwon S, Kim YC, Park JY, Lee J, An JN, Kim CT, Oh S, Park S, Kim DK, Oh YK, Kim YS (2020) The long-term effects of metformin on patients with type 2 diabetic Kidney disease. Diabetes Care 43:948–955
Lee A, Morley JE (1998) Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res 6:47–53. https://doi.org/10.1002/j.1550-8528.1998.tb00314.x
Lee J, Yesilkanal AE, Wynne JP, Frankenberger C, Liu J, Yan J, Elbaz M, Rabe DC, Rustandy FD, Tiwari P, Grossman EA, Hart PC, Kang C, Sanderson SM, Andrade J, Nomura DK, Bonini MG, Locasale JW, Rosner MR (2019) Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 568:254–258. https://doi.org/10.1038/s41586-019-1005-x
Li SN, Wang X, Zeng QT, Feng YB, Cheng X, Mao XB, Wang TH, Deng HP (2009) Metformin inhibits nuclear factor kappaB activation and decreases serum high-sensitivity C-reactive protein level in experimental atherogenesis of rabbits. Heart Vessels 24:446–453. https://doi.org/10.1007/s00380-008-1137-7
Li CJ, Tzeng YT, Chiu YH, Lin HY, Hou MF, Chu PY (2021) Pathogenesis and potential therapeutic targets for triple-negative breast cancer. Cancers (Basel). https://doi.org/10.3390/cancers13122978
Li X, Cai Y, Luo J, Ding J, Yao G, Xiao X, Tang Y, Liang Z (2021b) Metformin attenuates hypothalamic inflammation via downregulation of RIPK1-independent microglial necroptosis in diet-induced obese mice. Cell Death Discov 7:338. https://doi.org/10.1038/s41420-021-00732-5
Lin Y, Wang K, Ma C, Wang X, Gong Z, Zhang R, Zang D, Cheng Y (2018a) Corrigendum: evaluation of metformin on cognitive improvement in patients with non-dementia vascular cognitive impairment and abnormal glucose metabolism. Front Aging Neurosci 10:322. https://doi.org/10.3389/fnagi.2018.00322
Lin Y, Wang K, Ma C, Wang X, Gong Z, Zhang R, Zang D, Cheng Y (2018b) Evaluation of metformin on cognitive improvement in patients with non-dementia vascular cognitive impairment and abnormal glucose metabolism. Front Aging Neurosci 10:227. https://doi.org/10.3389/fnagi.2018.00227
Ling S, Xie H, Yang F, Shan Q, Dai H, Zhuo J, Wei X, Song P, Zhou L, Xu X, Zheng S (2017) Metformin potentiates the effect of arsenic trioxide suppressing intrahepatic cholangiocarcinoma: roles of p38 MAPK, ERK3, and mTORC1. J Hematol Oncol 10:59. https://doi.org/10.1186/s13045-017-0424-0
Liu YS, Chen CN, Chen ZG, Peng Y, Lin XP, Xu LL (2020) Vascular and metabolic effects of metformin added to insulin therapy in patients with type 1 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev 36:e3334. https://doi.org/10.1002/dmrr.3334
Liu S, Polsdofer EV, Zhou L, Ruan S, Lyu H, Hou D, Liu H, Thor AD, He Z, Liu B (2021) Upregulation of endogenous TRAIL-elicited apoptosis is essential for metformin-mediated antitumor activity against TNBC and NSCLC. Mol Ther Oncolytics 21:303–314. https://doi.org/10.1016/j.omto.2021.04.012
Løvvik TS, Carlsen SM, Salvesen Ø, Steffensen B, Bixo M, Gómez-Real F, Lønnebotn M, Hestvold KV, Zabielska R, Hirschberg AL, Trouva A (2019) Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 7:256–266
Lu H, Han X, Ren J, Ren K, Li Z, Zhang Q (2021a) Metformin attenuates synergic effect of diabetes mellitus and Helicobacter pylori infection on gastric cancer cells proliferation by suppressing PTEN expression. J Cell Mol Med 25:4534–4542. https://doi.org/10.1111/jcmm.15967
Lu T, Li M, Zhao M, Huang Y, Bi G, Liang J, Chen Z, Zheng Y, Xi J, Lin Z, Zhan C, Jiang W, Wang Q, Tan L (2021b) Metformin inhibits human non-small cell lung cancer by regulating AMPK-CEBPB-PDL1 signaling pathway. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-021-03116-x
Lustig RH, Schmidt LA, Brindis CD (2012) Public health: the toxic truth about sugar. Nature 482:27–29. https://doi.org/10.1038/482027a
Ma WQ, Sun XJ, Zhu Y, Liu NF (2021) Metformin attenuates hyperlipidaemia-associated vascular calcification through anti-ferroptotic effects. Free Radic Biol Med 165:229–242. https://doi.org/10.1016/j.freeradbiomed.2021.01.033
Ma T, Tian X, Zhang B, Li M, Wang Y, Yang C, Wu J, Wei X, Qu Q, Yu Y, Long S, Feng JW, Li C, Zhang C, Xie C, Wu Y, Xu Z, Chen J, Yu Y, Huang X, He Y, Yao L, Zhang L, Zhu M, Wang W, Wang ZC, Zhang M, Bao Y, Jia W, Lin SY, Ye Z, Piao HL, Deng X, Zhang CS, Lin SC (2022) Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 603:159–165. https://doi.org/10.1038/s41586-022-04431-8
Mandwie M, Karunia J, Niaz A, Keay KA, Musumeci G, Rennie C, Mcgrath K, Al-Badri G, Castorina A (2021) Metformin treatment attenuates brain inflammation and rescues PACAP/VIP neuropeptide alterations in mice fed a high-fat diet. Int J Mol Sci. https://doi.org/10.3390/ijms222413660
Masarwa R, Brunetti VC, Aloe S, Henderson M, Platt RW, Filion KB (2021) Efficacy and safety of metformin for obesity: a systematic review. Pediatrics. https://doi.org/10.1542/peds.2020-1610
Misirkic Marjanovic MS, Vucicevic LM, Despotovic AR, Stamenkovic MM, Janjetovic KD (2021) Dual anticancer role of metformin: an old drug regulating AMPK dependent/independent pathways in metabolic, oncogenic/tumorsuppresing and immunity context. Am J Cancer Res 11:5625–5643
Mohan M, Al-Talabany S, Mckinnie A, Mordi IR, Singh JSS, Gandy SJ, Baig F, Hussain MS, Bhalraam U, Khan F, Choy AM, Matthew S, Houston JG, Struthers AD, George J, Lang CC (2019) A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur Heart J 40:3409–3417. https://doi.org/10.1093/eurheartj/ehz203
Moses RG (2010) Repaglinide/metformin fixed-dose combination to improve glycemic control in patients with type 2 diabetes: an update. Diabetes Metab Syndr Obes 3:145–154. https://doi.org/10.2147/dmsott.s6621
Mossmann D, Park S, Hall MN (2018) mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer 18:744–757. https://doi.org/10.1038/s41568-018-0074-8
Munoz LE, Huang L, Bommireddy R, Sharma R, Monterroza L, Guin RN, Samaranayake SG, Pack CD, Ramachandiran S, Reddy SJC, Shanmugam M, Selvaraj P (2021) Metformin reduces PD-L1 on tumor cells and enhances the anti-tumor immune response generated by vaccine immunotherapy. J Immunother Cancer. https://doi.org/10.1136/jitc-2021-002614
Murff HJ, Roumie CL, Greevy RA, Hackstadt AJ, Mcgowan LED, Hung AM, Grijalva CG, Griffin MR (2018) Metformin use and incidence cancer risk: evidence for a selective protective effect against liver cancer. Cancer Causes Control 29:823–832. https://doi.org/10.1007/s10552-018-1058-4
Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ (2002) Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51:2074–2081. https://doi.org/10.2337/diabetes.51.7.2074
Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B (2014) Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 41:61–68. https://doi.org/10.3233/JAD-131901
Palazon A, Tyrakis PA, Macias D, Velica P, Rundqvist H, Fitzpatrick S, Vojnovic N, Phan AT, Loman N, Hedenfalk I, Hatschek T, Lovrot J, Foukakis T, Goldrath AW, Bergh J, Johnson RS (2017) An HIF-1alpha/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell 32:669–683. https://doi.org/10.1016/j.ccell.2017.10.003
Piskovatska V, Stefanyshyn N, Storey KB, Vaiserman AM, Lushchak O (2019) Metformin as a geroprotector: experimental and clinical evidence. Biogerontology 20:33–48. https://doi.org/10.1007/s10522-018-9773-5
Podhorecka M, Ibanez B, Dmoszynska A (2017) Metformin - its potential anti-cancer and anti-aging effects. Postepy Hig Med Dosw (online) 71:170–175. https://doi.org/10.5604/01.3001.0010.3801
Pugliese G, Penno G, Natali A, Barutta F, Di Paolo S, Reboldi G, Gesualdo L, De Nicola L, Italian Diabetes S, The Italian Society Of N (2019) Diabetic kidney disease: new clinical and therapeutic issues. joint position statement of the italian diabetes society and the Italian society of nephrology on "the natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function. Nutr Metab Cardiovasc Dis 29:1127–1150. https://doi.org/10.1016/j.numecd.2019.07.017
Reitz KM, Marroquin OC, Zenati MS, Kennedy J, Korytkowski M, Tzeng E, Koscum S, Newhouse D, Garcia RM, Vates J, Billiar TR (2020) Association between preoperative metformin exposure and postoperative outcomes in adults with type 2 diabetes. JAMA Surg 155:e200416
Rezvanian H, Adibi N, Siavash M, Kachuei A, Shojaee-Moradie F, Asilian A (2009) Increased insulin sensitivity by metformin enhances intense-pulsed-light-assisted hair removal in patients with polycystic ovary syndrome. Dermatology 218:231–236. https://doi.org/10.1159/000187718
Rippe JM, Angelopoulos TJ (2016) Sugars, obesity, and cardiovascular disease: results from recent randomized control trials. Eur J Nutr 55:45–53. https://doi.org/10.1007/s00394-016-1257-2
Salani B, Maffioli S, Hamoudane M, Parodi A, Ravera S, Passalacqua M, Alama A, Nhiri M, Cordera R, Maggi D (2012) Caveolin-1 is essential for metformin inhibitory effect on IGF1 action in non-small-cell lung cancer cells. FASEB J 26:788–798. https://doi.org/10.1096/fj.11-192088
Saleh R, Nair VS, Murshed K, Nada MA, Elkord E, Shaheen R (2021) Correction to: transcriptome of CD8(+) tumor-infiltrating T cells: a link between diabetes and colorectal cancer. Cancer Immunol Immunother 70:2639–2640. https://doi.org/10.1007/s00262-021-02903-w
Samaras K, Makkar S, Crawford JD, Kochan NA, Wen W, Draper B, Trollor JN, Brodaty H, Sachdev PS (2020) Metformin use is associated with slowed cognitive decline and reduced incident dementia in older adults with Type 2 diabetes: the Sydney memory and ageing study. Diabetes Care 43:2691–2701
Sardu C, D’onofrio N, Torella M, Portoghese M, Mureddu S, Loreni F, Ferraraccio F, Panarese I, Trotta MC, Gatta G, Galdiero M, Sasso FC, D’amico M, De Feo M, Balestrieri ML, Paolisso G, Marfella R (2021) Metformin therapy effects on the expression of sodium-glucose cotransporter 2, leptin, and sirt6 levels in pericoronary fat excised from pre-diabetic patients with acute myocardial infarction. Biomedicines. https://doi.org/10.3390/biomedicines9080904
Sarmento-Cabral A, L-López F, Gahete MD, Castano JP, Luque RM (2017) Metformin reduces prostate tumor growth, in a diet-dependent manner, by modulating multiple signaling pathways. Mol Cancer Res 15:862–874. https://doi.org/10.1158/1541-7786.MCR-16-0493
Schernthaner G, Brand K, Bailey CJ (2022) Metformin and the heart: Update on mechanisms of cardiovascular protection with special reference to comorbid type 2 diabetes and heart failure. Metabolism 130:155160. https://doi.org/10.1016/j.metabol.2022.155160
Seneviratne A, Cave L, Hyde G, Moestrup SK, Carling D, Mason JC, Haskard DO, Boyle JJ (2021) Metformin directly suppresses atherosclerosis in normoglycaemic mice via haematopoietic adenosine monophosphate-activated protein kinase. Cardiovasc Res 117:1295–1308. https://doi.org/10.1093/cvr/cvaa171
Shi P, Liu W, Tala WH, Li F, Zhang H, Wu Y, Kong Y, Zhou Z, Wang C, Chen W, Liu R, Chen C (2017) Metformin suppresses triple-negative breast cancer stem cells by targeting KLF5 for degradation. Cell Discov 3:17010. https://doi.org/10.1038/celldisc.2017.10
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33. https://doi.org/10.3322/caac.21708
Sigala DM, Hieronimus B, Medici V, Lee V, Nunez MV, Bremer AA, Cox CL, Price CA, Benyam Y, Chaudhari AJ, Abdelhafez Y, Mcgahan JP, Goran MI, Sirlin CB, Pacini G, Tura A, Keim NL, Havel PJ, Stanhope KL (2021) Consuming sucrose- or HFCS-sweetened beverages increases hepatic lipid and decreases insulin sensitivity in adults. J Clin Endocrinol Metab 106:3248–3264. https://doi.org/10.1210/clinem/dgab508
Snell-Bergeon JK (2017) Diabetes: Cardiovascular benefits of metformin in T1DM. Nat Rev Endocrinol 13:565–566. https://doi.org/10.1038/nrendo.2017.116
Soberanes S, Misharin AV, Jairaman A, Morales-Nebreda L, Mcquattie-Pimentel AC, Cho T, Hamanaka RB, Meliton AY, Reyfman PA, Walter JM, Chen CI, Chi M, Chiu S, Gonzalez-Gonzalez FJ, Antalek M, Abdala-Valencia H, Chiarella SE, Sun KA, Woods PS, Ghio AJ, Jain M, Perlman H, Ridge KM, Morimoto RI, Sznajder JI, Balch WE, Bhorade SM, Bharat A, Prakriya M, Chandel NS, Mutlu GM, Budinger GRS (2019) Metformin targets mitochondrial electron transport to reduce air-pollution-induced thrombosis. Cell Metab 29:335 e335-347. https://doi.org/10.1016/j.cmet.2018.09.019
Soliman GA, Shukla SK, Etekpo A, Gunda V, Steenson SM, Gautam N, Alnouti Y, Singh PK (2020) The synergistic effect of an ATP-competitive inhibitor of mtor and metformin on pancreatic tumor growth. Curr Dev Nutr 4:nzaa131. https://doi.org/10.1093/cdn/nzaa131
Stanhope KL (2016) Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci 53:52–67. https://doi.org/10.3109/10408363.2015.1084990
Strekalova E, Malin D, Rajanala H, Cryns VL (2017) Metformin sensitizes triple-negative breast cancer to proapoptotic TRAIL receptor agonists by suppressing XIAP expression. Breast Cancer Res Treat 163:435–447. https://doi.org/10.1007/s10549-017-4201-0
Sun C, Hu SH, Dong BQ, Jiang S, Miao F, Lei TC (2021) Metformin promotes the hair-inductive activity of three-dimensional aggregates of epidermal and dermal cells self-assembled in vitro. Skin Pharmacol Physiol. https://doi.org/10.1159/000521400
Tariq N, Ayub R, Alam AY, Rahim F, Raees SR (2007) Clinical diagnosis of polycystic ovarian syndrome and response to metformin therapy. J Coll Physicians Surg Pak 17:469–472
Thanigaimani S, Singh TP, Unosson J, Phie J, Moxon J, Wanhainen A, Golledge J (2021) Editor’s Choice - association between metformin prescription and abdominal aortic aneurysm growth and clinical events: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 62:747–756. https://doi.org/10.1016/j.ejvs.2021.06.013
Thein D, Christiansen MN, Mogensen UM, Bundgaard JS, Rorth R, Madelaire C, Fosbol EL, Schou M, Torp-Pedersen C, Gislason G, Kober L, Kristensen SL (2020) Add-on therapy in metformin-treated patients with type 2 diabetes at moderate cardiovascular risk: a nationwide study. Cardiovasc Diabetol 19:107. https://doi.org/10.1186/s12933-020-01078-5
Torres W, Nava M, Galbán N, Gómez Y, Morillo V, Rojas M, Cano C, Chacín M, DMarco L, Herazo Y, Velasco M, Bermúdez V, Rojas-Quintero J (2020) Anti-aging effect of metformin: a molecular and therapeutical perspective. Curr Pharm Des. https://doi.org/10.2174/1381612826666200716161610
Trinh SX, Nguyen HT, Saimuang K, Prachayasittikul V, Chan On W (2017) Metformin inhibits migration and invasion of cholangiocarcinoma cells. Asian Pac J Cancer Prev 18:473–477. https://doi.org/10.22034/APJCP.2017.18.2.473
Tseng CH (2021) Metformin use is associated with a lower risk of osteoporosis/vertebral fracture in Taiwanese patients with type 2 diabetes mellitus. Eur J Endocrinol 184:299–310
Van Bommel EJM, Ruiter D, Muskiet MHA, Van Baar MJB, Kramer MHH, Nieuwdorp M, Joles JA, Bjornstad P, Van Raalte DH (2020) Insulin sensitivity and renal hemodynamic function in metformin-treated adults with type 2 diabetes and preserved renal function. Diabetes Care 43:228–234. https://doi.org/10.2337/dc19-1651
Varghese S, Samuel SM, Varghese E, Kubatka P, Busselberg D (2019) High glucose represses the anti-proliferative and pro-apoptotic effect of metformin in triple negative breast cancer cells. Biomolecules. https://doi.org/10.3390/biom9010016
Wahdan-Alaswad RS, Cochrane DR, Spoelstra NS, Howe EN, Edgerton SM, Anderson SM, Thor AD, Richer JK (2014) Metformin-induced killing of triple-negative breast cancer cells is mediated by reduction in fatty acid synthase via miRNA-193b. Horm Cancer 5:374–389. https://doi.org/10.1007/s12672-014-0188-8
Wahdan-Alaswad R, Harrell JC, Fan Z, Edgerton SM, Liu B, Thor AD (2016) Metformin attenuates transforming growth factor beta (TGF-beta) mediated oncogenesis in mesenchymal stem-like/claudin-low triple negative breast cancer. Cell Cycle 15:1046–1059. https://doi.org/10.1080/15384101.2016.1152432
Wang J, Gallagher D, Devito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD (2012) Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 11:23–35. https://doi.org/10.1016/j.stem.2012.03.016
Wang L, Song Y, Wu GN, Yuan DM (2013) Association of the metformin with the risk of lung cancer: a meta-analysis. Transl Lung Cancer Res 2:259–263. https://doi.org/10.3978/j.issn.2218-6751.2013.08.01
Wang G, Dong Y, Liu H (2020a) Curcumol enhances the anti-tumor effects of metformin via suppressing epithelial-mesenchymal transition in triple-negative breast cancer. Ann Transl Med 8:946. https://doi.org/10.21037/atm-20-5438
Wang S, Lin Y, Xiong X, Wang L, Guo Y, Chen Y, Chen S, Wang G, Lin P, Chen H, Yeung SJ, Bremer E, Zhang H (2020b) Low-dose metformin reprograms the tumor immune microenvironment in human esophageal cancer: results of a phase II clinical trial. Clin Cancer Res 26:4921–4932. https://doi.org/10.1158/1078-0432.CCR-20-0113
Wen Q, Hu M, Lai M, Li J, Hu Z, Quan K, Liu J, Liu H, Meng Y, Wang S, Wen X, Yu C, Li S, Huang S, Zheng Y, Lin H, Liang X, Lu L, Mai Z, Zhang C, Wu T, Ng EHY, Stener-Victorin E, Ma H (2021) Effect of acupuncture and metformin on insulin sensitivity in women with polycystic ovary syndrome and insulin resistance: a three-armed randomized controlled trial. Hum Reprod. https://doi.org/10.1093/humrep/deab272
Westphal LP, Widmer R, Held U, Steigmiller K, Hametner C, Ringleb P, Curtze S, Martinez-Majander N, Tiainen M, Nolte CH, Scheitz JF, Erdur H, Polymeris AA, Traenka C, Eskandari A, Michel P, Heldner MR, Arnold M, Zini A, Vandelli L, Coutinho JM, Groot AE, Padjen V, Jovanovic DR, Bejot Y, Breniere C, Turc G, Seners P, Pezzini A, Magoni M, Leys D, Gilliot S, Scherrer MJ, Kagi G, Luft AR, Gensicke H, Nederkoorn P, Tatlisumak T, Engelter ST, Wegener S, Thrombolysis in Ischemic Stroke Patients Study G (2020) Association of prestroke metformin use, stroke severity, and thrombolysis outcome. Neurology 95:e362–e373. https://doi.org/10.1212/WNL.0000000000009951
Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G (2017) Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 23:850–858
Wu Y, Tu M, Huang Y, Liu Y, Zhang D (2020) Association of metformin with pregnancy outcomes in women with polycystic ovarian syndrome undergoing in vitro fertilization: a systematic review and meta-analysis. JAMA Netw Open 3:e2011995. https://doi.org/10.1001/jamanetworkopen.2020.11995
Xia W, Qi X, Li M, Wu Y, Sun L, Fan X, Yuan Y, Li J (2021) Metformin promotes anticancer activity of NK cells in a p38 MAPK dependent manner. Oncoimmunology 10:1995999. https://doi.org/10.1080/2162402X.2021.1995999
Xiao K, Liu F, Liu J, Xu J, Wu Q, Li X (2020) The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: a meta-analysis. J Clin Pharm Ther 45:783–792. https://doi.org/10.1111/jcpt.13167
Xie J, Xia L, Xiang W, He W, Yin H, Wang F, Gao T, Qi W, Yang Z, Yang X, Zhou T (2020) Metformin selectively inhibits metastatic colorectal cancer with the KRAS mutation by intracellular accumulation through silencing MATE1. Proc Natl Acad Sci USA 117:13012–13022
Xiong W, Sun KY, Zhu Y, Zhang X, Zhou YH, Zou X (2021) Metformin alleviates inflammation through suppressing FASN-dependent palmitoylation of Akt. Cell Death Dis 12:934. https://doi.org/10.1038/s41419-021-04235-0
Xu HY, Fang W, Huang ZW, Lu JC, Wang YQ, Tang QL, Song GH, Kang Y, Zhu XJ, Zou CY, Yang HL, Shen JN, Wang J (2017) Metformin reduces SATB2-mediated osteosarcoma stem cell-like phenotype and tumor growth via inhibition of N-cadherin/NF-kB signaling. Eur Rev Med Pharmacol Sci 21:4516–4528
Xu C, Li XF, Tian HY, Shi HJ, Zhang DD, Abasubong KP, Liu WB (2019) Metformin improves the glucose homeostasis of Wuchang bream fed high-carbohydrate diets: a dynamic study. Endocr Connect 8:182–194. https://doi.org/10.1530/EC-18-0517
Yang Z, Zhang X, Roberts RO, Roberts LR, Chaiteerakij R (2016) Metformin does not improve survival of cholangiocarcinoma patients with diabetes. Hepatology 63:667–668. https://doi.org/10.1002/hep.27821
Yuen N, Szulc-Lerch KU, Li YQ, Morshead CM, Mabbott DJ, Wong CS, Nieman BJ (2021) Metformin effects on brain development following cranial irradiation in a mouse model. Neuro Oncol 23:1523–1536. https://doi.org/10.1093/neuonc/noab131
Zaccardi F, Kloecker DE, Buse JB, Mathieu C, Khunti K, Davies MJ (2021) Use of metformin and cardiovascular effects of new classes of glucose-lowering agents: a meta-analysis of cardiovascular outcome trials in type 2 diabetes. Diabetes Care 44:e32–e34. https://doi.org/10.2337/dc20-2080
Zajda A, Huttunen KM, Sikora J, Podsiedlik M, Markowicz-Piasecka M (2020) Is metformin a geroprotector? A peek into the current clinical and experimental data. Mech Ageing Dev 191:111350. https://doi.org/10.1016/j.mad.2020.111350
Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA (2004) AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem 279:47898–47905. https://doi.org/10.1074/jbc.M408149200
Zell JA, Mclaren CE, Morgan TR, Lawson MJ, Rezk S, Albers CG, Chen WP, Carmichael JC, Chung J, Richmond E, Rodriguez LM, Szabo E, Ford LG, Pollak MN, Meyskens FL (2020) A phase IIa trial of metformin for colorectal cancer risk reduction among individuals with history of colorectal adenomas and elevated body mass index. Cancer Prev Res (phila) 13:203–212. https://doi.org/10.1158/1940-6207.CAPR-18-0262
Zemdegs J, Martin H, Pintana H, Bullich S, Manta S, Marques MA, Moro C, Laye S, Ducrocq F, Chattipakorn N, Chattipakorn SC, Rampon C, Penicaud L, Fioramonti X, Guiard BP (2019) Metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice by decreasing circulating branched-chain amino acids. J Neurosci 39:5935–5948. https://doi.org/10.1523/JNEUROSCI.2904-18.2019
Zemgulyte G, Tanaka S, Hide I, Sakai N, Pampuscenko K, Borutaite V, Rastenyte D (2021) Evaluation of the effectiveness of post-stroke metformin treatment using permanent middle cerebral artery occlusion in Rats. Pharmaceuticals (Basel). https://doi.org/10.3390/ph14040312
Zhang C, Ma S, Wu J, Luo L, Qiao S, Li R, Xu W, Wang N, Zhao B, Wang X, Zhang Y, Wang X (2020) A specific gut microbiota and metabolomic profiles shifts related to antidiabetic action: the similar and complementary antidiabetic properties of type 3 resistant starch from Canna edulis and metformin. Pharmacol Res 159:104985. https://doi.org/10.1016/j.phrs.2020.104985
Zhang Y, Liu C, Chen B, Chen F, Duan JY, Zhang MJ, Jiao J (2020b) Associations of impaired glucose metabolism with chronic peridontitis in pre-diabetes patients. Beijing Da Xue Xue Bao Yi Xue Ban 52:71–76
Zhu X, Shen J, Feng S, Huang C, Liu Z, Sun YE, Liu H (2020) Metformin improves cognition of aged mice by promoting cerebral angiogenesis and neurogenesis. Aging (Albany NY). https://doi.org/10.18632/aging.103693
Acknowledgements
This work was supported partly by The National Natural Science Foundation of China (81972366); The Natural Science Foundation of Guangdong (2022A1515012606); The Shenzhen Science and Technology Innovation Commission projects (JCYJ20220818101808018, KCXFZ202110203002406).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Zhang, M., Deng, D. et al. The function, mechanisms, and clinical applications of metformin: potential drug, unlimited potentials. Arch. Pharm. Res. 46, 389–407 (2023). https://doi.org/10.1007/s12272-023-01445-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-023-01445-2