Abstract
Cancer stem cells (CSCs) are believed to have an essential role in tumor resistance and metastasis; however, no therapeutic strategy for the selective elimination of CSCs has been established. Recently, several studies have shown that the metabolic regulation for ATP synthesis and biological building block generation in CSCs are different from that in bulk cancer cells and rather similar to that in normal tissue stem cells. To take advantage of this difference for CSC elimination therapy, many studies have tested the effect of blocking these metabolism. Two specific processes for lipid biosynthesis, i.e., fatty acid unsaturation and cholesterol biosynthesis, have been shown to be very effective and selective for CSC targets. In this review, lipid metabolism specific to CSCs are summarized. In addition, how monounsaturated fatty acid and cholesterol synthesis may contribute to CSC maintenance are discussed. Specifically, the molecular mechanism required for lipid synthesis and essential for stem cell biology is highlighted. The limit and preview of the lipid metabolism targeting for CSCs are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
What do we call cancer stem cells?
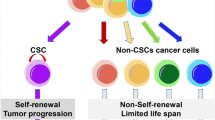
The possibility that all cancer cells may not be created equal was raised approximately 50 years ago, based on the finding that only a very small portion of tumor cells are able to generate other tumors in vivo (Southam 1961; Bruce and Van Der Gaag 1963; Bergsagel and Valeriote 1968). One explanation is that cancer cells are a heterogeneous cell population in a hierarchy, and only a small number of “stem-like” cells can develop tumors in vivo and they act similarly to tissue development (Ogawa et al. 1971; Park et al. 1971; Nowell 1976; Reya et al. 2001; Jung and Kim 2015). These cancer-initiating, “stem-like” cells were first isolated from leukemia cells (Bonnet and Dick 1997). They were shown to express the markers of normal hematopoietic stem cells (HSCs), demonstrating that these tumor-generating cells share many characteristics with tissue progenitor (or stem) cells. To date, all solid tumors are reported to include this stem-like cancer cell, which are now widely called cancer stem cells (CSCs) or tumor initiating cells (Fidler and Kripke 1977; Fidler and Hart 1982; Heppner 1984; Reya et al. 2001). Cells that include these CSCs were isolated from many tissues, including lung (Eramo et al. 2008), breast (Al-Hajj et al. 2003), colon (O’Brien et al. 2007), liver (Yang et al. 2008) and stomach (Nishii et al. 2009), the five main fatal cancers worldwide (UK_Cancer_Research 2018). Actually, these cells may contain only part of the “stem-ness” for the tissues from which the cancers originated. The fine molecular definition of those cells is still not clear, probably due to the heterogeneity of all cancer cells. CSCs may be characterized with expression of ALDH, some cell surface antigens (such as CD133, CD44, and CD34) (Kim and Ryu 2017) and enhanced signaling axis (Notch, Wnt, etc.) or specific nuclear proteins (SOX2, Hes1 and Hes5), which are basically the characteristics of the normal stem cells of the corresponding tissues. The most important characteristics of CSCs are the self-renewal activity in vitro and cancer forming ability in vivo (Jung and Kim 2015). It is now widely accepted that this small population may have stronger potential for metastasis and may be responsible for resistance as well (Kaplan et al. 2005; Jung and Kim 2015).
The resistance of CSCs
Currently, most cancer patients are treated with irradiation and/or chemotherapy that mainly focuses on the fast growing cells (Gerlinger et al. 2012). Some of the main characteristics of CSCs are that they are quiescent such as normal cells and the elevated expression of the ATP-binding cassette (ABC) transporter (Kim et al. 2002; Scharenberg et al. 2002; Dean et al. 2005; van Herwaarden et al. 2007). Since chemotherapeutic agents and radiation are more efficient on the bulk cultured, fast growing cells and the elevated amount of ABC transporters (including multidrug resistance (MDR) genes) pump out many alien substances (drugs) from cells, the CSCs may circumvent the conventional therapies. The CSCs and genomic instability may lead to incurable metastatic recurrences (Dean et al. 2005; Donnenberg and Donnenberg 2005), as illustrated in Fig. 1. The surviving CSCs now may differentiate to recurrent tumors in the original place or in a new distal organ (Mimeault et al. 2007; Todaro et al. 2007; Dylla et al. 2008; Li et al. 2008; Yang et al. 2008; Ansari et al. 2011). The recurrent or/and metastasized tumors likely developed from these CSCs may go through additional, increased, mutagenesis processes in a new microenvironment, will establish new cellular contexts that are quite different from the original primary tumors (Gerlinger et al. 2012; Jung and Kim 2015), and will be more difficult to treat. Therefore, the conventional chemotherapy and radiation therapy need to be combined in targeted therapy to eliminate CSCs as much as possible. Since the CSCs share many characteristics with normal cells, very precise targeting to CSCs should be developed to minimize adverse effects. Several strategies to block stem cell signaling have been developed, and some of them are being tested in clinical trials (Jung and Kim 2015). Recent findings in cancer metabolism, especially related to lipids, are of interest because these suggest the possibility of the development of new CSC-specific therapeutic targeting strategy.
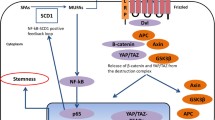
Cancer stem cells and the current strategy of cancer therapy. Cancer stem cells are slow growing quiescent cells therefore they can circumvent the conventional therapy that kills fast growing cells. It is required to eliminated not only the bulk cancer cells but also the cancer stem cells as well to avoid recurrence of tumors
Altered metabolism in CSCs
Glucose metabolism for ATP generation
Tumor cells are mostly under the hypoxic and low nutritional supplement condition due to their fast proliferation with inadequate blood vessel formation. Cancer cells known to use mainly glycolysis for ATP generation, not mitochondrial oxidative phosphorylation (OXPHOS), probably to adapt to this hypoxic condition; however, most cancer cells undergo glycolytic energy production even under oxygen-rich conditions as well, which is called the “Warburg Effect” (Warburg 1956). These aerobic, glycolytic cancers consume much more glucose than normal cells because aerobic glycolysis is a less efficient energy production system than OXPHOS (~ 1/16). In addition to the glucose avidity, cancer cells excessively require glutamine for their growth (Matsuno et al. 1986; Shyh-Chang et al. 2013). The glutamine consumption in the TCA cycle in tumors cells may provide the carbon backbone and nitrogen required for the amino acid, nucleotide, and lipid biosynthesis (Shyh-Chang et al. 2013). Through these two events, cancer cells rewire their metabolism program to generate ATP, provide building blocks for cellular compartments for proliferation and maintain their cancerous potential from many challenges from the outside (Vander Heiden et al. 2009; Danhier et al. 2017; Yi et al. 2018).
The microenvironment of tissue stem cells may be hypoxic, and stem cells prefer glycolytic energy metabolism to mitochondrial oxidative phosphorylation. CSCs are also similar to tissue stem cells, suggesting that both cell types should deal with similar microenvironmeta1 cues. While most of the normal cells in the aerobic condition use OXPHOS for energy metabolism, stem cells appear to be different. It appears that not just a passive adaptation process to the hypoxic environment but an active regulation process is required for their stem-ness. The introduction of stem cell factors into fibroblasts to generate iPS (induced pluripotent stem cells) shifted the main energy metabolism from OXPHOS to glycolysis (Folmes et al. 2011; Yi et al. 2018). The mitochondrial structure changes to have fewer cristae in iPS, and this glycolytic reprograming for energy metabolism is required for the stem-ness of iPS (Folmes et al. 2011; Zhang et al. 2017a, b). This reprograming allows the cells to avoid reactive oxygen species (ROS) production, which impairs genomic integrity and self-renewal potential (Shyh-Chang et al. 2013; Ito and Suda 2014; Chen et al. 2016a, b) because ROS is generated by OXPHOS (Li et al. 2017a, b). Human embryonic stem (ES) cells are also glycolytic due to low cytochrome c oxidase (COX) expression, which is essential for mitochondrial electron transport in complex IV. The master regulator in hypoxia, hypoxia-inducible factor 1-alpha (HIF1-alpha), is essential for cancer adaptation to a hypoxic environment and plays pivotal roles in early ES cells (Zhou et al. 2012); however, some tissue stem cells prefer OXPHOS (Bartesaghi et al. 2015), showing the plasticity of metabolic reprograming in stem cells. The finding that aerobic glycolysis is also essential for stem-like potential maintenance in breast cancer (Ciavardelli et al. 2014; Peng et al. 2018), colon cancer (Emmink et al. 2013), osteosarcomas (Palorini et al. 2014), and embryonic carcinoma (Vega-Naredo et al. 2014) also showed the aerobic glycolysis-preferring metabolic similarity between normal stem cells and CSCs. The metabolism difference in the bulk cells and CSCs in lung cancers was demonstrated biochemically after the isolation of the CSC population (Liu et al. 2014), which also supports the notion that CSCs prefer glycolysis, while the bulk cancers mainly use OXPHOS for ATP generation; however, not all CSCs require aerobic glycolysis (Dando et al. 2015). Studies on glioblastomas (Janiszewska et al. 2012), ovarian cancers (Pasto et al. 2014), and pancreatic cancers (Viale et al. 2014) showed that CSCs may depends more on mitochondrial OXPHOS rather than glycolysis. These contradictory results may have come from not only the heterogeneity issues but also the plasticity, as CSCs may flexibly adapt their metabolic program (Zhang et al. 2016). It appears that the ability of CSCs and stem cells to reprogram the ATP-generating metabolic pathway from one to another depends on the microenvironment changes, such as nutrient and oxygen levels (Sancho et al. 2015; Zhang et al. 2016).
Lipid metabolism
In addition to glucose metabolism, lipid metabolism is also related to the stem cell properties in normal tissue and cancers. The loss of peroxisome proliferator-activated receptor (PPAR) δ, which is important for lipid metabolism, results in defects in maintaining HSCs, and its agonist rescues the defect. Fatty acid metabolism is also important for the energy production and biosynthesis for cellular compartments. The inhibition of mitochondrial fatty acid oxidation (FAO) induces the loss of HSCs (Ito et al. 2012). Since PPAR activates the expression of many genes governing FAO, these results suggest that the PPAR-FAO metabolic axis may be required for stem cell maintenance (Clapham and Arch 2007; Narkar et al. 2008; Ito et al. 2012). In addition, fatty acid synthesis and oxidation are essential for the maintenance of adult tissue stem cells and CSCs from multiple organs (Folmes et al. 2013; Knobloch et al. 2013; Brandi et al. 2017; Wang et al. 2018a, b). Unsaturated fatty acid generation by a cellular enzyme is required for CSCs and normal stem cells from many tissues (Ben-David et al. 2013; Song et al. 2017; Stoffel et al. 2017). Collectively, fatty acid metabolism, both in anabolic and catabolic pathways, is tightly regulated in CSCs to maintain the self-renewal and resistance activity (Brandi et al. 2017).
De novo lipogenesis is more active in CSCs than in bulk growing tumor cells in glioblastoma multiform (GBM) (Yasumoto et al. 2016) and is required for stem cell renewal in breast cancer (Pandey et al. 2013). The excess lipid generated by the cells may be stored in lipid droplets. The lipid droplet contains various fatty acids and cholesterols with triacylglycerol and may serve as a reservoir of lipids in cells. The lipid droplet is induced by HIF-1 (Gimm et al. 2010) and may contribute to colorectal cancers (Du et al. 2017). Lipid droplets were induced in CSC-like cells in colorectal cancers (Tirinato et al. 2015), prostate cancers (Yue et al. 2014) breast cancers (de Gonzalo-Calvo et al. 2015) and ovarian cancers (Li et al. 2017a, b). The increased lipid droplets may contribute as storages of an alternative energy source and protect the stem cells from stressful peroxidation in the hypoxic niche (Bailey et al. 2015). Even though lipid synthesis, oxidation and glucose metabolism should closely interact systemically and intracellularly, the exact interactions in CSCs are not well known yet.
Vulnerable targets of CSCs in lipid metabolism
Monounsaturated fatty acid synthesis in CSCs
SCD, or stearoyl-CoA desaturase (Δ-9-desaturase), is an enzyme that catalyzes the biosynthesis of monounsaturated fatty acids (MUFAs) from saturated fatty acids. SCD introduces a single double bond at the 9, 10 position of long-chain acyl-CoAs(Paton and Ntambi 2009). Palmitic acid (16:0) and stearic acid (18:0) are converted to palmitoleic acid (16:1) and oleic acid (18:1), respectively, by this enzyme (Enoch et al. 1976; Ntambi 1999; Paton and Ntambi 2009). In humans, the cis-palmitoleate biosynthesis by SCD1 occurs mainly in the liver and adipose tissue, and the products are incorporated into phospholipids, triglycerides, waxes, and cholesterol esters (Paton and Ntambi 2009). The genes for SCD have been cloned from many species, from yeast to humans (Paton and Ntambi 2009). Unlike the mouse, which has 4 SCD genes (scd1, scd2, scd3 and scd4), humans have only 2 SCD genes (SCD1 and SCD5). The human SCD1 has high homology with all 4 murine SCD genes, while SCD5 has relatively low homology with the mouse genes, suggesting that the role of human SCD5 is different from that of murine SCDs. The ubiquitous expression of SCD1 in most tissues, including liver and adipocytes, but limited expression of SCD5 in the brain suggests that SCD1 is the main enzyme governing the Δ-9 desaturation of fatty acids in the whole human body. Indeed, the SCD1 siRNA was as efficient as the pharmacological intervention in CSC targeting in a variety of stem-like cells from many cancer tissues, supporting the idea that the major desaturase in human cells may be SCD1, not SCD5 (Martin and Cravatt 2009); however, differential roles of SCD1 and SCD5 have also been suggested as promigratory and prosurvival in breast cancer, respectively (Angelucci et al. 2018). SCD5 has also been shown to induce noncanonical WNT, Wnt5a, while reducing canonical WNT, Wnt7b, suggesting that a complicated, reciprocal, regulatory mechanism exists in WNT regulation by SCDs (Zhang et al. 2017a, b).
The importance of the SCD1 enzyme in CSCs was originally raised by the elevated expression of SCD1 in CSC-enriched groups and its requirement in CSC cultures of lung cancers, ovarian cancers and breast cancers (Noto et al. 2013; Colacino et al. 2016; Lobello et al. 2016). Normal stem cells also elevated SCD1 expression and required its function for stem-like activity in mesenchymal stem cells (Lu et al. 2014), pluripotent stem cells (Ben-David et al. 2013) and hair stem cells (Stoffel et al. 2017). Strikingly, Caenorhabditis elegans germ cells require the worm homologous gene of SCD1, suggesting that the requirement of MUFA for stem cell maintenance has been conserved in evolution for a long time.
Li et al., as well as our own group, first demonstrated that CSCs have more MUFA than the bulk cells in ovarian cancer and GBM, respectively (Li et al. 2017a, b; Song et al. 2017). These two studies demonstrated that the increased composition of MUFAs may be essential for cancer stem cell stem-ness. In addition, the demonstration of the distinguished lipidomics profiles of cancer stem cells compared to bulk cancer cell populations in GBM using mass spectrometry (Song et al. 2017) suggested that the lipid component changes may be important determinants for the cell fate of cancer stem cells. Several reports have shown that the SCD1 inhibition selectively eliminated the CSC population in lung, brain, lymphatic and colon cancers (Zhang et al. 2012; Noto et al. 2013; Li et al. 2017a, b; Song et al. 2017) and that effect was reverted by the supplementation of oleic acid, a main product of SCD1 enzyme-mediated reactions. EGFR signaling, which may be a key signaling pathway for most CSC cultures, phosphorylates SCD1 Tyr55 for stabilization and regulates the lipid unsaturation in lung cancer (Zhang et al. 2017a, b).
Function of MUFA in CSCs
Palmitoleic acid (palmitoleate) generally means the cis-Δ9 form (C16:1Δ9 or C16:1 n7). This cis, 16-carbon MUFA should be generated by the enzyme SCD1 from palmitic acid or be supplemented from diet (Paton and Ntambi 2009). The trans-isomer of palmitoleic acid is palmitelaidic acid (or trans-palmitoleic acid) and exits much less than palmitoleic acid in the human body because they are not generated by the human enzyme. The palmitelaidic acid in the circulation should be supplemented from diet and associated with lower insulin resistance, the presence of atherogenic dyslipidemia, and the incidence of diabetes (Mozaffarian et al. 2010). The fatty acid bound to Wnt was identified as cis-Δ9 palmitoleate (C16:1Δ9) (Takada et al. 2006; Zheng et al. 2016). Therefore, SCD1 is required for the Wnt cis-Δ9 palmitoleoylation in the absence of an exogenous supplement of cis-MUFAs, palmitoleic acid or oleic acid (Paton and Ntambi 2009; Rios-Esteves and Resh 2013).
Palmitoleic acid (16:1) was mentioned as a lipokine, and it was suggested that this may behave such as a lipid hormone because it is generated by white adipose tissue to communicate with distal organs, such as the liver and muscles (Cao et al. 2008). Palmitoleic acid treatment activates mitochondria and increases oxygen consumption, fatty acid oxidation and ATP production in adipocytes (Cruz et al. 2018). Palmitoleic acid also induces insulin secretion and inhibits insulin mRNA expression in pancreatic β-cells (Maedler et al. 2003; Yang and Gong 2017). The concentration of palmitoleic acid is also important for endothelial function (Sarabi et al. 2001; Kenny et al. 2002), the regulation of endoplasmic reticulum stress (Akazawa et al. 2010), the regulation of high-fat induced inflammation (Chan et al. 2015) and the regulation of the cell cycle with growth factor stimulation (Koeberle et al. 2012). Palmitoleic acid is the main lipid component contributing to the regulation of SCD-1 expression and also the main product of this enzyme activity. Palmitoleic acid (16:1) suppresses SCD1 transcription, while palmitic acid (16:0) activates it. Interestingly, the SCD1 protein is degraded by palmitoleic acid and stabilized by palmitic acid. This reciprocal regulation suggests that the ratio of two 16-carbon fatty acids (mono-unsaturated vs fully saturated) may regulate the amount of SCD1, which has a central role in de novo lipid synthesis (Cao et al. 2008). The levels of palmitoleic acid in the diet are quite low, and the concentration in tissues and the circulation is maintained at a minimum. Due to the low basal level in the diet and circulation, and the rapid changes due to regulation, palmitoleic acid may be appropriate for an important regulatory signal messenger over palmitate, which is more abundant in the tissues and circulation. Another main MUFA generated by SCD1, oleic acid, is very abundant in many tissues and not easy to change quickly. Therefore, oleic acid may not be appropriate for tightly regulating endocrine signaling or the rate-limiting modulator of a key intercellular signaling molecule (Cao et al. 2008), such as WNT or sonic hedgehog (Shh). It may be possible that palmitoleic acid is generated from diet-originated oleic acid if the biosynthesis is defected.
Cholesterol synthesis in CSCs
Cholesterol is generated by multiple biosynthetic processes or acquired from the diet. The biosynthetic process of cholesterol and its role are summarized in Fig. 2. The synthesized cholesterol can be used for many biomaterials, such as steroid hormones, and the metabolic intermediates in the synthetic process may also be utilized for the different biological synthetic pathways. Epidemiological correlation with blood cholesterol level and tumor incidence or mortality have been accumulated. Some studies have shown a causative, positive correlation (Batty et al. 2011; Pelton et al. 2012; Shafique et al. 2012), and the use of statins, the most widely used medicine for lowering blood cholesterol, was also correlated with reduced cancer incidence (Murtola et al. 2014) and a better prognosis (Nielsen et al. 2012); however, there are some opposing reports showing no correlation (Pedersen et al. 2000) or even a negative correlation (Matsuzaki et al. 2002). TCGA data analyses and many recent clinical reports, however, strongly support a positive role of cholesterol in cancer progress and worse prognosis (Kuzu et al. 2016).
The lipid anabolism of cholesterol and fatty acid. Acetyl-CoA is used for both pathways. The metabolites in cholesterol pathway can be used for other biosynthesis. The drugs available for the shown enzymes are presented as red letters. The red star marked are the 3 genes out of 5 genes screened as essential genes by Song et al. (2017)
By screening using siRNAs targeting ~ 5000 drug-targetable molecules, we identified 5 molecules that are required for the self-renewal of cancer stem-like cells but dispensable for bulk cancer cell growth. Two of these were essential components of cholesterol biosynthesis. The blocking of cholesterol synthesis using the siRNAs of key enzymes or a HMG-CoA reductase inhibitors, atorvastatin, selectively eliminated the stem cells of GBM, colorectal, and lung cancer cells (Song et al. 2017). A high-fat diet resulting in a total cholesterol increase in the circulation enhances in vivo GBM tumor growth, which is suppressed by statin treatment (Song et al. 2017). Simvastatin inhibits the expression of stem-ness-related genes and the metastatic invasion of embryonic carcinomas, hepatoblastomas and breast adenocarcinomas (Torres et al. 2015; Tate et al. 2017). More importantly, the low concentration of statins did not change the lipidomics profile in the bulk cancer cells and shifted the profiles from that of CSCs to that of bulk cultured cells (Song et al. 2017). Cholesterol enhances the self-renewal of colorectal cancer cells, upregulates the expression of stem-ness genes, and may be connected to the MAPK signaling pathway (Wang et al. 2017a, b). These results strongly suggest a positive and important role of cholesterol in the biological activity of CSCs, including self-renewal and its maintenance.
The signaling required for stem cell maintenance
Notch, Shh, WNT, and more in CSC biology
WNT signaling pathway is one of the most important signaling pathways for many stem cell-like activities. It is well studied in cancer biology because its related cellular signaling component APC (tumor suppressor gene) is often mutated in colon cancer, resulting in the activation of the oncogenic β catenin transcription factor in the majority of colon cancer cases; however, WNT is also important for many types of tissue stem cells (Kelly et al. 2011; ten Berge et al. 2011) and CSCs from the cancers of many tissues (Holland et al. 2013). The protein should be secreted to bind the membrane receptor Frizzled, and then, the intracellular protein complex APC/Axin/GSK3β is disrupted to stabilize and release β-catenin to the nucleus. Other than this canonical pathway, some noncanonical pathways have also been identified, including the WNT-activated transcription factor YAP/TAZ, which is also downstream of Hippo suppressive signaling (Park et al. 2015). This noncanonical WNT signaling is important for stem cell self-renewal and that of CSCs (Lian et al. 2010; Cordenonsi et al. 2011). The noncanonical pathway may be mediated through the small GTPase Rho, which requires a fatty acid conjugation, or isopreneylation (Foster et al. 1996). The blockage of WNT signaling also block CSC self-renewal, and several strong candidate drugs for this blockade strategy are under clinical evaluation (Jung and Kim 2015).
Notch signaling may be the most important signaling for stem cell population maintenance (Bigas and Porcheri 2018). It is required for stem cell self-renewal, as blocking Notch signaling by the cellular protein Numb on one side of the cell results in the cell undergoing asymmetric division, leading to the differentiation of that side daughter cells (Rhyu et al. 1994; Gonczy 2008) while the other side daughter cells remain as stem cells. Losing the Notch signal makes all stem cells initiate differentiation, leading to the depletion of stem cells (Shen et al. 1997; Kim and Shen 2008). Notch also plays the most essential role in CSCs (Mine et al. 2009; Fan et al. 2010). Notch signaling was shown to be essential for cancer stem cells in multiple tissues in mouse in vivo models. Notch is not a secreted protein but a trans-membrane protein that binds to another trans-membrane ligand, such as Delta, in the neighboring cells. This binding makes the γ-secretase cleave the Notch peptide and release the Notch intracellular domain, NICD, which translocates to the nucleus and activates many genes essential for stem-ness maintenance (Schroeter et al. 1998). Targeting this Notch activation with γ-secretase inhibitors dramatically eliminates CSCs from many cancer tissues, suggesting that it may be an effective approach for targeting CSCs (Fan et al. 2006), and there are some reagents under clinical evaluation (Jung and Kim 2015).
Another important CSC-regulating secreted protein, the sonic hedgehog protein (Shh), binds to Patched on the membrane, releasing the bound transmembrane protein Smoothened. Then, downstream activation of the transcription factor GLI, which was restricted to the membrane, is released to move into the nucleus (Milla et al. 2012). Shh pathway inhibition has been shown to reduce neuroblastoma and medulloblastoma CSCs (Schiapparelli et al. 2011; Wang et al. 2012). Many inhibitors for blocking Shh signaling have been developed. Small molecules, such as PF-04449913, saridegib, and vismodegib, have been tested for their clinical benefits (Jung and Kim 2015). Figure 3 shows the CSC regulating signaling related to the lipid modification and possible intervention points of those.
Main signaling modulated by lipids and essential for cancer stem cells. The palmitoleate and palmitate modification on the signaling molecules and the geranyl-geranylation (or fanasylation) of small GTPase proteins are marked. The cholesterol binding to Shh and lipid raft are also presented. All of those signaling eventually proceed to the nucleus to activate many genes required for cancer stem cells. The pharmacological intervention on cholesterol and MUFA generation are shown on the target signaling molecules
CSC signaling is directly modulated by MUFA
Fatty acylation of proteins is a covalent binding of fatty acids to serine or cysteine residues in proteins (Resh 2006). This posttranslational process regulates a variety of functions, including the membrane targeting, trafficking and signaling of those target proteins. The hydrophobic carbohydrate chains added to the protein functions, such as to anchor the lipid membranes and/or direct the trafficking and interactions with other proteins/lipids (Linder and Deschenes 2007). Interestingly, most of these use fully saturated fatty acid chains. Stearoylation (18:0) of the human transferrin receptor 1 (TFR1) is required for proper mitochondrial function (Senyilmaz et al. 2015). More than one hundred proteins have been identified to be palmitoylated (16:0) (Martin and Cravatt 2009) and myristoylated (14:0) (Thinon et al. 2014), demonstrating that these two events may do an important portion of protein acylation (Zheng et al. 2016) and the conjugated biological events. They regulate a wide range of biological activity in signaling. Synaptic proteins, such as SNAP26, may be regulated by palmitoylation for intercellular signaling (Greaves and Chamberlain 2011), and kinases, such as Src, may be regulated by myristoylation in intracellular signaling (Kamps et al. 1985; Shoji and Kubota 1989).
One very important intercellular signaling protein modulated by fatty acids is Wnt. Wnt3a was originally identified as palmitoylated (16:0) on Cys77 (Willert et al. 2003). This modification appears to be important for the biological function but not essential for secretion. Independent research demonstrated that Wnt3a is modified with a monounsaturated (16:1) fatty acid, palmitoleic acid, at Ser209 (Takada et al. 2006). They further demonstrated that this modification is essential for the transportation from the endoplasmic reticulum (ER) to the plasma membrane by showing that the palmitoleoylation mutant Wnt3a (Ser209Ala) failed to be secreted and rather accumulated in ER. The palmitoleoylation on Ser209 in Wnt3a is mediated by the enzyme Porcupine (PORCN), which is a membrane-bound O-acyltransferase (Takada et al. 2006). The inhibition of PORCN with small molecules demonstrated that the enzyme is essential for Wnt secretion and the following downstream signal transduction. Therefore, the suppression of this enzyme’s activity may be a selective and potent approach to target cancer and cancer stem-ness (Liu et al. 2013). Additionally, the cleavage of the palmitoleate group by a specific carboxylesterase suppressed the WNT activity (Kakugawa et al. 2015). This palmitoleate group binding may also be required for direct interaction with the Frizzled receptor and is also conserved for other Wnt family proteins. This MUFA modification of WNT is conserved in the worm, fly and mammals (Rocheleau et al. 1997; Janda et al. 2012).
Another very important intercellular stem cell signaling molecule is Shh, one of three hedgehog proteins in humans. The cleaved and secreted Shh peptide is purified as the N-terminal Cys24 palmitoylated, cleaved form (N-peptide), and that acylation is essential for the biological activity of Shh (Pepinsky et al. 1998). Since Wnt is palmitoylated and palmitoleoylated, and since the biological roles of these two modifications appear to be different, the possibility of palmitoleoylation on Shh may be possible; however, SCD1, which is the main enzyme for palmitoleate synthesis, is not required for the fatty acylation of Shh. Porcupine, the acyltransferase of Wnt, did not acylate the Shh peptide using MUFA (Rios-Esteves and Resh 2013). The N-terminus acylation of Shh was mediated by Hedgehog acyltransferase (Hhat), a membrane-bound, O-acyltransferase family of proteins (Pepinsky et al. 1998; Buglino and Resh 2008), and the inhibition of Hhat activity suppressed (Petrova et al. 2013) the signaling by Shh. It may be reasonable to assume that Shh is not modulated by SCD1-generated MUFA, such as palmitoleic acid. Wnt proteins are currently the only confirmed palmitoleoylated major signaling proteins (Zheng et al. 2016), while many proteins are reported to be palmitoylated, as previously mentioned.
CSC signaling modulated by the cholesterols
After Shh is translated, the signaling peptide is cleaved in the ER, followed by its cleavage at Gly257, making the N-peptide and C-peptide. Then, the cholesterol is covalently bound to the new C-terminal end Gly257of the N-peptide. This cholesterol-bound N-peptide now can be palmitoylated at the Cys85 residue, the new N-terminal of this N-peptide (Riobo 2012). The genetic defects in the cholesterol biosynthesis are connected to a class of genetically determined anatomical defects, termed holoprosencephaly (HPE). HPE are also very typical defects derived from the dominant mutation in Shh signaling. Cholesterol depletion blocks the autoprocessing of Shh and results in signaling blockage (Roessler et al. 1997; Guy 2000; Gofflot et al. 2001). The Smoothened translocation from the endosome to the cilia is tightly regulated by cholesterol as well (Rohatgi et al. 2007; Blassberg and Jacob 2017; Xiao et al. 2017); therefore, cholesterol metabolism should be closely connected to Shh signaling through the cholesterol conjugation.
The N-terminal C2 domain of the Notch ligand interacts with lipids in the plasma membrane and Notch (Suckling et al. 2017). A low sterol diet suppressed the Notch pathway in Drosophila (Obniski et al. 2018); however, this component of Notch signaling is not directly modified by cholesterol or other lipids. Notch cleavage, which requires γ-secretase, is mediated at the ‘lipid-rafts’, a small, submicroscopic domains. They are present in plasma and other membranes, and many proteins and glycoproteins are concentrated in these regions (Bretscher and Munro 1993). Cholesterol levels are a key factor in determining raft structure and stability (Silvius 2003). Lipid–rafts are known to exclude unsaturated phospholipids but are enriched in cholesterol (Silvius 2003; Hakobyan and Heuer 2014). The change in the lipid-raft structure by cholesterol composition may be a critical modulator of γ-secretase-mediated Notch signaling (Osenkowski et al. 2008). Collectively, the cholesterol metabolism should have a critical role in Notch signaling, and various transmembrane signaling related to the lipid-raft structure still needs to be unveiled (Levental and Veatch 2016) in addition to Shh signaling (Simons and Ikonen 1997).
An intermediate of cholesterol biosynthesis, farnesyl pyrophosphate, may be used for many other biosynthetic processes. The small G-protein is farnesylated to get to the membrane, and this process is essential for the signal transduction mediated by the G-protein. Many oncogenic receptor tyrosine kinases (RTK) require Ras protein for signaling, and EGFR signaling is especially essential for CSC maintenance. In addition, the noncanonical pathway of WNT signaling requires another G-protein, Rho, which requires farnesylation or geranyl–geranylation, as well as other small GTPases. Therefore, the cholesterol biosynthesis that generated the metabolites used for this key signal transduction may be essential for the CSC maintenance, not the final sterol products. Indeed, statins inhibit the YAP/TAZ action through the blocking of Rho geranyl-geranylation (Sorrentino et al. 2014).
To target fatty acids or cholesterols or both?
The biosynthesis of fatty acids and sterols may be independently regulated; however, the two pathways can also be regulated together by Sterol regulatory element-binding proteins (Ye and DeBose-Boyd 2011). In addition, as previously described in this review, both pathways are strongly associated with CSCs’ roles or/and maintenance, possibly through the molecular signaling CSC should maintain. The blocking of either pathway may be sufficient to suppress CSCs (Song et al. 2017); however, lipid biosynthesis is globally controlled by metabolic regulation. Therefore, the two pathways should have a point to crosstalk in CSC signaling. Recently, an interesting study made a connection between phospholipid remodeling and cholesterols in stem cell growth. The loss of lysophosphatidylcholine acyltransferase-3 (Lpcat3) enhances cholesterol biosynthesis, and the excess cholesterol increases intestinal stem cell proliferation in vivo and ex vivo, resulting in the promotion of tumorigenesis (Wang et al. 2018a, b). Lpcaf3 is an enzyme that catalyzes the reacylation of lysophospholipids (one fatty acid chain) to phospholipids (two fatty acid chains). Lpcat3 mediates lipid and glucose homeostasis by reducing mitochondrial fatty acid oxidation in the liver (Cash and Hui 2016). The suppression of Lpcat3 may increase intracellular lysophospholipid levels, leading to disrupted lipid homeostasis, an increase in lipid synthesis (Li et al. 2012), and failure to appropriately maintain the lipid bilayer structure. Lysophospholipids appear to regulate the maintenance of many stem cells (Whetton et al. 2003; Pebay et al. 2007). Though the mechanism leading to cholesterol production by Lpcaf3 is unknown, the in vivo connection of the two lipid synthesis pathways resulting in stem cell enrichment is interesting because cholesterol synthesis and fatty acid unsaturation are the most vulnerable processes for CSC targeting (Mancini et al. 2018).
The lipid-raft is a dynamic complex that contains many subgroups of fatty acids and sterols. Cholesterol associates with greater affinity with unsaturated phosphatidylcholines than with unsaturated phosphatidylethanolamines (Yeagle and Young 1986), suggesting that different phospholipids and acyl groups may change the affinity to cholesterol. The lipid-rafts should be involved in many transmembrane cell signaling processes in addition to the membranous secretase-mediated processes (Simons and Ikonen 1997; Di Vizio et al. 2008; Osenkowski et al. 2008; Roy et al. 2011; Levental and Veatch 2016). Therefore, the lipid composition in the raft should be critical for cell signaling, which may be essential for CSCs.
In recent years, the targeting of SCD1 was suggested as a potential CSC-targeted therapeutic strategy by multiple research groups. As described, the enzyme SCD1 is now believed as a key enzyme for CSC maintenance through MUFA synthesis. Since the palmitoleoylation of WNT is essential for WNT secretion, targeting SCD1 inhibits β-catenin accumulation in breast cancer cell nuclei (Mauvoisin et al. 2013). Dai et al., showed that SCD1 inhibition blocked the Akt/GSK3β/β-catenin signaling, leading to the apoptosis of glioma cells and may sensitize the effect of temozolomide (Dai et al. 2017). SCD1 inhibition also reduced the FA uptake protein CD36 (Zhao et al. 2017), suggesting that secondary lipid availability restriction may be a result. ER stress-enhanced autophagy was reported in lung CSCs due to SCD1 blocking, and this may suggest that intracellular membrane composition may lead to ER functional abnormalities (Pisanu et al. 2017). Downstream of the noncanonical WNT signaling, the YAP/TAZ pathway is mediated by SCD1, along with the canonical β-catenin pathway (Noto et al. 2013); however, YAP/TAZ is also regulated by Hippo signaling independent of WNT activity. From the loss- and gain-of-function screening, miRNA targeting SCD1 was identified as miR-600 and shown to suppress canonical WNT signaling in human cancer specimens as well (Pisanu et al. 2017). Blocking SCD1 in colon cancer cells induced apoptosis by promoting ceramide synthesis, suggesting a WNT-independent role (Chen et al. 2016a, b). The positive feedback between SCD1 and NFκB has also been reported in ovarian CSCs, suggesting a role of NFκB-dependent signaling in CSC maintenance (Li et al. 2017a, b). The elevated expression and function of SCD1 is recognized as a hallmark of CSCs in variety of tissue origins. The elevated contents of MUFA may also be detected in most CSCs. Since the effect of SCD1 inhibition should be systemic and can regulate lipid metabolism in the liver and muscles, the inhibitor may have an impact on the whole-body metabolism as well. Since several clinical trials are currently underway for noncancer conditions, the safety of cancer treatments may be evaluated soon.
Targeting cholesterol synthesis may lead to a wide range of biological events because sterol synthesis metabolism is connected to many biologically active components. Compared to SCD1 inhibition, clinical conditions for the pharmacological blocking of cholesterol synthesis has been well-established. Hydroxy-methyl-glutharyl-coenzyme A reductase (HMG-CoAR) is the rate-limiting enzyme for cholesterol biosynthesis. Statins are one of the most widely used classes of drugs for blood cholesterol maintenance worldwide. Statins have a mevalonate moiety that binds to the reaction pocket of HMG-CoAR and then blocks the activity. One of the strong oncogenes, Myc, induced a transcriptome that is associated with the mevalonate suppressed gene expression profile, showing the reciprocal interaction of the lipid control and one master oncogene action (Wang et al. 2017a, b). Supplementation of oleic acid in the CSC culture medium enriched CSCs dramatically, even when statin was present to block the CSC renewal (Song et al. 2017) suggest there are some crosstalk may exist between MUFA and cholesterol biosynthesis pathways in CSC renewal.
The intermediate metabolites are used for farnesylation or geranyl-geranylation, which are essential for Ras and Rho family of small guanosine triphosphates (GTPases) tethering to the membrane. Therefore, the multifunction of GTPases from G protein-coupled receptors to RTK signaling may be influenced by statin treatment (Mullen et al. 2016). Indeed, simvastatin treatment suppresses the protein geranyl-geranylation (Ginestier et al. 2012). As expected from this finding, a geranyl-geranylation inhibitor can also suppress many CSC activities. Blocking of the localization of RhoA to the membrane suppresses p27Kip localization to the nucleus, leading to the Rb activation. Interestingly, the HMG-CoAR suppression also leads to YAP/TAZ inactivation through the failure of RhoA geranyl-geranylation. SCD1 and HMG-CoAR inhibition meet at the YAP/TAZ molecule that controls the tissue overgrowth and is suppressed by Hippo signaling (Sorrentino et al. 2014). Other than statins, bisphosphonates also suppress the cholesterol metabolism and hamper the synthesis of geranyl-geranyl pyrophosphate or farnesyl pyrophosphate, leading to the elimination of breast CSCs (Buhler et al. 2016). Supplementation with oleic acid in the CSC culture medium enriched the CSCs dramatically, even when statins blocked the CSC renewal (Song et al. 2017), also suggesting that the two signaling pathways may combine.
Conclusions
In this review, the altered metabolism of CSCs and recent findings that show that MUFA and cholesterol metabolism are two very promising targets for the selective elimination of CSCs are summarized. CSCs likely prefer similar glucose and lipid metabolism as stem cells of normal tissues. Compared to the other fast-growing cells in the tumor, the quiescent CSCs may be able to handle with this low efficiency and will be relatively free from the dangerous ROS production from OXPHOS. CSCs may get their energy from fatty acid β-oxidation in mitochondria, which is elevated and essential for CSCs to maintain their potential. These distinguishable metabolism characteristics of CSCs may be utilized for the selective targeting of CSCs; however, the detailed mechanism and meaning of this differential regulation of lipid and glucose metabolism in CSCs is not yet known. Therefore, further intensive studies need to be performed for potential therapeutic targeting on this modulated signaling, but the plasticity in the regulation of metabolism may be a strong obstacle.
The promising and vulnerable target on lipid metabolism is in the unsaturation process of fatty acids. SCD1 activity increases in variety of CSCs, and its requirement in CSCs suggests that MUFA generation may be an excellent target for CSC therapy. This is very relevant, considering SCD1′s role in WNT signaling, which is critical in CSC biology. However, the consequence of blocking systemic metabolism regulators in normal body cells are not clarified. Inhibitors, however, may function directly in CSCs, and the systemic level of MUFA may be manageable with diet supplementation. Song et al. showed that the targeting of CSCs with atorvastatin effectively suppressed the cancer cell growth in vivo in the supplementation of high-fat diet, demonstrating that the statin directly functioned in the cancer cells. Currently, several SCD1 inhibitors are being developed, and clinical evaluations may follow for safety and efficacy.
Cholesterol metabolism in CSCs as a promising and selective target for therapy was also raised based on epidemiological data and in vitro and in vivo preclinical data. The lipid-raft may be tightly regulated by the content of cholesterol, and it may change the signaling cascade across the membrane. The γ-secretase-mediated Notch signaling may be the 1st candidate changed by the cholesterol metabolism defect. Shh signaling may also be affected by the cholesterol shortage; however, the small GTPase protein modulation also requires this metabolic process and crosstalk with noncanonical WNT and RTK downstream. The blocking of LSS1, which generates cholesterol from squalene, eliminated most GBM CSCs (Song et al. 2017), demonstrating that cholesterol synthesis is still essential for CSCs. Targeting cholesterol synthesis for cancer therapy is currently being investigated in more than 50 clinical trials (NIH 2018). Widely accumulated data for statins and bisphosphonates may be useful for evaluating the safety.
Though these two targets, cholesterol metabolism and MUFA synthesis, are very effective in support of relevant molecular mechanisms, the clinical efficacy or these two therapeutic strategies has not yet been demonstrated. Indeed, the clinical relevance of CSC targeting has not been sufficiently proven either. While the presence of this population in most cancers is now widely recognized, the molecular characteristics of these cells and the effective isolation of these cells have not been well-established. It may be caused from the plasticity of these CSCs, which actively respond to the environment and even modulate the niche. CSCs can be reversely established from the differentiated bulk cancer cells. The genomic instability that all cancers have may still contribute to this plasticity. Therefore, CSC targeting with one specific molecule may not be successfully achieved as we hoped; however, we will continue to pursue the targeting of metabolism in CSC populations, which can be combined with other conventional and targeted therapy. Since the metabolic reprograming of cancers and CSCs is currently being increasingly unveiled, and since many metabolic syndrome management strategies are already available, we may soon find an effective approach to better handle this disease.
References
Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ (2010) Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol 52(4):586–593
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7):3983–3988
Angelucci C, D’Alessio A, Iacopino F, Proietti G, Di Leone A, Masetti R, Sica G (2018) Pivotal role of human stearoyl-CoA desaturases (SCD1 and 5) in breast cancer progression: oleic acid-based effect of SCD1 on cell migration and a novel pro-cell survival role for SCD5. Oncotarget 9(36):24364–24380
Ansari J, Hussain SA, Alhasso A, Mahmood R, Ansari A, Glaholm J (2011) Role of second-line systemic treatment post-docetaxel in metastatic castrate resistant prostate cancer- current strategies and future directions. Anticancer Agents Med Chem 11(3):296–306
Bailey AP, Koster G, Guillermier C, Hirst EM, MacRae JI, Lechene CP, Postle AD, Gould AP (2015) Antioxidant role for lipid droplets in a stem cell niche of drosophila. Cell 163(2):340–353
Bartesaghi S, Graziano V, Galavotti S, Henriquez NV, Betts J, Saxena J, Minieri V, Deli A, Karlsson A, Martins LM, Capasso M, Nicotera P, Brandner S, De Laurenzi V, Salomoni P (2015) Inhibition of oxidative metabolism leads to p53 genetic inactivation and transformation in neural stem cells. Proc Natl Acad Sci USA 112(4):1059–1064
Batty GD, Kivimaki M, Clarke R, Davey Smith G, Shipley MJ (2011) Modifiable risk factors for prostate cancer mortality in London: forty years of follow-up in the Whitehall study. Cancer Causes Control 22(2):311–318
Ben-David U, Gan QF, Golan-Lev T, Arora P, Yanuka O, Oren YS, Leikin-Frenkel A, Graf M, Garippa R, Boehringer M, Gromo G, Benvenisty N (2013) Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell 12(2):167–179
Bergsagel DE, Valeriote FA (1968) Growth characteristics of a mouse plasma cell tumor. Cancer Res 28(11):2187–2196
Bigas A, Porcheri C (2018) Notch and Stem Cells. Adv Exp Med Biol 1066:235–263
Blassberg R, Jacob J (2017) Lipid metabolism fattens up hedgehog signaling. BMC Biol 15(1):95
Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3(7):730–737
Brandi J, Dando I, Pozza ED, Biondani G, Jenkins R, Elliott V, Park K, Fanelli G, Zolla L, Costello E, Scarpa A, Cecconi D, Palmieri M (2017) Proteomic analysis of pancreatic cancer stem cells: functional role of fatty acid synthesis and mevalonate pathways. J Proteom 150:310–322
Bretscher MS, Munro S (1993) Cholesterol and the Golgi apparatus. Science 261(5126):1280–1281
Bruce WR, Van Der Gaag H (1963) A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature 199:79–80
Buglino JA, Resh MD (2008) Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J Biol Chem 283(32):22076–22088
Buhler H, Hoberg C, Fakhrian K, Adamietz IA (2016) Zoledronic acid inhibits the motility of cancer stem-like cells from the human breast cancer cell line MDA-MB 231. In Vivo 30(6):761–768
Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS (2008) Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134(6):933–944
Cash JG, Hui DY (2016) Liver-specific overexpression of LPCAT3 reduces postprandial hyperglycemia and improves lipoprotein metabolic profile in mice. Nutr Diabetes 6:e206
Chan KL, Pillon NJ, Sivaloganathan DM, Costford SR, Liu Z, Theret M, Chazaud B, Klip A (2015) Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK). J Biol Chem 290(27):16979–16988
Chen CL, Uthaya Kumar DB, Punj V, Xu J, Sher L, Tahara SM, Hess S, Machida K (2016a) NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab 23(1):206–219
Chen L, Ren J, Yang L, Li Y, Fu J, Tian Y, Qiu F, Liu Z, Qiu Y (2016b) Stearoyl-CoA desaturase-1 mediated cell apoptosis in colorectal cancer by promoting ceramide synthesis. Sci Rep 6:19665
Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, De Cola A, Scavo E, Carollo R, D’Agostino D, Forli F, D’Aguanno S, Todaro M, Stassi G, Di Ilio C, De Laurenzi V, Urbani A (2014) Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis 5:e1336
Clapham JC, Arch JR (2007) Thermogenic and metabolic antiobesity drugs: rationale and opportunities. Diabetes Obes Metab 9(3):259–275
Colacino JA, McDermott SP, Sartor MA, Wicha MS, Rozek LS (2016) Transcriptomic profiling of curcumin-treated human breast stem cells identifies a role for stearoyl-coa desaturase in breast cancer prevention. Breast Cancer Res Treat 158(1):29–41
Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, Daidone MG, Dupont S, Basso G, Bicciato S, Piccolo S (2011) The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147(4):759–772
Cruz MM, Lopes AB, Crisma AR, de Sa RCC, Kuwabara WMT, Curi R, de Andrade PBM, Alonso-Vale MIC (2018) Palmitoleic acid (16:1n7) increases oxygen consumption, fatty acid oxidation and ATP content in white adipocytes. Lipids Health Dis 17(1):55
Dai S, Yan Y, Xu Z, Zeng S, Qian L, Huo L, Li X, Sun L, Gong Z (2017) SCD1 confers temozolomide resistance to human glioma cells via the Akt/GSK3beta/beta-Catenin signaling axis. Front Pharmacol 8:960
Dando I, Dalla Pozza E, Biondani G, Cordani M, Palmieri M, Donadelli M (2015) The metabolic landscape of cancer stem cells. IUBMB Life 67(9):687–693
Danhier P, Banski P, Payen VL, Grasso D, Ippolito L, Sonveaux P, Porporato PE (2017) Cancer metabolism in space and time: beyond the Warburg effect. Biochim Biophys Acta 1858(8):556–572
de Gonzalo-Calvo D, Lopez-Vilaro L, Nasarre L, Perez-Olabarria M, Vazquez T, Escuin D, Badimon L, Barnadas A, Lerma E, Llorente-Cortes V (2015) Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: a molecular and clinicopathological study. BMC Cancer 15:460
Dean M, Fojo T, Bates S (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5(4):275–284
Di Vizio D, Solomon KR, Freeman MR (2008) Cholesterol and cholesterol-rich membranes in prostate cancer: an update. Tumori 94(5):633–639
Donnenberg VS, Donnenberg AD (2005) Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol 45(8):872–877
Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI, Campbell S, Welford SM (2017) HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat Commun 8(1):1769
Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S, Clarke MF, Hoey T, Lewicki J, Gurney AL (2008) Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE 3(6):e2428
Emmink BL, Verheem A, Van Houdt WJ, Steller EJ, Govaert KM, Pham TV, Piersma SR, Borel Rinkes IH, Jimenez CR, Kranenburg O (2013) The secretome of colon cancer stem cells contains drug-metabolizing enzymes. J Proteom 91:84–96
Enoch HG, Catala A, Strittmatter P (1976) Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J Biol Chem 251(16):5095–5103
Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R (2008) Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 15(3):504–514
Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, Eberhart CG (2006) Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res 66(15):7445–7452
Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, Nikkhah G, Dimeco F, Piccirillo S, Vescovi AL, Eberhart CG (2010) NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28(1):5–16
Fidler IJ, Hart IR (1982) Biological diversity in metastatic neoplasms: origins and implications. Science 217(4564):998–1003
Fidler IJ, Kripke ML (1977) Metastasis results from preexisting variant cells within a malignant tumor. Science 197(4306):893–895
Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A (2011) Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 14(2):264–271
Folmes CD, Park S, Terzic A (2013) Lipid metabolism greases the stem cell engine. Cell Metab 17(2):153–155
Foster R, Hu KQ, Lu Y, Nolan KM, Thissen J, Settleman J (1996) Identification of a novel human Rho protein with unusual properties: GTPase deficiency and in vivo farnesylation. Mol Cell Biol 16(6):2689–2699
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366(10):883–892
Gimm T, Wiese M, Teschemacher B, Deggerich A, Schodel J, Knaup KX, Hackenbeck T, Hellerbrand C, Amann K, Wiesener MS, Honing S, Eckardt KU, Warnecke C (2010) Hypoxia-inducible protein 2 is a novel lipid droplet protein and a specific target gene of hypoxia-inducible factor-1. FASEB J 24(11):4443–4458
Ginestier C, Monville F, Wicinski J, Cabaud O, Cervera N, Josselin E, Finetti P, Guille A, Larderet G, Viens P, Sebti S, Bertucci F, Birnbaum D, Charafe-Jauffret E (2012) Mevalonate metabolism regulates Basal breast cancer stem cells and is a potential therapeutic target. Stem Cells 30(7):1327–1337
Gofflot F, Gaoua W, Bourguignon L, Roux C, Picard JJ (2001) Expression of Sonic Hedgehog downstream genes is modified in rat embryos exposed in utero to a distal inhibitor of cholesterol biosynthesis. Dev Dyn 220(2):99–111
Gonczy P (2008) Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol 9(5):355–366
Greaves J, Chamberlain LH (2011) Differential palmitoylation regulates intracellular patterning of SNAP25. J Cell Sci 124(Pt 8):1351–1360
Guy RK (2000) Inhibition of sonic hedgehog autoprocessing in cultured mammalian cells by sterol deprivation. Proc Natl Acad Sci USA 97(13):7307–7312
Hakobyan D, Heuer A (2014) Key molecular requirements for raft formation in lipid/cholesterol membranes. PLoS ONE 9(2):e87369
Heppner GH (1984) Tumor heterogeneity. Cancer Res 44(6):2259–2265
Holland JD, Klaus A, Garratt AN, Birchmeier W (2013) Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol 25(2):254–264
Ito K, Suda T (2014) Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 15(4):243–256
Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, Pandolfi PP (2012) A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med 18(9):1350–1358
Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC (2012) Structural basis of Wnt recognition by Frizzled. Science 337(6090):59–64
Janiszewska M, Suva ML, Riggi N, Houtkooper RH, Auwerx J, Clement-Schatlo V, Radovanovic I, Rheinbay E, Provero P, Stamenkovic I (2012) Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev 26(17):1926–1944
Jung Y, Kim WY (2015) Cancer stem cell targeting: are we there yet? Arch Pharmacal Res 38(3):414–422
Kakugawa S, Langton PF, Zebisch M, Howell S, Chang TH, Liu Y, Feizi T, Bineva G, O’Reilly N, Snijders AP, Jones EY, Vincent JP (2015) Notum deacylates Wnt proteins to suppress signalling activity. Nature 519(7542):187–192
Kamps MP, Buss JE, Sefton BM (1985) Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci USA 82(14):4625–4628
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438(7069):820–827
Kelly KF, Ng DY, Jayakumaran G, Wood GA, Koide H, Doble BW (2011) beta-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell 8(2):214–227
Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR (2002) The role of gap junctions in mediating endothelium-dependent responses to bradykinin in myometrial small arteries isolated from pregnant women. Br J Pharmacol 136(8):1085–1088
Kim WT, Ryu CJ (2017) Cancer stem cell surface markers on normal stem cells. BMB Rep 50(6):285–298
Kim WY, Shen J (2008) Presenilins are required for maintenance of neural stem cells in the developing brain. Mol Neurodegener 3:2
Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K (2002) The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res 8(1):22–28
Knobloch M, Braun SM, Zurkirchen L, von Schoultz C, Zamboni N, Arauzo-Bravo MJ, Kovacs WJ, Karalay O, Suter U, Machado RA, Roccio M, Lutolf MP, Semenkovich CF, Jessberger S (2013) Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature 493(7431):226–230
Koeberle A, Shindou H, Harayama T, Shimizu T (2012) Palmitoleate is a mitogen, formed upon stimulation with growth factors, and converted to palmitoleoyl-phosphatidylinositol. J Biol Chem 287(32):27244–27254
Kuzu OF, Noory MA, Robertson GP (2016) The role of cholesterol in cancer. Cancer Res 76(8):2063–2070
Levental I, Veatch S (2016) The continuing mystery of lipid rafts. J Mol Biol 428(24 Pt A):4749–4764
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100(9):672–679
Li Z, Ding T, Pan X, Li Y, Li R, Sanders PE, Kuo MS, Hussain MM, Cao G, Jiang XC (2012) Lysophosphatidylcholine acyltransferase 3 knockdown-mediated liver lysophosphatidylcholine accumulation promotes very low density lipoprotein production by enhancing microsomal triglyceride transfer protein expression. J Biol Chem 287(24):20122–20131
Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, Matei D, Cheng JX (2017a) Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell 20(3):303–314
Li X, Fang P, Yang WY, Chan K, Lavallee M, Xu K, Gao T, Wang H, Yang X (2017b) Mitochondrial ROS, uncoupled from ATP synthesis, determine endothelial activation for both physiological recruitment of patrolling cells and pathological recruitment of inflammatory cells. Can J Physiol Pharmacol 95(3):247–252
Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, Ding S, Guan KL (2010) The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev 24(11):1106–1118
Linder ME, Deschenes RJ (2007) Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol 8(1):74–84
Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, Li J, Tompkins C, Pferdekamper A, Steffy A, Cheng J, Kowal C, Phung V, Guo G, Wang Y, Graham MP, Flynn S, Brenner JC, Li C, Villarroel MC, Schultz PG, Wu X, McNamara P, Sellers WR, Petruzzelli L, Boral AL, Seidel HM, McLaughlin ME, Che J, Carey TE, Vanasse G, Harris JL (2013) Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci USA 110(50):20224–20229
Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL, Hu Y, Wang P, Ju HQ, Xu RH, Huang P (2014) Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death Differ 21(1):124–135
Lobello N, Biamonte F, Pisanu ME, Faniello MC, Jakopin Z, Chiarella E, Giovannone ED, Mancini R, Ciliberto G, Cuda G, Costanzo F (2016) Ferritin heavy chain is a negative regulator of ovarian cancer stem cell expansion and epithelial to mesenchymal transition. Oncotarget 7(38):62019–62033
Lu Y, Zhou Z, Tao J, Dou B, Gao M, Liu Y (2014) Overexpression of stearoyl-CoA desaturase 1 in bone marrow mesenchymal stem cells enhance the expression of induced endothelial cells. Lipids Health Dis 13:53
Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY (2003) Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 52(3):726–733
Mancini R, Noto A, Pisanu ME, De Vitis C, Maugeri-Sacca M, Ciliberto G (2018) Metabolic features of cancer stem cells: the emerging role of lipid metabolism. Oncogene 37(18):2367–2378
Martin BR, Cravatt BF (2009) Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods 6(2):135–138
Matsuno T, Satoh T, Suzuki H (1986) Prominent glutamine oxidation activity in mitochondria of avian transplantable hepatoma induced by MC-29 virus. J Cell Physiol 128(3):397–401
Matsuzaki M, Kita T, Mabuchi H, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H (2002) Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia. Circ J 66(12):1087–1095
Mauvoisin D, Charfi C, Lounis AM, Rassart E, Mounier C (2013) Decreasing stearoyl-CoA desaturase-1 expression inhibits beta-catenin signaling in breast cancer cells. Cancer Sci 104(1):36–42
Milla LA, Gonzalez-Ramirez CN, Palma V (2012) Sonic Hedgehog in cancer stem cells: a novel link with autophagy. Biol Res 45(3):223–230
Mimeault M, Hauke R, Mehta PP, Batra SK (2007) Recent advances in cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Cell Mol Med 11(5):981–1011
Mine T, Matsueda S, Li Y, Tokumitsu H, Gao H, Danes C, Wong KK, Wang X, Ferrone S, Ioannides CG (2009) Breast cancer cells expressing stem cell markers CD44 + CD24 lo are eliminated by Numb-1 peptide-activated T cells. Cancer Immunol Immunother 58(8):1185–1194
Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS (2010) Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med 153(12):790–799
Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ (2016) The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer 16(11):718–731
Murtola TJ, Visvanathan K, Artama M, Vainio H, Pukkala E (2014) Statin use and breast cancer survival: a nationwide cohort study from Finland. PLoS ONE 9(10):e110231
Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM (2008) AMPK and PPARdelta agonists are exercise mimetics. Cell 134(3):405–415
Nielsen SF, Nordestgaard BG, Bojesen SE (2012) Statin use and reduced cancer-related mortality. N Engl J Med 367(19):1792–1802
NIH (2018) ClinicalTrials.gov. https://clinicaltrials.gov/ct2/home
Nishii T, Yashiro M, Shinto O, Sawada T, Ohira M, Hirakawa K (2009) Cancer stem cell-like SP cells have a high adhesion ability to the peritoneum in gastric carcinoma. Cancer Sci 100(8):1397–1402
Noto A, Raffa S, De Vitis C, Roscilli G, Malpicci D, Coluccia P, Di Napoli A, Ricci A, Giovagnoli MR, Aurisicchio L, Torrisi MR, Ciliberto G, Mancini R (2013) Stearoyl-CoA desaturase-1 is a key factor for lung cancer-initiating cells. Cell Death Dis 4:e947
Nowell PC (1976) The clonal evolution of tumor cell populations. Science 194(4260):23–28
Ntambi JM (1999) Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res 40(9):1549–1558
Obniski R, Sieber M, Spradling AC (2018) Dietary lipids modulate notch signaling and influence adult intestinal development and metabolism in drosophila. Dev Cell 47(1):98–111
O’Brien CA, Pollett A, Gallinger S, Dick JE (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445(7123):106–110
Ogawa M, Bergsagel DE, McCulloch EA (1971) Differential effects of melphalan on mouse myeloma (adj. PC-5) and hemopoietic stem cells. Cancer Res 31(12):2116–2119
Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ (2008) Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem 283(33):22529–22540
Palorini R, Votta G, Balestrieri C, Monestiroli A, Olivieri S, Vento R, Chiaradonna F (2014) Energy metabolism characterization of a novel cancer stem cell-like line 3AB-OS. J Cell Biochem 115(2):368–379
Pandey PR, Xing F, Sharma S, Watabe M, Pai SK, Iiizumi-Gairani M, Fukuda K, Hirota S, Mo YY, Watabe K (2013) Elevated lipogenesis in epithelial stem-like cell confers survival advantage in ductal carcinoma in situ of breast cancer. Oncogene 32(42):5111–5122
Park CH, Bergsagel DE, McCulloch EA (1971) Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst 46(2):411–422
Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL (2015) Alternative Wnt signaling activates YAP/TAZ. Cell 162(4):780–794
Pasto A, Bellio C, Pilotto G, Ciminale V, Silic-Benussi M, Guzzo G, Rasola A, Frasson C, Nardo G, Zulato E, Nicoletto MO, Manicone M, Indraccolo S, Amadori A (2014) Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget 5(12):4305–4319
Paton CM, Ntambi JM (2009) Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab 297(1):E28–E37
Pebay A, Bonder CS, Pitson SM (2007) Stem cell regulation by lysophospholipids. Prostaglandins Other Lipid Mediat 84(3–4):83–97
Pedersen TR, Wilhelmsen L, Faergeman O, Strandberg TE, Thorgeirsson G, Troedsson L, Kristianson J, Berg K, Cook TJ, Haghfelt T, Kjekshus J, Miettinen T, Olsson AG, Pyorala K, Wedel H (2000) Follow-up study of patients randomized in the Scandinavian simvastatin survival study (4S) of cholesterol lowering. Am J Cardiol 86(3):257–262
Pelton K, Freeman MR, Solomon KR (2012) Cholesterol and prostate cancer. Curr Opin Pharmacol 12(6):751–759
Peng F, Wang JH, Fan WJ, Meng YT, Li MM, Li TT, Cui B, Wang HF, Zhao Y, An F, Guo T, Liu XF, Zhang L, Lv L, Lv DK, Xu LZ, Xie JJ, Lin WX, Lam EW, Xu J, Liu Q (2018) Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene 37(8):1062–1074
Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, Taylor FR, Wang EA, Galdes A (1998) Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem 273(22):14037–14045
Petrova E, Rios-Esteves J, Ouerfelli O, Glickman JF, Resh MD (2013) Inhibitors of Hedgehog acyltransferase block Sonic Hedgehog signaling. Nat Chem Biol 9(4):247–249
Pisanu ME, Noto A, De Vitis C, Morrone S, Scognamiglio G, Botti G, Venuta F, Diso D, Jakopin Z, Padula F, Ricci A, Mariotta S, Giovagnoli MR, Giarnieri E, Amelio I, Agostini M, Melino G, Ciliberto G, Mancini R (2017) Blockade of Stearoyl-CoA-desaturase 1 activity reverts resistance to cisplatin in lung cancer stem cells. Cancer Lett 406:93–104
Resh MD (2006) Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol 2(11):584–590
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414(6859):105–111
Rhyu MS, Jan LY, Jan YN (1994) Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76(3):477–491
Riobo NA (2012) Cholesterol and its derivatives in Sonic Hedgehog signaling and cancer. Curr Opin Pharmacol 12(6):736–741
Rios-Esteves J, Resh MD (2013) Stearoyl CoA desaturase is required to produce active lipid-modified Wnt proteins. Cell Rep 4(6):1072–1081
Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC (1997) Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell 90(4):707–716
Roessler E, Belloni E, Gaudenz K, Vargas F, Scherer SW, Tsui LC, Muenke M (1997) Mutations in the C-terminal domain of Sonic Hedgehog cause holoprosencephaly. Hum Mol Genet 6(11):1847–1853
Rohatgi R, Milenkovic L, Scott MP (2007) Patched1 regulates hedgehog signaling at the primary cilium. Science 317(5836):372–376
Roy M, Kung HJ, Ghosh PM (2011) Statins and prostate cancer: role of cholesterol inhibition vs prevention of small GTP-binding proteins. Am J Cancer Res 1(4):542–561
Sancho P, Burgos-Ramos E, Tavera A, Bou Kheir T, Jagust P, Schoenhals M, Barneda D, Sellers K, Campos-Olivas R, Grana O, Viera CR, Yuneva M, Sainz B Jr, Heeschen C (2015) MYC/PGC-1alpha balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab 22(4):590–605
Sarabi M, Vessby B, Millgard J, Lind L (2001) Endothelium-dependent vasodilation is related to the fatty acid composition of serum lipids in healthy subjects. Atherosclerosis 156(2):349–355
Scharenberg CW, Harkey MA, Torok-Storb B (2002) The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 99(2):507–512
Schiapparelli P, Shahi MH, Enguita-German M, Johnsen JI, Kogner P, Lazcoz P, Castresana JS (2011) Inhibition of the sonic hedgehog pathway by cyplopamine reduces the CD133+/CD15+ cell compartment and the in vitro tumorigenic capability of neuroblastoma cells. Cancer Lett 310(2):222–231
Schroeter EH, Kisslinger JA, Kopan R (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393(6683):382–386
Senyilmaz D, Virtue S, Xu X, Tan CY, Griffin JL, Miller AK, Vidal-Puig A, Teleman AA (2015) Regulation of mitochondrial morphology and function by stearoylation of TFR1. Nature 525(7567):124–128
Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS (2012) Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years’ follow up. BMC Cancer 12:25
Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S (1997) Skeletal and CNS defects in Presenilin-1-deficient mice. Cell 89(4):629–639
Shoji S, Kubota Y (1989) Function of protein myristoylation in cellular regulation and viral proliferation. Yakugaku Zasshi 109(2):71–85
Shyh-Chang N, Daley GQ, Cantley LC (2013) Stem cell metabolism in tissue development and aging. Development 140(12):2535–2547
Silvius JR (2003) Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochem Biophys Acta 1610(2):174–183
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387(6633):569–572
Song M, Lee H, Nam MH, Jeong E, Kim S, Hong Y, Kim N, Yim HY, Yoo YJ, Kim JS, Cho YY, Mills GB, Kim WY, Yoon S (2017) Loss-of-function screens of druggable targetome against cancer stem-like cells. FASEB J 31(2):625–635
Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, Rosato A, Piccolo S, Del Sal G (2014) Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 16(4):357–366
Southam CM (1961) Applications of immunology to clinical cancer. Past attempts and future possibilities. Cancer Res 21:1302–1316
Stoffel W, Schmidt-Soltau I, Jenke B, Binczek E, Hammels I (2017) Hair growth cycle is arrested in SCD1 deficiency by impaired wnt3a-palmitoleoylation and retrieved by the artificial lipid barrier. J Investig Dermatol 137(7):1424–1433
Suckling RJ, Korona B, Whiteman P, Chillakuri C, Holt L, Handford PA, Lea SM (2017) Structural and functional dissection of the interplay between lipid and Notch binding by human Notch ligands. EMBO J 36(15):2204–2215
Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S (2006) Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell 11(6):791–801
Tate R, Zona E, De Cicco R, Trotta V, Urciuoli M, Morelli A, Baiano S, Carnuccio R, Fuggetta MP, Morelli F (2017) Simvastatin inhibits the expression of stemnessrelated genes and the metastatic invasion of human cancer cells via destruction of the cytoskeleton. Int J Oncol 51(6):1851–1859
ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, Siu RK, Nusse R (2011) Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol 13(9):1070–1075
Thinon E, Serwa RA, Broncel M, Brannigan JA, Brassat U, Wright MH, Heal WP, Wilkinson AJ, Mann DJ, Tate EW (2014) Global profiling of co- and post-translationally N-myristoylated proteomes in human cells. Nat Commun 5:4919
Tirinato L, Liberale C, Di Franco S, Candeloro P, Benfante A, La Rocca R, Potze L, Marotta R, Ruffilli R, Rajamanickam VP, Malerba M, De Angelis F, Falqui A, Carbone E, Todaro M, Medema JP, Stassi G, Di Fabrizio E (2015) Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells 33(1):35–44
Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G (2007) Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 1(4):389–402
Torres CG, Olivares A, Stoore C (2015) Simvastatin exhibits antiproliferative effects on spheres derived from canine mammary carcinoma cells. Oncol Rep 33(5):2235–2244
UK_Cancer_Research (2018). https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer/incidence#heading-Zero
van Herwaarden AE, Wagenaar E, Merino G, Jonker JW, Rosing H, Beijnen JH, Schinkel AH (2007) Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol Cell Biol 27(4):1247–1253
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033
Vega-Naredo I, Loureiro R, Mesquita KA, Barbosa IA, Tavares LC, Branco AF, Erickson JR, Holy J, Perkins EL, Carvalho RA, Oliveira PJ (2014) Mitochondrial metabolism directs stemness and differentiation in P19 embryonal carcinoma stem cells. Cell Death Differ 21(10):1560–1574
Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, Yuan M, Asara JM, Wang YA, Heffernan TP, Kimmelman AC, Wang H, Fleming JB, Cantley LC, DePinho RA, Draetta GF (2014) Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 514(7524):628–632
Wang X, Venugopal C, Manoranjan B, McFarlane N, O’Farrell E, Nolte S, Gunnarsson T, Hollenberg R, Kwiecien J, Northcott P, Taylor MD, Hawkins C, Singh SK (2012) Sonic hedgehog regulates Bmi1 in human medulloblastoma brain tumor-initiating cells. Oncogene 31(2):187–199
Wang C, Li P, Xuan J, Zhu C, Liu J, Shan L, Du Q, Ren Y, Ye J (2017a) Cholesterol Enhances Colorectal Cancer Progression via ROS Elevation and MAPK Signaling Pathway Activation. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 42(2):729–742
Wang X, Huang Z, Wu Q, Prager BC, Mack SC, Yang K, Kim LJY, Gimple RC, Shi Y, Lai S, Xie Q, Miller TE, Hubert CG, Song A, Dong Z, Zhou W, Fang X, Zhu Z, Mahadev V, Bao S, Rich JN (2017b) MYC-regulated mevalonate metabolism maintains brain tumor-initiating cells. Cancer Res 77(18):4947–4960
Wang B, Rong X, Palladino END, Wang J, Fogelman AM, Martin MG, Alrefai WA, Ford DA, Tontonoz P (2018a) Phospholipid remodeling and cholesterol availability regulate intestinal stemness and tumorigenesis. Cell Stem Cell 22(2):206–220
Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, Somlo G, Jandial R, Ann D, Hanash S, Jove R, Yu H (2018b) JAK/STAT3-regulated fatty acid beta-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab 27(1):136–150
Warburg O (1956) On the origin of cancer cells. Science 123(3191):309–314
Whetton AD, Lu Y, Pierce A, Carney L, Spooncer E (2003) Lysophospholipids synergistically promote primitive hematopoietic cell chemotaxis via a mechanism involving Vav 1. Blood 102(8):2798–2802
Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR 3rd, Nusse R (2003) Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423(6938):448–452
Xiao X, Tang JJ, Peng C, Wang Y, Fu L, Qiu ZP, Xiong Y, Yang LF, Cui HW, He XL, Yin L, Qi W, Wong CC, Zhao Y, Li BL, Qiu WW, Song BL (2017) Cholesterol modification of smoothened is required for hedgehog signaling. Mol Cell 66(1):154–162
Yang Y, Gong L (2017) Palmitoleate inhibits insulin transcription by activating the ERK1/2 pathway in rat pancreatic beta-cells. Exp Ther Med 13(6):2805–2811
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST (2008) Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 13(2):153–166
Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, Ogata M, Katsuyama Y, Sadahiro H, Suzuki M, Owada Y (2016) Inhibition of fatty acid synthase decreases expression of stemness markers in glioma stem cells. PLoS ONE 11(1):e0147717
Ye J, DeBose-Boyd RA (2011) Regulation of cholesterol and fatty acid synthesis. Cold Spring Harbor Perspect Biol 3(7):a004754
Yeagle PL, Young JE (1986) Factors contributing to the distribution of cholesterol among phospholipid vesicles. J Biol Chem 261(18):8175–8181
Yi M, Li J, Chen S, Cai J, Ban Y, Peng Q, Zhou Y, Zeng Z, Peng S, Li X, Xiong W, Li G, Xiang B (2018) Emerging role of lipid metabolism alterations in Cancer stem cells. J Exp Clin Cancer Res 37(1):118
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X, Ratliff TL, Cheng JX (2014) Cholesteryl ester accumulation induced by PTEN loss and PI3 K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab 19(3):393–406
Zhang H, Li H, Ho N, Li D, Li S (2012) Scd1 plays a tumor-suppressive role in survival of leukemia stem cells and the development of chronic myeloid leukemia. Mol Cell Biol 32(10):1776–1787
Zhang H, Badur MG, Divakaruni AS, Parker SJ, Jager C, Hiller K, Murphy AN, Metallo CM (2016) Distinct metabolic states can support self-renewal and lipogenesis in human pluripotent stem cells under different culture conditions. Cell Rep 16(6):1536–1547
Zhang C, Skamagki M, Liu Z, Ananthanarayanan A, Zhao R, Li H, Kim K (2017a) Biological significance of the suppression of oxidative phosphorylation in induced pluripotent stem cells. Cell Rep 21(8):2058–2065
Zhang J, Song F, Zhao X, Jiang H, Wu X, Wang B, Zhou M, Tian M, Shi B, Wang H, Jia Y, Pan X, Li Z (2017b) EGFR modulates monounsaturated fatty acid synthesis through phosphorylation of SCD1 in lung cancer. Mol Cancer 16(1):127
Zhao J, Zhi Z, Wang C, Xing H, Song G, Yu X, Zhu Y, Wang X, Zhang X, Di Y (2017) Exogenous lipids promote the growth of breast cancer cells via CD36. Oncol Rep 38(4):2105–2115
Zheng B, Jarugumilli GK, Chen B, Wu X (2016) Chemical probes to directly profile palmitoleoylation of proteins. ChemBioChem 17(21):2022–2027
Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, Blau CA, Horwitz MS, Hockenbery D, Ware C, Ruohola-Baker H (2012) HIF1alpha induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J 31(9):2103–2116
Acknowledgements
This study was supported by the grant from National Research Foundation (NRF) of Korea (NRF-2018R1D1A1B07045153 and NRF-2015R1D1A1A01056594) funded by the Korean government. I appreciate Dr. YK Kim for the illustration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, WY. Therapeutic targeting of lipid synthesis metabolism for selective elimination of cancer stem cells. Arch. Pharm. Res. 42, 25–39 (2019). https://doi.org/10.1007/s12272-018-1098-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-018-1098-z