Abstract
Toxicarioside N (Tox N), a natural product extract from Antiaris toxicaria, has been reported to induce apoptosis in human gastric cancer cells. However, the mechanism and actual role of autophagy in Tox N-induced apoptosis of human gastric cancer cells remains poorly understood. In the current study, we demonstrated that Tox N could induce autophagy by inhibiting the Akt/mTOR signaling pathway in SGC-7901 cells. Moreover, we found that the inhibition of autophagy by 3-methyladenine, an autophagy inhibitor, enhanced Tox N-induced apoptotic cell death. However, the stimulation of autophagy by rapamycin, an autophagy activator, remarkably suppressed Tox N-induced apoptosis, suggesting that autophagy plays a protective role in Tox N-induced apoptosis. Thus, the results from this study suggested that Tox N combination with an autophagy inhibitor might be a promising strategy to enhance the anticancer activity of Tox N for the treatment of human gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer, a malignant disease with poor prognosis, is a most common cancer with high morbidity and mortality worldwide (Torre et al. 2015; Charalampakis et al. 2018). Due to lack of the specific symptoms in the early gastric cancer, most patients are found to be at advanced stages after diagnosis (Foukakis et al. 2007). Currently, the treatment of patients with advanced-stage gastric cancer is usually based on surgery associated with chemotherapy, but the treatment outcome for gastric cancer remains unsatisfactory (Van Cutsem et al. 2016). Therefore, there is an urgent demand to develop effective therapeutic agents with fewer side effects in advanced-stage gastric cancer treatment.
Medicinal plants are rich in bioactive compounds and over 60% of all currently used therapeutic agents originated from natural compounds (Cragg and Pezzuto 2016). Toxicarioside N (Tox N) is a natural product extracted from the seeds of Antiaris toxicaria in the Hainan province, China. Tox N is a cardenolide compound with a special structure that was first identified by our cooperative laboratory (Zuo et al. 2013). Traditionally, toxicaria compounds are used to treat congestive heart failure and arrhythmia (Newman et al. 2008; Shi et al. 2010). A recent study from our group showed that Tox N exhibits potent anticancer activity in the human gastric cancer cell line SGC-7901 (Zhao et al. 2018), yet the detailed mechanisms of cell death induced by Tox N are not well understood.
Autophagy is an evolutionarily conserved catabolic process that degrades and recycles cellular components, such as misfolded/unfolded proteins and unnecessary/damaged organelles, to maintain intracellular homeostasis and protect cells against environmental stresses. Recently, autophagy has gained increasing attention due to its important regulatory role in cell death (Levin-Salomon et al. 2014). Growing evidence has demonstrated that autophagy is related to various diseases including cancer (Chang et al. 2011; Zheng et al. 2012), and can be active in cancer cells treated with chemotherapeutic compounds (Cho et al. 2014). However, the functional role of autophagy in cancer cells is controversial. In most cases, autophagy protects cancer cells (Singh et al. 2018); however, in some conditions, autophagy activation can promote tumor cell death (Fulda and Kogel 2015). Generally, the induction of autophagy is tightly regulated by various signaling pathways, and one of them is the classic Akt/mTOR signaling pathway that causes the inhibition of autophagy (Zou et al. 2017). Although there is growing interest in modulating autophagy to treat cancer cells combined with anti-cancer agents, little is known about the regulatory role of autophagy in Tox N-induced apoptosis against human gastric cancer cells.
Our preliminary study showed that Tox N induced apoptosis in SGC-7901 cells by activating the p38 MAPK pathway (Zhao et al. 2018). In the present study, we demonstrated that Tox N induced protective autophagy in SGC-7901 cells by inhibiting the Akt/mTOR pathway. The inhibition of autophagy increased the Tox N-induced apoptosis in SGC-7901 cells, where its activation suppressed the cells apoptosis. Therefore, the com-bined treatment of Tox N with an autophagic inhibitor could enhance the efficiency of Tox N as an anti-cancer drug against human gastric cancer cells.
Materials and methods
Cell culture, reagents and antibodies
Human gastric cancer cell line SGC-7901 was purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences, Shanghai Institute of Cell Biology (Shanghai, China). Tox N was extracted and purified from the seeds of Antiaris toxicaria in our laboratory (Zuo et al. 2013). The purified Tox N was dissolved in DMSO (1 mg/mL) and stored at − 20 °C for subsequent experiments in this study. RPMI 1640, fetal bovine serum, streptomycin, and penicillin were purchased from Gibco (Grand Island, NY, USA). The Annexin V-FITC (fluorescein isothiocyanate)/PI (propidium iodide) Apoptosis kit was obtained from the Beyotime Institute of Biotechnology (Beyotime, Jiangsu, China). 3-MA and Rap were obtained from Sigma (St. Louis, MO, USA). The following antibodies were used in this study: the phosphorylated forms of Akt and mTOR were purchased from Cell Signaling Technology (Danvers, MA, USA), and the antibodies of SQSTM1/p62, LC3, Beclin-1, caspase-3 and PARP were purchased from Abcam (Cambridge, MA, USA).
Cell grouping and drug treatment
Cells were cultured in RPMI1640 supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. The experiment had the following six groups: control group, 3-MA group, Rap group, Tox N group, Tox N combined 3-MA group (Tox N + 3-MA), Tox N combined Rap group (Tox N + Rap). The drug dosage of the control group was zero; 5 mmol/L 3-MA was adopted in the 3-MA group; 100 nmol/L Rap was adopted in the Rap group; and 30 nM Tox N was adopted in the Tox N group.
Immunofluorescence staining for evaluation of autophagy
Cells were grown on sterile cover slips in a 6-well plate at a concentration of 0.5–1 × 105 cell/mL for 24 h. The cells were fixed with 4% paraformaldehyde for 30 min at room temperature, incubated with 0.1% Triton X-100 for permeabilization and then blocked with 5% skim milk in PBS for 30 min. After washing with PBS, the cells were incubated with LC3-II polyclonal antibody overnight at 4 °C. Subsequently, the treated cells were washed three times with PBS and incubated with Alexa Fluor 488—conjugated goat anti-rabbit IgG at room temperature for 1 h. Finally, the nuclei were counterstained with Hoechst for 5 min and observed under a confocal laser scanning microscope (Olympus Corporation, Tokyo, Japan).
Western blot
Cells were harvested and lysed in RIPA lysis buffer (Beyotime, Beijing, China) with protease inhibitors. The supernatant fractions were collected and centrifuged at 12,000×g for 15 min at 4 °C, and the protein concentration was determined by a bicinchoninic acid (BCA) kit (Beyotime, shanghai, China). Equal amounts of protein (40 μg/sample) were separated by 12% SDS-PAGE gels and transferred onto the PVDF membranes. The membranes were blocked with 5% non-fat milk at 37 °C for 1 h to block non-specific binding and were probed with different primary antibodies against specific proteins at 4 °C overnight. After being washed with PBST, the membranes were incubated with anti-IgG secondary antibody conjugated to horseradish peroxidase at room temperature for 1 h. The reactive proteins were observed by an ECL chemiluminescence system (Eastman-Kodak, NY, USA) after the final washing. The polyclonal antibody specific to β-actin was used as an internal control.
Apoptosis analysis
In this study, MTT, Hoechst 33,258, AnnexinV-FITC/PI and the western blot were used to analyze apoptosis as mentioned in our previous study (Zhao et al. 2018).
Treatment with Akt inhibitor
To further investigate whether the Akt/mTOR signaling pathway was involved in a Tox N-induced autophagy, SGC-7901 cells were treated with 8 mM MK-2206 (an Akt inhibitor), and incubated with Tox N for 24 h. The autophagy-related proteins were determined.
Statistical analysis
All experiments were repeated three times, independently, and the data were analyzed using the Graph Pad Prism software. Statistical analysis was employed using Student’s t test. A p** < 0.01 was considered to be statistically significant.
Results
Tox N induced autophagy in SGC-7901 cells
An increasing number of reports have suggested that appropriate autophagy can be considered as a target for anticancer therapy (Rubinsztein et al. 2012; Li et al. 2013). Therefore, we first investigated whether autophagy was induced by Tox N in SGC-7901 cells. SGC-7901 cells were treated with different concentrations of Tox N (0, 7.5, 15, 30 nM), and the western blot analysis was performed to detect the conversion of LC3-I to lipidated LC3-II, which is a hallmark of autophagy induction and is closely related to the autophagosomes membrane. Next, we detected the expression level of SQSTM1 and Beclin-1 (two autophagy related proteins). As shown in Fig. 1a, Tox N treatment markedly up-regulated LC3-II conversion, elevated the expression of Beclin1 and decreased the expression of SQSTM in a dose-dependent manner. To examine the effect of Tox N on autophagy, we also treated cells with 30 nM Tox N for different lengths of time. The results shown in Fig. 1b indicated that the Tox N-induced autophagy occurred in a time-dependent manner.
Tox N induced autophagy in SGC-7901 cells. a The expression levels of LC3-II/LC3-I, Beclin1 and SQSTM1 were examined after the cells were treated with different concentrations of Tox N for 24 h. b The expression level of LC3-II/LC3-I, Beclin1 and SQSTM1 were examined after cells were treated with 30 nM Tox N at different time points. c SGC-7901 cells were treated with 0, 7.5, 15 and 30 nM Tox N for 24 h and stained with anti-LC3 antibody to detect the LC3 puncta and Hoechst for nuclei by immunofluorescence used a confocal microscopy. d The numbers of LC3-II positive cells in each group were counted from at least 100 random fields. All the data shown in this figure are representative of at least three independent experiments. **p < 0.01
The autophagy induction was further evaluated by monitoring the distribution of endogenous LC3 puncta using immunofluorescence staining. Confocal microscopic analysis indicated that after being incubated with Tox N, the LC3 green fluorescent puncta numbers in cytoplasm increased significantly with increasing concentrations of Tox N (Fig. 1c), and the numbers of LC3 puncta were increased compared with those of the control groups in SGC-7901 cells (Fig. 1d).
The effect of Tox N on autophagic flux in SGC-7901 cells
Autophagy consists of a multistep process, including the formation of autophagosome and their fusion with lysosomes to form autophagy lysosomes and degradation (Fulda and Kogel 2015). Thus, the increase in the level of LC3-II and the number of LC3-containing autophagosomes after Tox N treatment could be attributed to either increased autophagic capacity at an early step or the decreased turnover of autophagosomes at a late stage. Therefore, we used autophagy inhibitors 3-MA and CQ to investigate the effect of Tox N on autophagic flux in SGC-7901 cells. As shown in Fig. 2a, we found that the combinatorial treatment with 3-MA significantly decreased the LC3-II conversion in Tox N-treated cells and suppressed the Tox N-induced formation of LC3 puncta (Fig. 2b, c). The results indicated that Tox N induced the formation of autophagosomes in the SGC-7901 cells. The treatment with CQ, a lysosomal inhibitor, led to the accumulation of SQSTM1 and LC3-II conversion (Fig. 2d), and significantly increased the endogenous LC3-II accumulation (Fig. 2e, f). Altogether, these results suggested that Tox N induced autophagic flux in SGC-7901 cells.
Tox N induced autophagy flux in SGC-7901 cells. a SGC-7901 cells were treated with Tox N (30 nM) in the absence or presence of 3-MA for 24 h, and the cell lysates were used to detect the expression of LC3-I and LC3-II by Western blotting. b SGC-7901 cells were treated with 30 nM Tox N in the absence or presence of 3-MA for 24 h and stained with anti-LC3 antibody to detect the LC3 puncta and Hoechst for nuclei by immunofluorescence using confocal microscopy. c The numbers of LC3-II positive cells in each group were counted from at least 100 random fields. d SGC-7901 cells were treated with Tox N (30 nM) in the absence or presence of CQ for 24 h, and the cell lysates were used to detect the expression of LC3-I and LC3-II and SQSTM1 by western blot. e SGC-7901 cells were treated with 30 nM Tox N in the absence or presence of CQ for 24 h and stained with anti-LC3 antibody to detect the LC3 puncta and Hoechst for nuclei by immunofluorescence used a confocal microscopy. f The numbers of LC3-II positive cells in each group were counted from at least 100 random fields. All the data shown in this figure are representative of at least 3 independent experiments, **p < 0.01
Tox N induced a protective autophagy in SGC-7901 cells
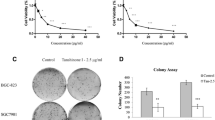
In our previous study, we demonstrated that Tox N induced apoptosis in SGC-7901 cells through multiple methods (Zhao et al. 2018). In the present study, to explore the role that autophagy plays in Tox N-induced apoptotic cell death, an autophagy inhibitor 3-MA and an autophagy activator Rap were used. As shown in Fig. 3a, b, a combinatorial treatment with 3-MA can enhance the inhibition ratio of cell proliferation, and cell typical apoptotic morphology cells caused by Tox N became more pronounced after 3-MA pretreatment (Fig. 3c, d). Then, AnnexinV-FITC/PI double staining also showed that the pretreatment of 3-MA enhanced Tox N-induced apoptosis (Fig. 3e, f). Moreover, apoptosis-associated proteins were determined by western blot analysis, as shown in Fig. 3g, h, and the cleaved forms of PARP and caspase-3 were potentiated by autophagy inhibition. In contrast, Rap attenuated Tox N-induced apoptotic cell death. Overall, the results indicated that Tox N-induced autophagy plays a protective role against Tox N-induced apoptosis in human gastric cancer cells.
Autophagy regulated Tox N-induced apoptosis in SGC-7901 cells. a SGC-7901 cells were treated with control, 3-MA, Rap in the presence or absence of Tox N (30 nM) for 24 h. Cell viability was measured by MTT assay. b Cell proliferation was measured by colony formation assay. c, d Typical cell apoptotic morphology was observed. e, f Apoptosis was determined using an Annexin-V-FITC/PI double staining assay. g, h The expression of caspase-3, cleaved caspase-3, PARP and cleaved PARP were assessed by western blot analysis. All the data shown in this figure are representative of at least three independent experiments, ** p < 0.01
Tox N induced autophagy by inhibiting the Akt/mTOR pathway
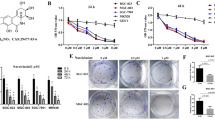
The Akt/mTOR signaling pathway has been considered as a major negative regulator of cellular autophagy (Zou et al. 2017). To determine the possible mechanism of Tox N-mediated autophagy in SGC-7901 cells, we measured the expression levels of the phosphorylated forms of Akt/mTOR pathway-related proteins, including p-Akt, p-mTOR. As shown in Fig. 4a, the expression of phosphorylated Akt and mTOR were decreased in SGC-7901 cells after Tox N treatment, suggesting that the Akt/mTOR signaling pathway was involved in Tox N-induced autophagy. The results indicated that Tox N suppressed the Akt/mTOR pathway in SGC-7901 cells and was involved in Tox N-induced autophagy in SGC-7901 cells.
Tox N induced autophagy by inhibiting the Akt-mTOR pathway. a SGC-7901 cells were treated with the indicated concentrations of Tox N for 24 h. Cell lysates were used to detect the expression of phosphorylated Akt, mTOR. b Cells were treated with or without Tox N (30 nM) in combination with MK-2206 (an Akt inhibitor) for 24 h. Cell lysates were used to detect the expression of phosphorylated Akt (P-Akt), LC3-1 and LC3-II, respectively. c, d Cells were treated with control or Tox N (30 nM) in combination with or without MK-2206 for 24 h. Cells were stained with ant-LC3 antibody for detection of LC3 puncta and Hoechst for nuclei by confocal microscopy. All the data shown in this figure are representative of at least three independent experiments, **p < 0.01
To further verify whether the suppression of the Akt/mTOR pathway is involved in Tox N-induced autophagy, we inhibited the Akt/mTOR pathway by MK-2206 in SGC-7901 cells. The effects of MK-2206 on the role of autophagy were examined, as shown in Fig. 4b. Akt inhibition by MK-2206 produced markedly increased the level of LC3-II conversion in the Tox N-treated cells compared to that in the controls, and the LC3 green fluorescent puncta numbers in the cytoplasm increased significantly after incubation with MK-2206. The results indicated that the Akt/mTOR signaling pathway was involved in Tox N-induced autophagy in the SGC-7901 cells.
Discussion
Tox N, as a natural cardenolide glycoside, is extracted from the seeds of Antiaris toxicaria, which is widespread throughout many tropical areas in the Southeast Asia. Traditionally, cardenolide glycosides are used in the treatment of congestive heart failure and as anti-arrhythmic agents (Newman et al. 2008; Shi et al. 2010). However, recent studies have demonstrated that certain cardenolides extracted from some plants and animals exhibit anticancer activities in various cancer cell lines (Prassas and Diamandis 2008; Prassas et al. 2011). The reports from our group have proved that cardenolide compounds isolated from the latex and seeds of Antiaris toxicaria possess significant cytotoxicity (Huang et al. 2012; Zhao et al. 2018). Our previous study showed that Tox N exhibited the potential anticancer activities against human gastric cancer SGC-7901 cells. However, whether Tox N could trigger autophagy in SGC-7901 cells and the role of autophagy in Tox N-induced apoptosis still must be elucidated.
There is some evidence that many antitumor agents can induce autophagy in various cancer cells (Huang et al. 2017; Brothers et al. 2018). The role of autophagy on the survival of cancer cells is paradoxical. Autophagy can promote cancer cell survival by removing dysfunctional organelles and recycling nutrients upon anticancer treatment. However, accumulating evidence indicates that numerous chemotherapeutic agents may trigger autophagic cell death, and thus, increase the efficacy of chemotherapeutic efficacy in cancer therapy (Dou et al. 2016; Liu et al. 2017). Therefore, further studies are required to explore the exact role of autophagy in Tox N-induced apoptosis. In the present study, we demonstrated that Tox N was able to induce autophagy in SGC-7901 cells and that autophagy played a protective role of in Tox N-induced apoptosis.
In the present study, we observed that the formation of LC3 puncta aggregation increased in cytoplasm of SGC-7901 cells after Tox N treatment and that the protein levels of LC3-II, a reliable marker of autophagy (Tanida and Waguri 2010), were markedly up-regulated after cell treatment with Tox N. Moreover, the protein level of SQSTM1, a substrate of autophagy (Vitale et al. 2015), was significantly down-regulated, indicating that autophagy was triggered by this drug. To the best of our knowledge, these results demonstrated for the first time that Tox N induced autophagy in SGC-7901 cells. To testify the exact effect of autophagy in Tox N-induced apoptosis, we used the autophagy inhibitor 3-MA and autophagy inducer Rap to regulate the level of autophagy in the present study. According to the analyses, the pretreatment with 3-MA could effectively increase the inhibitory effect of cell proliferation and up-regulate the levels of cleaved caspase-3 and cleaved PARP. Conversely, Rap attenuated Tox N-induced cell apoptosis. These results suggested that Tox N-induced autophagy plays a protective role in SGC-7901 cells and that the combinatory use of an autophagic inhibitor may improve Tox N-induced apoptotic cell death in SGC-7901 cells.
Multiple signaling pathways have been proved to be involved in autophagy; the Akt/mTOR pathway is one pathway, and is well-known as the major negative regulator of autophagy (Zou et al. 2017). Accumulating reports indicate that extracts from Chinese medicines have anticancer effects by inducing autophagy and apoptosis through blocking the Akt/mTOR signaling pathway (Lee et al. 2014; Zhang et al. 2015).The results from the present study showed that the phosphorylation levels of Akt and mTOR were significantly decreased after cells were incubated with Tox N in SGC-7901 cells, which suggested that the Akt/mTOR signaling pathway was involved in the autophagic process induced by Tox N. To better understand the Tox N-induced autophagy in SGC-7901 cells through blocking the Akt/mTOR signaling pathway, the effects of Akt inhibitor (MK-2206) on the Akt/mTOR signaling pathway were evaluated. The results showed that the inhibition of Akt/mTOR by MK-2206 substantially activated Tox N-induced autophagy, and Tox N-induced autophagy by blocking the Akt/mTOR signaling pathway.
Thus, the results of the present study revealed that Tox N could induce autophagy by blocking the Akt/mTOR signaling pathway in SGC-7901 cells and that autophagy played a protective role in SGC-7901 cells. These findings suggest that the combined autophagy inhibitor with Tox N treatment can be a promising and effective therapy to treat human gastric malignancies.
References
Brothers KM, Kowalski RP, Tian S, Kinchington PR, Shanks RMQ (2018) Bacteria induce autophagy in a human ocular surface cell line. Exp Eye Res 168:12–18
Chang YT, Tseng HC, Huang CC, Chen YP, Chiang HC, Chou FP (2011) Relative down-regulation of apoptosis and autophagy genes in colorectal cancer. Eur J Clin Invest 41:84–92
Charalampakis N, Economopoulou P, Kotsantis I, Tolia M, Schizas D, Liakakos T, Elimova E, Ajani JA, Psyrri A (2018) Medical management of gastric cancer: a 2017 update. Cancer Med 7:123–133
Cho KH, Park JH, Kwon KB, Lee YR, So HS, Lee KK, Lee SY, Moon SR, Yang SH (2014) Autophagy induction by low-dose cisplatin: the role of p53 in autophagy. Oncol Rep 31:248–254
Cragg GM, Pezzuto JM (2016) Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract. 2:41–59
Dou Q, Chen HN, Wang K, Yuan K, Lei Y, Li K, Lan J, Chen Y, Huang Z, Xie N, Zhang L, Xiang R, Nice EC, Wei Y, Huang C (2016) Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. Cancer Res 76:4457–4469
Foukakis T, Lundell L, Gubanski M, Lind PA (2007) Advances in the treatment of patients with gastric adenocarcinoma. Acta Oncol 46:277–285
Fulda S, Kögel D (2015) Cell death by autophagy: emerging molecular mechanisms and implications for cancer therapy. Oncogene 34:5105–5113
Huang FY, Mei WL, Li YN, Tan GH, Dai HF, Guo JL, Wang H, Huang YH, Zhao HG, Zhou SL, Lin YY (2012) Toxicarioside A inhibits tumor growth and angiogenesis: involvement of TGF-beta/endoglin signaling. PLoS ONE 7:e50351
Huang YH, Sun Y, Huang FY, Li YN, Wang CC, Mei WL, Dai HF, Tan GH, Huang C (2017) Toxicarioside O induces protective autophagy in a sirtuin-1-dependent manner in colorectal cancer cells. Oncotarget. 8:52783–52791
Lee HW, Jang KS, Choi HJ, Jo A, Cheong JH, Chun KH (2014) Celastrol inhibits gastric cancer growth by induction of apoptosis and autophagy. BMB Rep. 47:697–702
Levin-Salomon V, Bialik S, Kimchi A (2014) DAP-kinase and autophagy. Apoptosis 19:346–356
Li X, Xu HL, Liu YX, An N, Zhao S, Bao JK (2013) Autophagy modulation as a target for anticancer drug discovery. Acta Pharmacol Sin 34:612–624
Liu C, Liao JZ, Li PY (2017) Traditional Chinese herbal extracts inducing autophagy as a novel approach in therapy of nonalcoholic fatty liver disease. World J Gastroenterol 23:1964–1973
Newman RA, Yang P, Pawlus AD, Block KI (2008) Cardiac glycosides as novel cancer therapeutic agents. Mol Interv. 8:36–49
Prassas I, Diamandis EP (2008) Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 7:926–935
Prassas I, Karagiannis GS, Batruch I, Dimitromanolakis A, Datti A, Diamandis EP (2011) Digitoxin-induced cytotoxicity in cancer cells is mediated through distinct kinase and interferon signaling networks. Mol Cancer Ther 10:2083–2093
Rubinsztein DC, Codogno P, Levine B (2012) Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 11:709–730
Shi LS, Liao YR, Su MJ, Lee AS, Kuo PC, Damu AG, Kuo SC, Sun HD, Lee KH, Wu TS (2010) Cardiac glycosides from Antiaris toxicaria with potent cardiotonic activity. J Nat Prod 73:1214–1222
Singh SS, Vats S, Chia AY, Tan TZ, Deng S, Ong MS, Huang RY, Shen HM, Manjithaya R, Kumar AP (2018) Dual role of autophagy in hallmarks of cancer. Oncogene 37:1142–1158
Tanida I, Waguri S (2010) Measurement of autophagy in cells and tissues. Methods Mol Biol 648:193–214
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulen J, Jemal A (2015) Global cancer statistics, 2012. Cancer Epidemiol Biomark Prev 65:87–108
Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H (2016) Gastric cancer. Lancet 388:2654–2664
Vitale I, Manic G, Dandrea V, De Maria R (2015) Role of autophagy in the maintenance and function of cancer stem cells. Int J Dev Biol 59:95–108
Zhang Y, Zhou ZW, Jin H, Hu C, He ZX, Yu ZL, Ko KM, Yang T, Zhang X, Pan SY, Zhou SF (2015) Schisandrin B inhibits cell growth and induces cellular apoptosis and autophagy in mouse hepatocytes and macrophages: implications for its hepatotoxicity. Drug Des Devel Ther. 9:2001–2027
Zhao HG, Zhou SL, Lin YY, Dai HF, Huang FY (2018) Toxicarioside N induces apoptosis in human gastric cancer SGC-7901 cell by activating the p38MAPK pathway. Arch Pharm Res. 41:71–78
Zheng HY, Zhang XY, Wang XF, Sun BC (2012) Autophagy enhances the aggressiveness of human colorectal cancer cells and their ability to adapt to apoptotic stimulus. Cancer Biol Med. 9:105–110
Zou N, Wei Y, Li F, Yang Y, Cheng X, Wang C (2017) The inhibitory effects of compound Muniziqi granule against B16 cells and harmine induced autophagy and apoptosis by inhibiting Akt/mTOR pathway. BMC Complement Altern Med. 17:517
Zuo WJ, Dong WH, Jing C, Zhao YX, Chen HQ, Mei WL, Dai HF (2013) Two new strophanthidol cardenolides from the seeds of Antiaris toxicaria. Phytochem Lett 6:1–4
Acknowledgements
The authors acknowledge the financial support provided by The National Natural Science Foundation of China, Project Nos 81560484 and 81460557, Hainan province natural science foundation of China, Project No. 817147.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhao, HG., Zhou, SL., Lin, YY. et al. Autophagy plays a protective role against apoptosis induced by toxicarioside N via the Akt/mTOR pathway in human gastric cancer SGC-7901 cells. Arch. Pharm. Res. 41, 986–994 (2018). https://doi.org/10.1007/s12272-018-1049-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-018-1049-8