Abstract
Tamsulosin, a selective antagonist of the α1-adrenoceptor, is primarily metabolized by CYP3A4 and CYP2D6, and tamsulosin exposure is significantly increased according to the genetic polymorphism of CYP2D6. In this study, we investigated the effects of diltiazem, a moderate inhibitor of CYP3A4, on the pharmacokinetics of tamsulosin in subjects with different CYP2D6 genotypes. Twenty-three healthy Korean male subjects with CYP2D6*wt/*wt (*wt = *1 or *2) and CYP2D6*10/*10 were enrolled in the prospective, open-label, two-phase parallel pharmacokinetic study. On the first day of study (day 1), each subject received a single 0.2 mg oral dose of tamsulosin. After a washout period of 1 week, on day 8, the subjects were given a 60 mg oral dose of diltiazem three times daily for four days. On day 10, 1 h after the morning dose of diltiazem, they received a single 0.2 mg oral dose of tamsulosin. The pharmacokinetic parameters of tamsulosin in those with and without diltiazem treatment were compared in subjects with different CYP2D6 genotypes. After diltiazem treatment, the Cmax and AUCinf of tamsulosin in each CYP2D6 genotype group were significantly increased (p < 0.0001 for all). The CL/F of tamsulosin was also significantly decreased after diltiazem treatment (both p < 0.0001). However, diltiazem did not affect the t1/2 of tamsulosin in each genotype group. In conclusion, diltiazem significantly increases exposure to tamsulosin regardless of the genotype of CYP2D6. Dose adjustment in the daily maintenance dose of tamsulosin may improve tolerability and safety in patients receiving diltiazem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tamsulosin ((R)-5-(2-{[2-(2-Ethoxyphenoxy)ethyl]amino}propyl)-2-methoxybenzene-1-sulfonamide) is a selective antagonist of the α1-adrenoceptor that has a relatively high affinity for the α1A-subtype and, to a lesser extent, for the α1D-subtype adrenoceptor (Michel et al. 1998; Taguchi et al. 1998). Because the α1A-adrenoceptor is the most abundant and functionally important subtype in the human prostate (Michel and Vrydag 2006), tamsulosin is primarily used to treat lower urinary tract symptoms that suggest benign prostatic hyperplasia (BPH).
Tamsulosin is well absorbed in humans with almost 100% bioavailability when administered as a modified-released (MR) formulation under fasting conditions (van Hoogdalem et al. 1997). It undergoes hepatic metabolism in humans, resulting in five primary metabolites, M-1 to M-4 and AM-1 (Soeishi et al. 1996). Among these, cytochrome P450 (CYP) 3A4 and CYP2D6 form the M-1, M-2 and AM-1 and M-3 and M-4 metabolites (Kamimura et al. 1998), respectively, which are further metabolized to glucuronide or sulfate conjugated forms (Soeishi et al. 1996). Except for AM-1, all metabolites have a high affinity for the α1-adrenoceptor, but all are less pharmacologically active than the parent compound (Taguchi et al. 1997). In addition, the metabolites are present in low abundance (~ 9%) in plasma; thus, the pharmacokinetics of tamsulosin is responsible for the overall pharmacological effects of the drug (Soeishi et al. 1996).
BPH is a common condition in elderly men; thus, patients using tamsulosin may also receive concomitant medications for other conditions including cardiovascular, pulmonary, and/or gastrointestinal diseases (Franco-Salinas et al. 2010). Therefore, tamsulosin may be subject to drug-drug interactions, especially when co-administered with CYP3A4 or CYP2D6 inhibitors or inducers. Several in vitro and in vivo studies of tamsulosin drug-drug interactions have been reported. An in vitro study with human liver microsomes showed that ketoconazole, erythromycin, and verapamil, which are specific inhibitors of CYP3A4, substantially reduced the formation of the tamsulosin metabolites AM-1, M-1, and M-2. Meanwhile, quinidine, a specific inhibitor of CYP2D6, extensively inhibited M-3 and M-4 formation (Kamimura et al. 1998). In an in vivo study, the CYP inhibitor cimetidine significantly altered oral clearance and the area under the plasma concentration–time curve from time zero extrapolated to infinity (AUCinf) for tamsulosin (Miyazawa et al. 2002). In addition, co-administration of ketoconazole (a potent CYP3A4 inhibitor) and paroxetine (a potent CYP2D6 inhibitor) were each shown to significantly increase the mean values of peak plasma concentration (Cmax) and AUCinf of tamsulosin. Ketoconazole increased the AUCinf of tamsulosin by almost three-fold over tamsulosin alone (Troost et al. 2011). More recently, the drug-drug interaction between tamsulosin and two 5α-reductase inhibitors, finasteride (Chu et al. 2015) and dutasteride (Li et al. 2015), were investigated. These two drugs are both established substrates for CYP3A4, but the combination did not show significant alteration of tamsulosin pharmacokinetics.
Diltiazem, a calcium channel blocker, is widely used to treat mild-to-moderate hypertension, chronic stable and variant angina pectoris, supraventricular arrhythmia, and other cardiovascular disorders (Chaffman and Brogden 1985). Previous studies have reported that diltiazem inhibits the CYP3A4-dependent metabolism of drugs such as cyclosporine, midazolam, and triazolam (Backman et al. 1994; Bleck et al. 1996; Varhe et al. 1996).
The CYP enzymes are genetically polymorphic, and pharmacokinetics and pharmacodynamics of many substrate drugs were affected in various degrees by these genetic polymorphisms (Ma et al. 2012; Hirota et al. 2013; Preissner et al. 2013; Chaudhry et al. 2014; Probst-Schendzielorz et al. 2015; Byeon et al. 2015; Lee et al. 2016; Kim et al. 2017, 2018). Tamsulosin exposure is also significantly increased based on genetic polymorphism of CYP2D6 (Choi et al. 2012). Thus, the pharmacokinetic drug interactions of tamsulosin can vary according to the genetic polymorphisms of CYP2D6. In Korean population, two functional alleles (CYP2D6*1 and *2) and one reduced functional variant allele (CYP2D6*10) account for 93.9% of the allele frequency (Unpublished data). In the present study, the effects of diltiazem, a moderate CYP3A4 inhibitor, on the pharmacokinetics of tamsulosin were evaluated in subjects with CYP2D6*wt/*wt (*wt = *1 or *2) and *10/*10 genotypes.
Materials and methods
Subjects
Twenty-three healthy Korean male subjects with different CYP2D6 genotypes, 13 subjects with CYP2D6*wt/*wt, and 10 with CYP2D6*10/*10, participated in this study. Analyses of various CYP2D6 genotypes were performed by polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP) and long-range PCR methods, as previously described (Johansson et al. 1994, 1996; Mendoza et al. 2001). Written informed consent was obtained from all subjects prior to the study. All subjects were in good health as determined by their medical histories, physical examinations and routine laboratory tests (blood chemistry, hematology, and urine analysis). Subjects were not permitted to take any medication, consume alcohol or caffeine-containing beverages, or smoke for 7 days before and during the study. The study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the School of Pharmacy, Sungkyunkwan University, Suwon, Korea.
Study protocol
Prospective, open-label, two-phase parallel pharmacokinetic study was performed. On the first day of study (phase 1), each subject received a single 0.2 mg oral dose of tamsulosin (Harnal-D® 0.2 mg orally disintegrating tablet; Astellas Pharma Inc. Korea, Seoul, Korea) with 150 ml of water after an overnight fast. After a washout period of 1 week, in phase 2, the subjects were given a 60 mg oral dose of diltiazem starting on day 8 (Herben® 30 mg tablet (2 tablets); CJ Pharma, Seoul, Korea) three times daily for 4 days. This dose was previously shown to achieve full-inhibition of intestinal or hepatic CYP3A4. On day 10, a single 0.2 mg oral dose of tamsulosin was administered 1 h after the morning dose of diltiazem with 150 ml water, to avoid competition in the intestinal absorption phase, after an overnight fast. A fasting state was maintained for 4 h after administering the tamsulosin. Venous blood samples (7 ml) were obtained before and 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36 and 48 h after tamsulosin administration. All blood samples were collected in sodium heparin tubes and were centrifuged immediately. The separated plasma samples were stored at − 70 °C until needed. The blood pressure and heart rate of each subject was measured immediately after each blood sampling in the same arm (from 0 to 24 h after tamsulosin administration) by the same investigator using an automatic electronic manometer.
Determination of plasma tamsulosin concentrations
Plasma concentrations of tamsulosin were determined using a validated high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (Choi et al. 2012). To each 500 μl plasma sample, 10 μl of an internal standard solution (diphenhydramine 100 ng/ml in methanol) was added. After vortex mixing, 2 ml of methyl tert-butyl ether was added, and the solution was vigorously mixed for 30 s. Each sample was centrifuged at 3000 rpm for 10 min. The organic layer was transferred to a clean glass tube and evaporated at 50 °C under a mild stream of nitrogen gas. The residue was reconstituted with 300 μl mobile phase and a 10 μl sample was injected for LC-MS/MS analysis. The calibration standard curve of tamsulosin ranged from 0.01 to 20 ng/ml, with a lower limit of quantification (LLOQ) of 0.01 ng/ml. The accuracy and precision of this analytical method were determined by the replication of five sets of QC samples with four different tamsulosin concentrations (0.01, 0.03, 0.9 and 18 ng/ml) within 1 day or on five consecutive days. The intra-day and inter-day mean accuracy for tamsulosin were 97.2–106.2 and 98.4–103.7%, respectively. The coefficients of variation (intra-day and inter-day validation) were 1.7–6.0 and 1.8–5.3%, respectively.
Pharmacokinetic analysis
The pharmacokinetic parameters were estimated using non-compartmental methods with the BA Calc 2007 analysis program (KFDA, Seoul, Korea). Actual blood sampling times were used for the analysis. Observed values were used for Cmax and time to reach Cmax (tmax). Area under the plasma concentration–time curve (AUC) was calculated using the linear trapezoidal rule. The elimination rate constant (ke) was estimated from the least-squares regression slope of the terminal plasma concentration. The AUCinf was calculated by AUCinf = AUC + Ct/ke (Ct is the last plasma concentration measured). The elimination half-life (t1/2) was calculated as ln 2/ke, and the apparent oral clearance (CL/F) of tamsulosin was calculated by CL/F = dose/AUCinf.
Statistical analysis
The number of subjects in each treatment group was estimated to be sufficient to detect a 50% difference in the AUCinf of tamsulosin between groups with a statistical power of at least 80% (α level of 0.05). Power and sample size were calculated with the Power and Sample Size Program, PS (version 3.1.2) (Dupont and Plummer 1998). All pharmacokinetic data were expressed as the mean ± standard deviation (SD), except for tmax, which was presented as the median value and range. Differences in pharmacokinetic parameters of tamsulosin with and without diltiazem treatment were assessed using paired t test or Wilcoxon signed-rank sum test after normality and equal variance tests. The pharmacokinetic parameters of tamsulosin between CYP2D6*wt/*wt subjects without diltiazem treatment and CYP2D6*10/*10 subjects with diltiazem treatment were compared using Student’s t test or Mann–Whitney rank sum test as appropriate. Data were analyzed using the statistical program Sigmastat for Windows (version 11, Systat Software Inc., Chicago, IL). The p values of less than 0.05 were considered statistically significant.
Results
All subjects completed the study according to the protocol, and no clinically undesirable signs and/or symptoms that could be attributed to the administration of tamsulosin were observed throughout the study period. The mean age, height, and body weight of these subjects were 22.5 ± 1.8 years, 176.5 ± 5.6 cm, and 70.1 ± 7.3 kg, respectively.
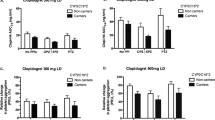
Mean plasma concentration–time profiles of tamsulosin in two different CYP2D6 genotype groups after a single oral 0.2 mg dose of tamsulosin with and without diltiazem treatment were depicted in Fig. 1. In subjects with the CYP2D6*wt/*wt genotype, the Cmax and AUCinf of tamsulosin were increased by 1.7- and 1.6-fold, respectively, and the CL/F of tamsulosin was decreased by 40% after diltiazem treatment (p < 0.0001 for all). In subjects with the CYP2D6*10/*10 genotype, the Cmax and AUCinf of tamsulosin were increased by 1.6-fold and 1.9-fold, respectively, and the CL/F of tamsulosin was decreased by 48% after diltiazem treatment (p < 0.0001 for all). However, these changes between the two different genotype groups were not statistically different. Changes in the t1/2 of tamsulosin due to diltiazem co-administration were not significant in either genotype group (Table 1).
The CYP2D6*10/*10 subjects with diltiazem treatment showed 2.0-fold higher Cmax, 1.5-fold longer t1/2, 68% lower CL/F, and 3.0-fold higher AUCinf values for tamsulosin than those in CYP2D6*wt/*wt subjects without diltiazem treatment, respectively (p < 0.0001 for all) (Table 1, Fig. 2).
Discussion
Tamsulosin is primarily indicated for treating BPH, which occurs only in men. Thus, our study exclusively included male subjects. Indirect comparisons of several previous studies showed that young healthy subjects (aged 18–44 years) had similar pharmacokinetics as elderly subjects (aged > 60 years) in the fasted state (Franco-Salinas et al. 2010). Considering these results, the ages of the subjects (19–27 years) included in our pharmacokinetic study were acceptable. In addition, although the recommended tamsulosin dose in many Western countries is 0.4 mg/day (FLOMAX label information 2016), 0.2 mg/day is preferred in Asian countries (HARNAL-D label information 2017). Several studies have confirmed that 0.2 mg of tamsulosin/day is effective in treating BPH in Asians, including Koreans, which might be due to relatively small body weights compared to Caucasians (Lee 2003; Li et al. 2003; Park et al. 2004). Therefore, we used 0.2 mg/day in this study.
Diltiazem co-administration significantly affected tamsulosin pharmacokinetics. Higher Cmax and AUC values and a lower CL/F for tamsulosin were observed after diltiazem treatment in each CYP2D6 genotype group (p < 0.0001 for all). The CYP3A subfamily is mainly expressed and localized in the liver and intestinal epithelium. The total CYP3A content in the small intestine is estimated to be ~ 1% of that in the liver. However, enteric CYP3A is known to significantly contribute to the overall first-pass metabolism of several drugs, such as verapamil, midazolam, and nifedipine (von Richter et al. 2001; Pinto et al. 2005; Zhang et al. 2007). In addition, semiphysiologically-based pharmacokinetic models have shown that five oral doses of 60 mg of diltiazem three times daily inactivates a maximum of 55% and 90% liver and gut wall CYP3A4, respectively (Zhang et al. 2009). Based on these findings, we speculate that the effects of diltiazem on tamsulosin plasma concentrations can be attributed to both hepatic metabolism and intestinal absorption.
Although diltiazem is known to be both a substrate for and an inhibitor of CYP3A4, diltiazem is also metabolized by CYP2D6 isozyme (Molden et al. 2000, 2002). Because tamsulosin is primarily metabolized via CYP3A4 and CYP2D6 (Kamimura et al. 1998), it is possible that diltiazem may inhibit the biotransformation of tamsulosin via competitive inhibition of CYP2D6. However, an earlier in vitro study showed that the IC50 of diltiazem for CYP2D6 activity inhibition was above 150 μM (Ma et al. 2000). Considering the range of diltiazem (40–200 ng/ml) for therapeutic plasma levels, it is unlikely that diltiazem inhibits CYP2D6 significantly in clinical situations.
A previous study reported that CYP2D6 EM subjects who administered ketoconazole (a potent CYP3A4 inhibitor) or paroxetine (a potent CYP2D6 inhibitor) concomitantly had a 2.8- and 1.6-fold higher tamsulosin AUC, respectively (Troost et al. 2011). The U.S. FDA stated that patients known to be CYP2D6 poor metabolizers (PMs) have the potential for a significant increase in tamsulosin exposure when it is co-administered with a strong CYP3A4 inhibitor (FLOMAX label information 2016). In this study, although diltiazem is a moderate CYP3A4 inhibitor, it may also markedly increase tamsulosin plasma exposure in carriers of CYP2D6*wt/*wt and CYP2D6*10/*10. The effects of diltiazem on the pharmacokinetics of tamsulosin were not dependent on CYP2D6 genotype. However, it is noted that the subjects carrying the CYP2D6*10/*10 genotype with diltiazem treatment had around three-fold higher AUCinf values of tamsulosin compared with the CYP2D6*wt/*wt subjects without diltiazem treatment (Table 1, Fig. 2c, p < 0.0001). This indicates that the CYP2D6 genotype and diltiazem co-administration have an additive effect on the pharmacokinetics of tamsulosin.
As mentioned above, elderly patients who are prescribed tamsulosin may also receive concomitant drugs to treat co-existing conditions. For example, patients suffering from hypertension, angina, and/or arrhythmia may be prescribed diltiazem and tamsulosin. This combination causes prolonged plasma exposure to tamsulosin, leading to an increased risk of unexpected and occasionally life-threatening adverse drug events including hypotension, dizziness, fainting, hepatopathy, and jaundice (HARNAL-D label information 2017). Furthermore, caution is advised with co-administration of tamsulosin and agents with vasodilating effect, such as phosphodiesterase-5 (PDE5) inhibitors, which can potentially cause symptomatic hypotension (FLOMAX label information 2016). Additional vasodilation mediated by diltiazem may also exacerbate the tamsulosin-induced orthostatic hypotension and related symptoms.
For these safety matters, the systolic blood pressure, diastolic blood pressure, and heart rate of all subjects were measured after every blood sample collection. Although slight differences in the mean values for diastolic blood pressure between subjects with and without diltiazem treatment were observed in both genotype groups, these were not found to be clinically important (data not shown). However, multiple dosing of tamsulosin could lead to much higher tamsulosin plasma concentrations due to an accumulation effect, resulting in a greater orthostatic response than observed after a single dose (Troost et al. 2011). Thus, a multiple-dosing study will provide clearer information on the effects of diltiazem co-administration on the tolerability and safety of tamsulosin.
In conclusion, this study showed that diltiazem has a significant impact on the pharmacokinetics of tamsulosin according to increased Cmax and AUCinf, and decreased CL/F values and this effect was not affected by the genetic polymorphism of CYP2D6. Diltiazem likely increases the risk of drug-induced adverse events via prolonged plasma exposure of tamsulosin. Furthermore, significantly higher plasma tamsulosin concentrations are predicted when diltiazem is concomitantly administered in subjects with inherently decreased CYP2D6 enzymatic activity. Dose adjustment in the daily maintenance of tamsulosin may improve tolerability and safety in patients also receiving diltiazem.
References
Backman JT, Olkkola KT, Aranko K, Himberg JJ, Neuvonen PJ (1994) Dose of midazolam should be reduced during diltiazem and verapamil treatments. Br J Clin Pharmacol 37:221–225
Bleck JS, Thiesemann C, Kliem V, Christians U, Hecker H, Repp H, Frei U, Westhoff-Bleck M, Manns M, Sewing KF (1996) Diltiazem increases blood concentrations of cyclized cyclosporine metabolites resulting in different cyclosporine metabolite patterns in stable male and female renal allograft recipients. Br J Clin Pharmacol 41:551–556
Byeon JY, Kim YH, Na HS, Jang JH, Kim SH, Lee YJ, Bae JW, Kim IS, Jang CG, Chung MW, Lee SY (2015) Effects of the CYP2D6*10 allele on the pharmacokinetics of atomoxetine and its metabolites. Arch Pharm Res 38:2083–2091
Chaffman M, Brogden RN (1985) Diltiazem: a review of its pharmacological properties and therapeutic efficacy. Drugs 29:387–454
Chaudhry SR, Muhammad S, Eidens M, Klemm M, Khan D, Efferth T, Weisshaar MP (2014) Pharmacogenetic prediction of individual variability in drug response based on CYP2D6, CYP2C9 and CYP2C19 genetic polymorphisms. Curr Drug Metab 15(7):711–718
Choi CI, Bae JW, Jang CG, Lee SY (2012) Tamsulosin exposure is significantly increased by the CYP2D6*10/*10 genotype. J Clin Pharmacol 52:1934–1938
Chu N, Xu H, Wang G, Wang J, Chen W, Yuan F, Yang M, Li X (2015) Pharmacokinetic interaction of finasteride with tamsulosin hydrochloride: an open-label, randomized, 3-period crossover study in healthy Chinese male volunteers. Clin Ther 37:462–472
Dupont WD, Plummer WD Jr (1998) Power and sample size calculations for studies involving linear regression. Control Clin Trials 19:589–601
FLOMAX label information (2016) Boehringer Ingelheim Pharmaceuticals Incorporated, Ridgefield. http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Flomax%20Caps/Flomax.pdf. Accessed 19 Feb 2018
Franco-Salinas G, de la Rosette JJ, Michel MC (2010) Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations. Clin Pharmacokinet 49:177–188
HARNAL-D LABEL INFORMATION (2017) Astellas Pharma Korea Inc., Seoul. http://www.astellas.co.kr/product/pdf/harnald.pdf. Accessed 19 Feb 2018
Hirota T, Eguchi S, Ieiri I (2013) Impact of genetic polymorphisms in CYP2C9 and CYP2C19 on the pharmacokinetics of clinically used drugs. Drug Metab Pharmacokinet 28(1):28–37
Johansson I, Oscarson M, Yue QY, Bertilsson L, Sjöqvist F, Ingelman-Sundberg M (1994) Genetic analysis of the Chinese cytochrome P4502D locus: characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylation. Mol Pharmacol 46:452–459
Johansson I, Lundqvist E, Dahl ML, Ingelman-Sundberg M (1996) PCR-based genotyping for duplicated and deleted CYP2D6 genes. Pharmacogenetics 6:351–355
Kamimura H, Oishi S, Matsushima H, Watanabe T, Higuchi S, Hall M, Wood SG, Chasseaud LF (1998) Identification of cytochrome P450 isozymes involved in metabolism of the alpha1-adrenoceptor blocker tamsulosin in human liver microsomes. Xenobiotica 28:909–922
Kim SH, Kim DH, Byeon JY, Kim YH, Kim DH, Lim HJ, Lee CM, Whang SS, Choi CI, Bae JW, Lee YJ, Jang CG, Lee SY (2017) Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of celecoxib and its carboxylic acid metabolite. Arch Pharm Res 40(3):382–390
Kim MJ, Byeon JY, Kim YH, Kim SH, Lee CM, Jung EH, Chae WK, Lee YJ, Jang CG, Lee SY, Choi CI (2018) Effect of the CYP2D6*10 allele on the pharmacokinetics of clomiphene and its active metabolites. Arch Pharm Res 41(3):347–353
Lee E (2003) Comparison of tamsulosin and finasteride for lower urinary tract symptoms associated with benign prostatic hyperplasia in Korean patients. J Int Med Res 30:584–590
Lee HJ, Kim YH, Kim SH, Lee CM, Yang AY, Jang CG, Lee SY, Bae JW, Choi CI (2016) Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of zafirlukast. Arch Pharm Res 39(7):1013–1019
Li NC, Chen S, Yang XH, Du LD, Wang JY, Na YQ; Beijing Tamsulosin Study Group (2003) Efficacy of low-dose tamsulosin in chinese patients with symptomatic benign prostatic hyperplasia. Clin Drug Investig 23:781–787
Li H, Yang J, Zhao H, Fossler MJ, Wang C (2015) Effect of tamsulosin on the pharmacokinetics of dutasteride in Chinese male healthy volunteers. Clin Pharmacol Drug Dev 4:427–433
Ma B, Prueksaritanont T, Lin JH (2000) Drug interactions with calcium channel blockers: possible involvement of metabolite-intermediate complexation with CYP3A. Drug Metab Dispos 28:125–130
Ma JD, Lee KC, Kuo GM (2012) Clinical application of pharmacogenomics. J Pharm Pract 25(4):417–427
Mendoza R, Wan YJ, Poland RE, Smith M, Zheng Y, Berman N, Lin KM (2001) CYP2D6 polymorphism in a Mexican American population. Clin Pharmacol Ther 70:552–560
Michel MC, Vrydag W (2006) Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol 147:S88–S119
Michel MC, Grübbel B, Taguchi K, Verfürth F, Otto T, Kröpfl D (1998) Drugs for treatment of benign prostatic hyperplasia: affinity comparison at cloned alpha 1-adrenoceptor subtypes and in human prostate. J Auton Pharmacol 16:21–28
Miyazawa Y, Forrest A, Schentag JJ, Kamimura H, Swarz H, Ito Y (2002) Effect of concomitant administration of cimetidine hydrochloride on the pharmacokinetic and safety profile of tamsulosin hydrochloride 0.4 mg in healthy subjects. Curr Ther Res 63:15–26
Molden E, Asberg A, Christensen H (2000) CYP2D6 is involved in O-demethylation of diltiazem. An in vitro study with transfected human liver cells. Eur J Clin Pharmacol 56:575–579
Molden E, Asberg A, Christensen H (2002) Desacetyl-diltiazem displays severalfold higher affinity to CYP2D6 compared with CYP3A4. Drug Metab Dispos 30:1–3
Park CH, Chang HS, Oh BR, Kim HJ, Sul CK, Chung SK, Jung SI (2004) Efficacy of low-dose tamsulosin on lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a nonblind multicentre Korean study. Clin Drug Investig 24:41–47
Pinto AG, Wang YH, Chalasani N, Skaar T, Kolwankar D, Gorski JC, Liangpunsakul S, Hamman MA, Arefayene M, Hall SD (2005) Inhibition of human intestinal wall metabolism by macrolide antibiotics: effect of clarithromycin on cytochrome P450 3A4/5 activity and expression. Clin Pharmacol Ther 77:178–188
Preissner SC, Hoffmann MF, Preissner R, Dunkel M, Gewiess A, Preissner S (2013) Polymorphic cytochrome P450 enzymes (CYPs) and their role in personalized therapy. PLoS ONE 8(12):e82562
Probst-Schendzielorz K, Viviani R, Stingl JC (2015) Effect of Cytochrome P450 polymorphism on the action and metabolism of selective serotonin reuptake inhibitors. Expert Opin Drug Metab Toxicol 11(8):1219–1232
Soeishi Y, Matsushima H, Watanabe T, Higuchi S, Cornelissen K, Ward J (1996) Absorption, metabolism and excretion of tamsulosin hydrochloride in man. Xenobiotica 26:637–645
Taguchi K, Saitoh M, Sato S, Asano M, Michel MC (1997) Effects of tamsulosin metabolites at alpha-1 adrenoceptor subtypes. J Pharmacol Exp Ther 280:1–5
Taguchi K, Schäfers RF, Michel MC (1998) Radioreceptor assay analysis of tamsulosin and terazosin pharmacokinetics. Br J Clin Pharmacol 45:49–55
Troost J, Tatami S, Tsuda Y, Mattheus M, Mehlburger L, Wein M, Michel MC (2011) Effects of strong CYP2D6 and 3A4 inhibitors, paroxetine and ketoconazole, on the pharmacokinetics and cardiovascular safety of tamsulosin. Br J Clin Pharmacol 72:247–256
van Hoogdalem EJ, Soeishi Y, Matsushima H, Higuchi S (1997) Disposition of the selective alpha1A-adrenoceptor antagonist tamsulosin in humans: comparison with data from interspecies scaling. J Pharm Sci 86:1156–1161
Varhe A, Olkkola KT, Neuvonen PJ (1996) Diltiazem enhances the effects of triazolam by inhibiting its metabolism. Clin Pharmacol Ther 59:369–375
von Richter O, Greiner B, Fromm MF, Fraser R, Omari T, Barclay ML, Dent J, Somogyi AA, Eichelbaum M (2001) Determination of in vivo absorption, metabolism, and transport of drugs by the human intestinal wall and liver with a novel perfusion technique. Clin Pharmacol Ther 70:217–227
Zhang QY, Kaminsky LS, Dunbar D, Zhang J, Ding X (2007) Role of small intestinal cytochromes p450 in the bioavailability of oral nifedipine. Drug Metab Dispos 35:1617–1623
Zhang X, Quinney SK, Gorski JC, Jones DR, Hall SD (2009) Semiphysiologically based pharmacokinetic models for the inhibition of midazolam clearance by diltiazem and its major metabolite. Drug Metab Dispos 37:1587–1597
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2016R1A2B4007381).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest with respect to the authorship and/or publication of this article.
Rights and permissions
About this article
Cite this article
Byeon, JY., Lee, Y.J., Kim, YH. et al. Effects of diltiazem, a moderate inhibitor of CYP3A4, on the pharmacokinetics of tamsulosin in different CYP2D6 genotypes. Arch. Pharm. Res. 41, 564–570 (2018). https://doi.org/10.1007/s12272-018-1030-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-018-1030-6