Abstract
Our aim was to analyse the associations between carotid plaque burden (CPB), cardiovascular risk factors (CVRF), and surrogate markers of CV risk in subjects with metabolic syndrome (MetS). We consecutively included 75 asymptomatic outpatients with MetS components, <60 years old and non-smokers. We determined the presence of CVRF, left ventricular hypertrophy (LVH), carotid intima-media thickness (cIMT), albumin-creatinine ratio (ACR), coronary artery calcium score (CACS) and CPB by 3-dimensional vascular ultrasound (3DVUS) for comparison. A total of 50 (67%) subjects had MetS defined by harmonized criteria. A CPB >0 mm3 and a CACS >0 AU were the risk biomarkers most frequently observed (72% and 77%, respectively), followed by LVH (40%). CPB and CACS revealed association with cardiovascular risk (r = 0.308; p = 0.032 and r = 0.601 p < 0.01, respectively), and CPB also showed association with the burden of CVRF (r = 0.349; p = 0.014). CPB by 3DVUS was a prevalent CV risk marker, directly associated with CVRF and cardiovascular risk in MetS subjects.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS) is a broad entity that encompasses the presence of several cardiovascular risk factors (CVRF) with a shared physiopathological substrate, the insulin resistance [1]. However, the components that define MetS are not included in the most frequently used cardiovascular (CV) risk scores and a range of different cut-off points for CVRF are contemplated to ascertain its diagnosis [1,2,3]. These are some of the important limitations inherent to the classical risk scales applied to assess CV risk in the context of MetS. Consequently, different subclinical markers of CV risk have been proposed to help identify individuals with MetS at greater risk of developing clinical CV events, given their high prevalence and the associated risk of CV events compared with individuals without MetS [4,5,6].
Subclinical markers of CV risk include albuminuria, left ventricular hypertrophy and carotid artery alteration defined by carotid intima-media thickness [7]. At the same time, a growing body of evidence reinforces the potential value of detecting subclinical atherosclerosis with non-invasive imaging techniques to establish CV risk [8,9,10]. Indeed, recent clinical guidelines recommend to use imaging biomarkers based on the detection of atherosclerotic lesions, specifically carotid plaque burden (CPB) or coronary artery calcium score (CACS), over cIMT and prior markers [11,12,13]. With the advent of new three-dimensional vascular ultrasound (3DVUS) methods, it is now possible to accurately quantify atherosclerotic plaque burden at the individual level by carotid plaque volume assessment with very promising results [14, 15] comparable to other validated methods, such as CACS [16, 17]. Large population-based cohorts like the High Risk Plaque initiative and the PESA study have demonstrated a good correlation between 3D-CBP and CVRF [15] and the risk for developing cardiovascular events [17, 18]. However, evidence on CPB by 3DVUS is still limited, especially in MetS where, to the best of our knowledge, only one small study evaluated the correlation between CPB, cIMT and MetS components in a highly selected atypical population using an old 3D-like 2D-based US method [19]. In addition, no studies have compared 3D-CBP with previously established markers of CV risk like LVH and albuminuria.
The aim of the present study was to use 3DVUS to detect and quantify CPB in a cohort of asymptomatic patients with MetS components to determine, for the first time, its association with CVRF and estimated CV risk. In addition, we explored the potential value of CPB for CV risk assessment in MetS patients as compared with validated subclinical markers of CV risk.
Methods
Selection of Patients and Clinical Assessment
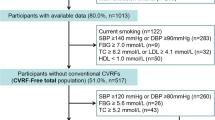
We conducted an observational prospective study including outpatients who presented components of MetS assessed in the CV Disease Prevention Unit at HM-Monteprincipe University Hospital in Madrid (Spain) during 2016–2017 period. In addition, eligible patients had to be below 60 years of age, non-smokers and have no previous history of cardiovascular events, in order to control for the effect of other factors unrelated to MetS on the development of atherosclerosis. Presence of MetS was diagnosed according to current harmonized criteria [1] if three or more of the following components were present: (a) abdominal obesity (abdominal perimeter > 88 cm in women; > 102 cm in men); (b) high blood pressure (BP) levels (systolic/diastolic BP > 130/85 mmHg) or antihypertensive therapy; (c) dysglycaemia (impaired fasting glycaemia > 100 mg/dl) or hypoglycaemic therapy; (d) atherogenic dyslipidaemia, considering the two components: low levels of high-density lipoproteins (HDL cholesterol) (< 40 mg/dl in men and 50 mg/dl in women) and high levels of triglycerides (> 150 mg/dl) or subjects taking lipid-lowering therapy. The presence of classical CVRF was determined as follows: (a) hypercholesterolemia: total cholesterol ≥240 mg/dl; LDL ≥160 mg/dl or use of lipid-lowering medication [2]; (b) hypertension: systolic BP ≥140 mmHg or diastolic BP ≥ 90 mmHg or taking hypotensive medication [20]; (c) diabetes: fasting glycaemia ≥126 mg/dl or Hb1Ac > 6.5% or use of hypoglycaemic medication [11, 12]. We defined two categorical scores according to (1) the number of components of MetS (from 0 to 5; including abdominal obesity, high blood pressure, dysglycaemia, low levels of high-density lipoproteins and high levels of triglycerides) and (2) the number of conventional CVRF (from 0 to 3, including hypercholesterolemia, hypertension and diabetes) present in each individual. Estimated CV risk at 10 years was calculated from the latest version of the ASCVD scale of the AHA/ACC, the data for which are obtained from the study with the most populational cohorts of reference [12].
Determination of Subclinical Markers of CV Risk and Subclinical Atherosclerosis
We evaluated carotid plaque burden by 3DVUS, carotid intima media thickness (cIMT), left ventricular hypertrophy (LVH) in echocardiography, albuminuria in urine measured by the albumin-creatinine ratio (ACR) and coronary artery calcium score (CACS) by CT as subclinical markers of CV risk. All markers were evaluated in all participants except CACS, which was evaluated in a subgroup of 41 individuals who agreed to undergo the test. All image markers were assessed by clinicians specialised in cardiovascular imaging, who were blind to the clinical data.
Evaluation of the presence and quantification of the 3D carotid plaques and the cIMT was carried out using a Philips Epiq ultrasound (Philips Health Care, Andover, MA, USA). Three-dimensional vascular ultrasound was performed with a volumetric linear array probe VL 13-5 2D/3D (Philips Health Care, Andover, MA, USA) according to a previously described protocol [15, 21]. Briefly, this consisted in conducting an automatic axial scan with a volumetric reconstruction of a segment approximately 6 cm in length (acquisition of 30°) of the vessel studied. 3D acquisitions focused on the carotid bulb, the mid-distal segment of the common carotid artery, the bulb and the proximal portion of the internal and external branches were explored. The analysis was conducted with special software for 3D Vascular Plaque Quantification-VPQ® that forms part of QLAB 10.5 application (Philips Health Care, Andover, MA, USA) to quantify carotid atherosclerosis plaque volume. Atherosclerotic plaque was defined according to the Mannheim criteria as a focal protrusion in the vessel lumen with a thickness greater than 50% of the adjacent cIMT, or diffuse thickening of the cIMT > 1.5 mm [22]. Then, carotid plaque burden (CPB) was calculated as the sum of all plaque volumes present in bilateral carotid 3D acquisitions. Presence of subclinical atherosclerosis by 3DVUS was defined, in accordance with previous studies [23], as the presence of any carotid plaque (qualitative) or CBP > 0 mm3 (quantitative). Supplementary Fig. 1 shows an atherosclerotic plaque detected by carotid 3DVUS. The cIMT was determined with electrocardiographic synchronisation in a longitudinal view of the carotid arteries, measuring in systole at the level of the posterior wall in the distal common portion of both carotid arteries at one centimetre from their bifurcation using the semiautomatic measuring software QLAB 10.5 (Philips Health Care, Andover, MA, USA). Abnormal cIMT was defined as a measurement above 0.9 mm because this value was significantly associated with an increased risk for CV disease [24]. The presence of LVH was assessed on the parasternal long axis view of a transthoracic cardiac ultrasound. A cut-off value of ≥11 mm for septal thickness was used to define the presence of LVH [25]. The mean urinary ACR was measured in a random sample of morning urine, establishing a cut-off value of ACR >30 mg/g [12, 26] for defining the presence of albuminuria. Quantification of the CACS was done using the Agatston method [27] in the images acquired using computed tomography equipment with 160 detectors (Aquilion, Toshiba Medical Systems Corp.) following a protocol without contrast, with low radiation dose and ECG synchronisation. Presence of subclinical atherosclerosis by CT was defined as the presence of any coronary calcification (qualitative) or CACS >0 (quantitative), according to prior evidence [23]. In line with recent guidelines and previous studies, subclinical atherosclerosis in the study patients was defined as the presence of any carotid plaque (CPB >0) by 3DVUS and/or the presence of any coronary calcifications (CACS >0). The study complied with the principles of the Helsinki declaration and was approved by the Ethical Research Committee of the University Hospital HM Montepríncipe (10.03.099-GHM), obtaining the informed consent of all participants.
Statistical Analysis
Results were expressed as means ± standard deviation (SD) for the quantitative variables and numbers and percentages for the qualitative variables. The distribution was determined by the Kolmogorov-Smirnov statistical test, applying a posteriori parametric or non-parametric tests depending on whether or not these followed a normal distribution. Between-group comparisons were done by the Student’s t and Chi [2] tests for independent samples, for quantitative and qualitative variables, respectively, or their non-parametric equivalents, as appropriate. The odds ratio association measure was used for qualitative variables and Pearson’s correlation coefficient for quantitative variables. Receiver-operator curves (ROC) were calculated for each biomarker to determine the presence of MetS as a surrogate parameter of increased CV risk. A linear regression model was used to study the correlation between markers of subclinical damage of MetS, CVRF and estimated risk. A p value <0.05 was considered statistically significant. The analysis was performed using the SPSS 20.0 computer software (SPSS Institute, France).
Results
Baseline Characteristics
A total of 75 patients were included in the study. Of the total sample, 50 presented MetS according to defined criteria and 25 did not meet criteria for MetS, without statistically significant differences in age and gender between the groups with and without MetS criteria (Table 1). Among the patients with MetS, abdominal obesity was the most common component (88%), followed by high blood pressure (82%) and impaired fasting glycaemia and hypertriglyceridaemia had similar frequencies (72% and 70%, respectively). Moreover, only a few patients (13%) met all 5 diagnostic criteria, with the largest groups fulfilling 3 or 4 criteria (27% and 27%, respectively). Regarding conventional CVRF, only 12 patients (16%) fulfilled criteria of diabetes and hypercholesterolaemia was the most common CVRF (43%). Only a small percentage of patients were taking statins (29%), with no significant differences between the groups with and without MetS (30% vs. 28%, p = 0.999). Given the limited age of inclusion into the study and the absence of smokers from the cohort, estimated CV risk at 10 years in the patients with MetS was low-intermediate (ASCVD risk at 10 years of 5.6 ± 4.4%). These data are recorded in detail in Table 1.
Markers of Subclinical CV Risk and Subclinical Atherosclerosis
The presence of biomarkers of subclinical CV risk and the quantification of these are described in Table 2. We detected the presence of subclinical markers in our cohort with the highest frequency by subclinical atherosclerosis markers, such as the presence of any carotid plaque (CBP >0) by 3DVUS and the presence of any coronary calcification (CACS >0) (63% and 65%, respectively), observing significant differences between the groups with and without criteria of MetS.
By contrast, the prevalence of markers of subclinical CV risk not directly related to plaque detection is very low (5% of cases with albuminuria and 5% of cases with elevated cIMT), and this was only detected in patients meeting MetS criteria. The prevalence of LVH was slightly higher (26%) and was also limited to subjects with MetS. Even after stratifying MetS patients by CVRFs, the prevalence of 3D-CPB was higher compared to the prevalence of LVH, cIMT and albuminuria (data not shown).
Subclinical atherosclerotic burden evaluated in continuum by CPB using 3DVUS and CACS by Agatston, together with ACR levels, were all higher in patients with MetS, whereas there were no significant differences in cIMT. Moreover, the areas under the curve for CPB by 3DVUS, CACS and ACR were all capable of identifying patients with MetS and, thus, with a higher CV risk, although the CACS values were slightly better than the others. The optimal cut off for CPB to diagnose metabolic syndrome was 3.55 mm3. However, this value should be interpreted with caution given the small sample size of our study (Supplementary Fig. 2).
Association Between Markers of Subclinical CV Risk, Subclinical Atherosclerosis and Components of MetS, Conventional CVRF and Risk Scales
Table 3 summarises the association between CVRF and markers that define MetS with subclinical CV risk markers. ACR was the marker with the greatest significant numbers of correlations with MetS components, especially in a positive sense with parameters associated with insulin resistance (impaired fasting glycaemia, Hb1Ac, abdominal perimeter) and negatively with HDL. LVH is associated with systolic and diastolic blood pressure and abdominal perimeter, but no significant association was found for cIMT. Regarding markers of subclinical atherosclerosis, CPB by 3DVUS was significantly correlated with triglycerides and high blood pressure, and CACS was correlated with fasting glycaemia. When studying the different biomarkers with the number of components of MetS, no significant correlation was found with any of the CV risk markers or with subclinical atherosclerosis (Fig. 1). By contrast, we found a significant association between CPB by 3DVUS and the number of CVRF (Fig. 2), and also with the ASCVD risk scale (Fig. 3). A relationship was also found between conventional CVRF and the risk estimated by ACR, although this was mainly observed with values under the pathological limit. The CACS was associated with estimated risk (Fig. 3), and no association was observed with the cIMT.
Association between the number of components of MetS, subclinical markers of CV risk and subclinical atherosclerosis. IMT, intima-media thickness in mm; CACS, coronary artery calcium score in Agatston units; Albumin-creatinine ratio in milligrammes per gramme; 3D carotid plaque burden in cubic millimetres
Association between the number of classical cardiovascular risk factors (dyslipidaemia, hypertension and diabetes mellitus), subclinical markers of CV risk and subclinical atherosclerosis. Same abbreviations and units as in Fig. 1
Association between CV risk estimated by the ASCVD risk scale at 10 years, subclinical markers of CV risk and subclinical atherosclerosis. Same abbreviations and units as in Fig. 1
Discussion
In the present study, we have shown that carotid plaque burden by 3D vascular ultrasound is highly prevalent in middle-aged patients with MetS and is associated with the presence of cardiovascular risk factors and estimated cardiovascular risk. Other findings were as follows: (1) CACS was also prevalent, followed by LVH, whereas albuminuria and abnormal cIMT were infrequent markers of cardiovascular risk among middle-aged MetS patients and (2) the quantification of specific cardiovascular biomarkers, such as 3D-CBP, CACS and ACR, could discriminate MetS patients from patients without MetS and revealed correlations with CVRF and estimated CV risk.
Most studies on MetS have focused on the study of subclinical markers of cardiovascular disease such as cIMT [28, 29], LVH [30] or ACR. Our work describes, for the first time, the value of determining atherosclerotic burden by 3D vascular ultrasound in MetS, and also provides a direct comparison with CACS and prior risk markers. Three-D carotid plaque burden is a prevalent CV risk marker, even after stratifying by CVRF, and is also associated with the number of CVRF and CV risk in these patients; however, we did not find a significant association with the number of MetS components. In line with this finding, according to the group of Brunek et al. [31], the detection of carotid plaques was closely influenced by the presence of MetS rather than by its separate components. A possible explanation for this could be that MetS components are, in fact, different manifestations of a common factor, insulin resistance. Hence, more importantly than considering each component individually is the fact that MetS favours the development of CVRF and subsequently, atherosclerosis. It has also been found that the addition of individual components of MetS to conventional risk scales does not improve their prediction [32], making it less important to find associations with its components. We believe that these results are important because they confirm links between CPB and the number of CVRF and cardiovascular risk in MetS patients, over individual MetS components.
Prior studies have addressed the relationship between MetS and carotid plaque presence, like the Northern Manhattan study by Rundek et al. [33], where MetS was associated with plaque presence in a multi-ethnic population-based cohort. Also, the study by Iglseder et al. [34] and the Tromsø study [35] confirmed this relationship especially in young to middle-aged patients. The beneficial aspects of using 3DVUS rely on the possibility to combine data on both plaque presence and plaque burden quantification, assessed as carotid plaque volume in our protocol. To our knowledge, only one study has assessed carotid plaque volume in individuals with MetS and compared it with cIMT finding a better association for cIMT than for volume, in contrast to our data [19]. However, this study was based on a highly selected population (a Canadian Ojee-Cree indigenous population subcohort of 166 patients) with a significantly higher prevalence of diabetes and lower participant´s age (mean age of 38.5 years old), compared to our study cohort. Moreover, this divergence from our results could be explained by the “pseudo-3D” method used by the authors that has been shown to be less accurate at detecting and quantifying small plaques, thus limiting its value in studies of early atherosclerosis in young populations [21]. In our study, we provide evidence on the usefulness of “real” 3D carotid plaque burden method in young adults with MetS to detect subclinical cardiovascular disease, over traditional markers like cIMT, LVH or ACR, and associations with cardiovascular risk.
Regarding the value of CACS in MetS, the most important evidence to date was reported in a recent publication of the MESA study [36]. The authors demonstrated its usefulness to detect MetS patients with an increased risk of developing a CV event when used in combination with traditional scales, and compared with other parameters such as cIMT or the ankle-brachial index. In line with these results, our study found associations with estimated CV risk for CACS but not for cIMT. Additionally, we found that CACS and CPB assessed by 3D ultrasound have similar prevalence and both of them were not associated with the number of MetS components but related to CV risk, reflecting possible similarities between both markers. However, our results should be interpreted with caution given that CACS was not available in all study population.
Of note, biomarkers quantification, rather than simply determining whether they are altered or not relative to a given cut off point, has shown to discriminate between patients with and without MetS in our study. This has been shown for CBP, CACS, and more especially for ACR, as this shows a direct and significant relationship with CVRF and CV risk, even from non-pathological values (<30 mg/g). This would suggest that subclinical cardiovascular disease might be considered as a continuum, and its quantitative assessment would best reflect CV risk.
Our work presents several limitations. It was an observational study with no possibility for follow-up to validate the prognostic value of CPB by 3DVUS, although there is substantial evidence for the prognostic value of the other parameters included in the study. Although improvement in CV events prediction by CBP could not be assessed directly (cross-sectional study), the presence of plaques could be considered a potential surrogate of future events [16, 17]. The sample of patients is small, which may have contributed to the low prevalence of markers of CV damage not directly associated with atherosclerotic plaque formation, limiting the statistical power in some comparisons. However, our results for non-atherosclerotic risk markers are similar to those of previous studies based on large cohorts and are probably related to the younger age of the patients recruited, meaning a shorter exposure time to develop subclinical CV disease [23, 37]. Moreover, our sample corresponded to a selected cohort of subjects with components of MetS but with a low prevalence of classical CVRF, owing to the exclusion of smokers and limiting the age of inclusion to subjects under 60 years old. By contrast, this makes the cohort more homogeneous, by removing the main atherosclerotic determinants not related to mechanisms of insulin resistance, such as smoking or advanced age [38], and it is better at accurately determining the intrinsic effects of MetS in premature subclinical CV disease. Nevertheless, we cannot exclude certain variation in the prevalence of CV risk markers explained by the differences and interactions between CVRF present in this specific population of young age and with a common underlying substrate, the insulin resistance. Also, CACS is only performed in a limited subgroup of patients, which also limits interpretation of the results. Data on left ventricular septal thickness was not available to be able to study the relationship of LVH quantitatively. However, we could include qualitative analysis of LVH (yes/no) assessed by echocardiography, which add value to previous studies in Mets where only electrocardiographic parameters were used. Additionally, current guidelines do not recommend quantitative assessment of LVH in this setting [39].
Conclusions
Detection of atherosclerosis by 3DVUS is a prevalent marker that identifies premature subclinical CV disease among subjects with MetS. Carotid plaque burden by 3D vascular ultrasound is highly prevalent in middle-aged patients with metabolic syndrome and is associated with the presence of cardiovascular risk factors and with subclinical cardiovascular disease.
Clinical Relevance
- We present the first evidence on the value of carotid plaque burden by 3D vascular ultrasound in patients with metabolic syndrome over current markers of subclinical CV risk like LVH, cIMT and albuminuria.
- Carotid plaque burden by 3D vascular ultrasound is highly prevalent in middle-aged patients with metabolic syndrome, and it is directly associated with the presence of cardiovascular risk factors and with cardiovascular risk.
- 3D ultrasound is an accessible technique that does not use radiation. It would be a simple technique to incorporate in the assessment of cardiovascular risk in young and middle-aged patients with MetS.
Abbreviations
- 3DVUS:
-
3-Dimensional vascular ultrasound
- ACR:
-
Albumin-creatinine ratio
- CACS:
-
Coronary artery calcium score
- cIMT:
-
Carotid intima-media thickness
- CPB:
-
Carotid plaque burden
- CT:
-
Computed tomography
- CV:
-
Cardiovascular
- CVRF:
-
Cardiovascular risk factors
- LVH:
-
Left ventricular hypertrophy
- MetS:
-
Metabolic syndrome
References
Alberti, K. G., Eckel, R. H., Grundy, S. M., et al. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation., 120(16), 1640–1645.
(2001). Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA., 285(19), 2486–2497.
Alberti, K. G., & Zimmet, P. Z. (1998). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine: a Journal of the British Diabetic Association., 15(7), 539–553.
Fernandez-Berges, D., Cabrera de Leon, A., Sanz, H., et al. (2012). Metabolic syndrome in Spain: Prevalence and coronary risk associated with harmonized definition and WHO proposal. DARIOS study. Revista Espanola de Cardiologia (English Edition), 65(3), 241–248.
Gami, A. S., Witt, B. J., Howard, D. E., et al. (2007). Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. Journal of the American College of Cardiology, 49(4), 403–414.
Lakka, H. M., Laaksonen, D. E., Lakka, T. A., et al. (2002). The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA., 288(21), 2709–2716.
Ingelsson, E., Sullivan, L. M., Murabito, J. M., et al. (2007). Prevalence and prognostic impact of subclinical cardiovascular disease in individuals with the metabolic syndrome and diabetes. Diabetes., 56(6), 1718–1726.
Yeboah, J., McClelland, R. L., Polonsky, T. S., et al. (2012). Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA., 308(8), 788–795.
Nambi, V., Chambless, L., Folsom, A. R., et al. (2010). Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: The ARIC (Atherosclerosis Risk In Communities) study. Journal of the American College of Cardiology, 55(15), 1600–1607.
Gepner, A. D., Young, R., Delaney, J. A., et al. (2015). Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circulation. Cardiovascular Imaging, 8(1), e002262.
Mach, F., Baigent, C., Catapano, A. L., et al. (2019). 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal, 41(1), 111–188.
Arnett, D. K., Blumenthal, R. S., Albert, M. A., et al. (2019). 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology, 74(10), 1376–1414.
Grundy, S. M., Stone, N. J., Bailey, A. L., et al. (2018). 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology, 73(24), e285–e350.
Wannarong, T., Parraga, G., Buchanan, D., et al. (2013). Progression of carotid plaque volume predicts cardiovascular events. Stroke., 44(7), 1859–1865.
Lopez-Melgar, B., Fernandez-Friera, L., Oliva, B., et al. (2017). Subclinical atherosclerosis burden by 3D ultrasound in mid-life: The PESA Study. Journal of the American College of Cardiology, 70(3), 301–313.
Baber, U., Mehran, R., Sartori, S., et al. (2015). Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: The BioImage Study. Journal of the American College of Cardiology, 65(11), 1065–1074.
Mortensen, M. B., Fuster, V., Muntendam, P., et al. (2016). A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: The BioImage Study. Journal of the American College of Cardiology, 68(9), 881–891.
Sillesen, H., Muntendam, P., Adourian, A., et al. (2012). Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC. Cardiovascular Imaging, 5(7), 681–689.
Pollex, R. L., Al-Shali, K. Z., House, A. A., et al. (2006). Relationship of the metabolic syndrome to carotid ultrasound traits. Cardiovascular Ultrasound, 4, 28.
Pearson, T. A., Palaniappan, L. P., Artinian, N. T., et al. (2013). American Heart Association Guide for Improving Cardiovascular Health at the Community Level, 2013 update: a scientific statement for public health practitioners, healthcare providers, and health policy makers. Circulation., 127(16), 1730–1753.
Lopez-Melgar, B., Fernandez-Friera, L., Sanchez-Gonzalez, J., et al. (2016). Accurate quantification of atherosclerotic plaque volume by 3D vascular ultrasound using the volumetric linear array method. Atherosclerosis., 248, 230–237.
Touboul, P. J., Hennerici, M. G., Meairs, S., et al. (2004). Mannheim intima-media thickness consensus. Cerebrovascular Diseases, 18(4), 346–349.
López-Melgar, B., Fernández-Friera, L., Oliva, B., et al. (2020). Short-term progression of multiterritorial subclinical atherosclerosis. Journal of the American College of Cardiology, 75(14), 1617–1627.
Stein, J. H., Korcarz, C. E., Hurst, R. T., et al. (2008). Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine, Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography., 21(2), 93–111 quiz 189-190.
Lang, R. M., Badano, L. P., Mor-Avi, V., et al. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal Cardiovascular Imaging, 16(3), 233–270.
Toto, R. D. (2004). Microalbuminuria: Definition, detection, and clinical significance. Journal of Clinical Hypertension (Greenwich, Conn.), 6(11 Suppl 3), 2–7.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology 1990;15(4):827-832.
Cuspidi, C., Sala, C., Provenzano, F., et al. (2018). Metabolic syndrome and subclinical carotid damage: a meta-analysis from population-based studies. Journal of Hypertension, 36(1), 23–30.
Paras, E., Mancini, G. B., & Lear, S. A. (2008). The relationship of three common definitions of the metabolic syndrome with sub-clinical carotid atherosclerosis. Atherosclerosis., 198(1), 228–236.
Li, N. Y., Yu, J., Zhang, X. W., et al. (2013). Features of left ventricular hypertrophy in patients with metabolic syndrome with or without comparable blood pressure: a meta-analysis. Endocrine., 43(3), 548–563.
Bonora, E., Kiechl, S., Willeit, J., et al. (2003). Carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the Bruneck study. Diabetes Care, 26(4), 1251–1257.
Wilson, P. W. (2004). Estimating cardiovascular disease risk and the metabolic syndrome: a Framingham view. Endocrinology and Metabolism Clinics of North America, 33(3), 467–481, v.
Rundek, T., White, H., Boden-Albala, B., Jin, Z., Elkind, M. S., & Sacco, R. L. (2007). The metabolic syndrome and subclinical carotid atherosclerosis: the Northern Manhattan Study. Journal of the Cardiometabolic Syndrome Winter, 2(1), 24–29.
Iglseder, B., Cip, P., Malaimare, L., Ladurner, G., & Paulweber, B. (2005). The metabolic syndrome is a stronger risk factor for early carotid atherosclerosis in women than in men. Stroke., 36(6), 1212–1217.
Herder, M., Arntzen, K. A., Johnsen, S. H., & Mathiesen, E. B. (2012). The metabolic syndrome and progression of carotid atherosclerosis over 13 years. The Tromso study. Cardiovascular Diabetology, 11, 77.
Zhao, Y., Evans, M. A., Allison, M. A., et al. (2019). Multisite atherosclerosis in subjects with metabolic syndrome and diabetes and relation to cardiovascular events: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis., 282, 202–209.
Fernandez-Friera, L., Penalvo, J. L., Fernandez-Ortiz, A., et al. (2015). Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: The PESA (Progression of early subclinical atherosclerosis) study. Circulation., 131(24), 2104–2113.
Li, S., Yun, M., Fernandez, C., et al. (2014). Cigarette smoking exacerbates the adverse effects of age and metabolic syndrome on subclinical atherosclerosis: The Bogalusa Heart Study. PLoS One, 9(5), e96368.
Whelton, P. K., Carey, R. M., Aronow, W. S., et al. (2017). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (Dallas, Tex.: 1979).
Acknowledgments
We want to thank Philips HealthCare® Iberia, especially the ultrasound team, for their technical support to be able to carry out this research work.
Funding
Dr. López-Melgar has received a grant from the Spanish Society of Cardiology “Proyecto de investigación traslacional en cardiología 2020” (SEC/FEC-INV-TRL 20/007). Dr. Díaz received fundings from Fundación de Investigación HM hospitales [I Convocatoria Intramural para grupos emergentes, 2016]. Dr. Fernández-Friera has received fundings from Comunidad de Madrid Government (AORTASANA-CM; B2017/BMD-3676), Fondo Social Europeo (FSE) and from Instituto de Salud Carlos III, Spain (PI15/02019). Sánchez-Vera, PhD has received fundings from CEU Universities-Banco Santander (MUSPB067).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human Subjects/Informed Consent Statement
All procedures followed were approved by the HM-Hospital ethic committee (10.03.099-GHM) and were in accordance with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Associate Editor Ana Barac oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1201 kb)
Rights and permissions
About this article
Cite this article
López-Melgar, B., Varona, J.F., Ortiz-Regalón, R. et al. Carotid Plaque Burden by 3-Dimensional Vascular Ultrasound as a Risk Marker for Patients with Metabolic Syndrome. J. of Cardiovasc. Trans. Res. 14, 1030–1039 (2021). https://doi.org/10.1007/s12265-021-10121-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-021-10121-z