Abstract
Transforming growth factor-β1 signaling pathways are known to involve in the development of post-infarction fibrosis, a process characterized by the aberrant activation, proliferation, and differentiation of fibroblasts, as well as the unbalanced turnover of extracellular matrix proteins. Recent studies have shown that Lefty1, a novel member of TGF-β superfamily, acts as a brake on the TGF-β signaling pathway in non-cardiac tissues. However, its role in myocardial infarction (MI)–induced fibrosis and left ventricular remodeling has not been fully elucidated. Here, for the first time, we reported that Lefty1 alleviated post-MI fibroblast proliferation, differentiation, and secretion through suppressing p-Smad2 and p-ERK1/2 signaling pathways in vivo and in vitro. In MI mice or TGF-β1-treated neonatal rat cardiac fibroblasts (CFBs), the expression of Lefty1 was upregulated. Adenovirus-mediated overexpression of Lefty1 significantly attenuated TGF-β1-induced CFBs’ proliferation, differentiation, and collagen production. Using the adeno-associated virus approach, we confirmed that Lefty1 attenuates MI-induced cardiac injury, as evidenced by the decreased infarct size and preserved cardiac function. These results highlight the importance of Lefty1 in the prevention of post-MI fibrosis and may help identify potential targets for therapeutic intervention of cardiac fibrosis.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI)–induced cardiac fibrosis (CF) plays a role in preventing cardiac rupture and maintaining the structural integrity of the heart in the early phase after MI. But in the long run, adverse and perpetual CF characterized by the aberrant activation, proliferation, and differentiation of fibroblasts, as well as unbalanced deposition and degradation of extracellular matrix (ECM), is associated with the development of heart failure (HF), malignant arrhythmias, and even sudden cardiac death, seriously endangering the prognosis of patients [1]. Currently, for most patients, pharmacological interventions are the primary means of containing post-MI fibrosis and left ventricular (LV) remodeling, but the complex signal cascades behind MI-induced CF are not well understood, hindering therapeutic translation.
Transforming growth factor-β1 (TGF-β1) signaling pathways are known to be implicated in the development of post-infarction fibrosis [2]. TGF-β1 exerts its fibrotic effects through its transmembrane receptors with serine/threonine kinase activity, type I receptor (TβRI; also known as activin receptor–like kinase, ALK) and type II receptor (TβRII) [3, 4]. Binding of active TGF-β1 to TβRII induces autophosphorylation of TβRII. Activated TβRII then recruits ALK1/5 and forms a heterologous complex with it on the cell membrane, which phosphorylates and activates the ALKs. ALK1/5 binding to the receptor-activated Smad proteins (R-Smads, mainly Smad2, 3) alters the conformation of R-Smads to form a dimer by phosphorylating the Ser residue at the C terminus. The R-Smads dimer then recruits the common Smad (Co-Smad, Smad4) to produce a trimer that can translocate into the nucleus and increase the transcription of genes associated with fibrogenesis. In addition to Smad-dependent or canonical pathways, non-canonical signaling pathways, such as MAPK, Rho-like GTPases, and PI3K-Akt, are also crucial for TGF-β1-mediated post-infarction fibrosis [2, 5, 6].
The left-right determination factor (Lefty), a novel member of TGF-β superfamily, possesses two variants in mice, namely, Lefty1 and Lefty2, which correspond to Lefty-B and Lefty-A in human, respectively [7]. A previous study has established that Lefty interferes with TGF-β1 signaling by inhibiting phosphorylation of Smad2 and its downstream events. Later, the active role of Lefty in extracellular matrix remodeling was recognized by Mason et al. in a fibrosarcoma model. They found that transfection of fibroblasts with Lefty significantly reduced collagen I mRNA expression and increased collagen cleavage [8]. Recent studies have shown that Lefty1 can alleviate epithelial-mesenchymal transition (EMT) and tubulointerstitial fibrosis by inhibiting TGF-β/Smad signaling pathway in unilateral ureteral obstruction (UUO) mice model [9, 10]. In endometrial cancer, the upregulation of Lefty suppresses Smad2-dependent cyclin A2 transcription to inhibit tumor cell proliferation [11]. Although Lefty1 acts as a repressor of the TGF-β signaling pathway, its role in MI-induced CF and LV remodeling has not been fully elucidated. Therefore, this study aimed to elucidate the potential role and mechanisms of Lefty1 in the pathogenesis of post-infarction fibrosis.
Material and Methods
Animal
Adult male C57BL/6 mice (body weight 20–24 g; mean age 8–10 weeks) and neonatal Sprague-Dawley rats (2–4 days old) were purchased from SiPeiFu (Beijing) Biotechnology Co., Ltd. (Beijing, China). All experimental procedures were conducted in compliance with both the Animal Care and Use Committee of Capital Medical University and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (the 8th Edition, NRC 2011).
Preparation of MI Model
The method of establishing mice MI model has been described previously [12]. Briefly, the mice were fasted for at least 12 h before operation. They were given anesthesia with 2% isoflurane inhalation and ventilated by endotracheal intubation. After routine disinfection, the left chest was dissected, and the heart was slightly squeezed out. The proximal left anterior descending branch of the left coronary artery was ligated with 6–0 silk sutures. It was confirmed that the operation was successful when the anterior wall of the LV immediately turned pale and the decreased activity of cardiac muscle. The sham-operated group received the same procedures without ligation.
Construction and Administration of Recombinant Adeno-Associated Viral Vector
Recombinant adeno-associated virus serotype 9 (rAAV9) vectors containing Lefty1 or green fluorescent protein (GFP) alone were constructed by Hanbio Biotechnology Co., Ltd. (Shanghai, China) according to the manufacturer’s protocol. The vectors (5 × 1012 viral particles per ml) were stored at − 80 °C before injection. After the MI model was achieved, rAAV9 vectors administration was performed immediately. According to the grouping, mice were injected with 100 μL of rAAV9-Lefty1 and rAAV9-GFP alone, respectively.
Primary Neonatal Rat Cardiac Fibroblasts Isolation and Culture
Neonatal rat cardiac fibroblasts (CFBs) were prepared from Sprague-Dawley rats as described previously [12]. Briefly, the hearts were harvested, chopped finely and washed with PBS, and then agitated gently in the collagenase II solution at 37 °C for 30 min, followed by filtration and centrifugation. The supernatant was discarded. The cells were resuspended in fresh Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, and cultured in a cell incubator with 5% CO2 for 90 min. At this time, CFBs had attached to the bottom of dishes. The CFBs within three passages were used for subsequent experiments.
Construction and Transfection of Recombinant Adenoviruses
Adenoviruses harboring Lefty1 short hairpin RNA (AdshLefty1) and containing Lefty1 were also constructed by Hanbio Biotechnology Co., Ltd. (Shanghai, China) according to the manufacturer’s protocol. Similar adenovirus vectors encoding short hairpin RNA (AdshRNA) or GFP gene (AdGFP) were used as control. Adenoviral infection was performed as described previously [10]. After 48 h of transfection, the CFBs were treated with phosphate-buffered saline (PBS) or TGF-β1 (10 ng/ml, HY-P7118, MCE) for 48 h and then whole cell extracts were prepared for analysis.
Histological and Immunofluorescence Staining
Heart tissue samples harvested from mice were fixed with 4% paraformaldehyde, embedded in paraffin, and transversely cut into 5 μm thickness. Masson staining was performed to highlight the fibers, and the extent of interstitial fibrosis was determined by fibrosis area / total administration area × 100%. The size of the scar was calculated by mean value of the percentage of the infarcted endocardial arc over the LV endocardial circumference and the percentage of the infarcted epicardial arc over the LV epicardial circumference. Immunofluorescence staining of α-SMA was performed using the methods described previously. Images acquisition and analysis were used by Image-Pro Plus.
MTT Assay
The CFBs were planted in 96-well plates at 3000 cells per well and exposed to PBS or TGF-β1 (10 ng/ml) for 48 h. Cell proliferation was quantified using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma, St. Louis, MO, USA). The optical density (OD) value of each well was detected using a spectrophotometer (BioTek, Elx800, Winooski, VT, USA) at 490 nm.
Echocardiography
Mice were examined with M-mode echocardiography (Vevo 770, Visual Sonics, Inc., Toronto, Ontario, Canada; RMV 707B probe, 15 MHz) before and 4 weeks after operation. Inhalational anesthesia with 2% isoflurane was adopted during the process of images acquisition. LV end-systolic diameter (LVSD) and end-diastolic diameter (LVDD), ejection fraction (EF), and fractional shortening (FS) were measured and averaged over three cardiac cycles.
Western Blotting Analysis
Protein samples were extracted from mice heart tissue or cultured CFBs with from M-PER Mammalian Protein Extraction Reagent (Thermo Fisher Scientific Inc) that contained protease inhibitors and phosphatase inhibitors, as described previously. The lysates (20 mg/lane) was separated by 10% SDS-PAGE gel and then transferred onto polyvinylidene fluoride (PVDF) membrane. At room temperature, the non-specific sites were blocked by 5% non-fat dried milk diluted with Tris-buffered saline Tween (TBST). One hour later, the membranes were incubated with primary antibodies against Lefty1 (ab22569, Abcam), collagen I (ab34710, Abcam), collagen III (ab7778, Abcam), Smad2/3 (#8695, Cell Signaling Technology), p-Smad2 (#18338, Cell Signaling Technology), p-Smad3 (#9520, Cell Signaling Technology), ERK1/2 (#4695S, Cell Signaling Technology), p-ERK1/2 (#9101S, Cell Signaling Technology), p38 MAPK (#8690S, Cell Signaling Technology), p-p38 MAPK (#9216S, Cell Signaling Technology), JNK (#9252S, Cell Signaling Technology), p-JNK (#4668S, Cell Signaling Technology), and GAPDH (#5174, Cell Signaling Technology) overnight at 4 °C. After thorough washing with TBST, the membranes were incubated with peroxidase-conjugated secondary antibodies labeled with peroxidase was incubated at 37 °C for 2 h. The bands were detected with enhanced chemiluminescence reagent on Gel Imaging System (Tanon 5200) and quantified by densitometry software (Image-Pro Plus).
Real-time PCR
TRIzol reagent (Invitrogen, USA) was used to extract the total RNA from cultured fibroblasts and heart tissues, and then, the total RNA was reverse transcribed into cDNA by the Prime-Script™RT reagent kit (TaKaRa, Japan) and subjected to quantitative real-time PCR (qRT-PCR). Reactions were performed with SYBR green (TaKaRa) and normalized to GAPDH expression in an ABI 7500 Fast (Applied Biosystems, USA). Primer pairs (5′ → 3′) used in this study included Collagen Iα F: AGGCTTCAGTGGTTTGGATG and R: CACCAACAGCACCATCGTTA; Collagen III F: CCCAACCCAGAGATCCCATT and R: GAAGCACAGGAGCAGGTGTAGA; α-SMA F: CTGGCATCGTGCTGGACTC and R: GCCCATCAGGCAACTCGTA; and Lefty1 F: ACTCAAGACCCTTTCAGGACAC and R: CAGCAGAGCCACAGGAATG.
Statistical Analysis
Data were presented as \( \overline{x}\pm SD \). Student’s t test was used to determine statistical significance between each group. A P value < 0.05 was considered statistically significant.
Results
Lefty1 Expression Is Upregulated in Response to MI or TGF-β1-Stimulation
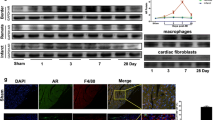
To investigate whether MI affects Lefty1 production, we resected heart tissues from the infarcted border zone in mice after experimental MI and the same area in sham-operated mice and detected Lefty1 protein expression by western blotting analysis (Fig. 1a and b). The results showed that compared with sham-operated mice, the expression level of Lefty1 protein in the MI group was significantly increased in the infarcted border zone at 2- and 4-week post-MI (P < 0.05). To further investigate whether stimulation of CFBs in vitro affects the generation of Lefty1, we next exposed CFBs to PBS or TGF-β1 (10 ng/ml) for cultivation. Consistent with our observation of progressively increased Lefty1 protein expression with MI, we observed robustly higher mRNA expression of Lefty1 in TGF-stimulated CFBs than in PBS-treated CFBs (Fig. 1c). These results suggest that Lefty1 may be involved in MI-induced CF.
Lefty1 expression is upregulated in MI mice and TGF-β1-stimulated CFBs. a Western blotting analysis of Lefty1 in mice LV samples from the sham-operated group and MI group (at 2 and 4 weeks) (n = 7 mice per group). b The histogram shows the average volume density corrected for GAPDH (n = 7 mice per group). *P < 0.05 vs sham group; #P < 0.05 vs MI (2 W) group. c Relative mRNA levels of Lefty1 in PBS or TGF-β1-treated CFBs (n = 3 batches of cells per group). *P < 0.05 vs PBS group
Overexpression of Lefty1 Attenuates TGF-β1-Induced CFBs’ Proliferation, Differentiation and Collagen Production
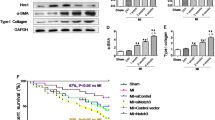
Next, we employed the adenoviral approach to investigate the regulatory effects of Lefty1 on CFBs. Western blotting analysis confirmed satisfactory overexpression and knockdown efficiency at 48 h after transfection (Fig. 2a). After successfully infected with AdshRNA, AdshLefty1, AdGFP, or AdshLefty1, the CFBs were treated with PBS or TGF-β1 (10 ng/ml) for 48 h. No differences in α-SMA (red) and DAPI (blue) deposition were observed in PBS-treated CFBs after adenovirus injection (upper panels in Fig. 2b and f). However, immunofluorescence staining indicated that compared with the PBS treatment group, AdshLefty1 administration increased α-SMA deposition in CFBs exposed to TGF-β1, while Lefty1 overexpression reversed this phenomenon (lower panels in Fig. 2b and f). Consistently, western blot analysis demonstrated that the protein levels of collagens I and III were significantly higher in AdshLefty1-infected CFBs. In contrast, the overexpression of Lefty1 considerably attenuated TGF-β1-induced collagen secretion (Fig. 2e and i). We further used the MTT assay to assess the proliferation of CFBs. As illustrated in Fig. 2d and h, the proliferative rate of CFBs was significantly declined after Lefty1 overexpression but elevated after transfected with AdshLefty1. These results indicate that Lefty1 may play roles in the function of CFBs in vitro.
Effect of Lefty1 on the proliferation, differentation and collagen production of cultured CFBs. a Western blotting analysis of overexpression and knockdown efficiency at 48 h after transfection. b Immunofluorescence staining of α-SMA in CFBs following AdshLefty1 infection. α-SMA and nuclei were stained with Cy5 (red) and DAPI (blue), respectively. c Quantification of each group shown in panel A. *P < 0.05 vs PBS group; #P < 0.05 vs AdshRNA. d, h Effects of Lefty1 on proliferation of CFBs determined by MTT assay. *P < 0.05 vs PBS group; #P < 0.05 vs AdshRNA or AdGFP group. e Western blotting analysis of collagen I and collagen III and quantification in AdshLefty1 infected CFBs (n = 3 batches of cells per group). *P < 0.05 vs PBS group; #P < 0.05 vs AdshRNA group. f Immunofluorescence staining of α-SMA in CFBs following AdLefty1 infection. g Quantification of each group shown in panel f. *P < 0.05 vs PBS group; #P < 0.05 vs AdGFP group. i Western blotting analysis of collagen I and collagen III and quantification in AdLefty1 infected CFBs (n = 3 batches of cells per group). *P < 0.05 vs PBS group; #P < 0.05 vs AdGFP group
rAAV9-Lefty1 Administration Ameliorates LV Fibrosis and Improves Cardiac Function in MI Mice
AAV9 vector is an ideal gene therapy tool for human diseases, with the ability to non-pathogenically transfect cells and ensure long-term transgenic expression [13]. Hence, we applied the recombinant AAV9 (rAAV9) vector to deliver GFP and the Lefty1 gene (rAAV9- Lefty1) into the heart through mice tail-vein and assessed whether rAAV9-Lefty1 administration could attenuate MI-induced CF in vivo. A satisfactory efficiency of infection was observed in the ventricles of mice injected with rAAV9-Lefty1, which showed a nearly 3-fold higher expression of Lefty1 compared with mice injected with rAAV9-GFP (Fig. 3a). The Masson’s staining was performed to evaluate the extent of fibrosis in LV. Four weeks after MI, the infarct size and the extent of interstitial fibrosis in the infarct border zone were markedly decreased in rAAV9-Lefty1 injected mice compared with rAAV9-GFP mice. Reducing interstitial fibrosis was found in the remote area in rAAV9-Lefty1 mice, while no statistical difference was found between the two groups (Fig. 3b and c). We further used real-time PCR to examine mRNA levels of collagen Iα, collagen III, and α-SMA. No differences in these fibrosis markers mRNA expression levels were found in sham-operated mice, while MI-induced fibrosis was notably mitigated in rAAV9-Lefty1 mice compared with rAAV9-GFP mice, as evidenced by significantly decreased collagen Iα, collagen III, and α-SMA mRNA levels in rAAV9-Lefty1 mice at 4 weeks post-MI (Fig. 3d). We also performed echocardiography to evaluate the cardiac parameters. No difference was found in baseline cardiac parameters between two sham- and MI-group. LVEF and FS were decreased 4 weeks after MI, but rAAV9-Lefty1 administration caused substantial improvements in EF and FS. LV dilation in rAAV9-Lefty1-injected mice was not notable than the rAAV9-GFP group (Fig. 3e).
Lefty1 mitigates MI-induced fibrosis in vitro. a Western blotting and quantitative analysis of overexpression efficiency after rAAV9-Lefty1 injection. b Representative histological images of the Masson trichrome staining of the overall short or long axis (upper panel), of the border zone (middle panel) and of the remote area (lower panel) after rAAV9-GFP (left) or rAAV9-Lefty1 (right) administration in sham operation group and MI group (n = 7 mice per group). c Quantitative analysis of the stained fibrotic areas of the overall short/long axis (right), of the border zone (middle) and of the remote area (left). *P < 0.05 vs. rAAV9-GFP group; NS: Not significant. d Real-Time PCR measuring collagen I (left), collagen III (middle) and α-SMA (right) mRNA level in the border zone of sham operated and MI mice (n = 7 mice per group). *P < 0.05 vs. PBS group; #P < 0.05 vs. rAAV9-GFP group. (E) EF, FS, LVDD, and LVSD were measured in rAAV9-GFP or rAAV9-Lefty1 MI mice at baseline and 4 weeks after MI (n = 7 mice per group). *P < 0.05 vs. PBS group; #P < 0.05 vs. rAAV9-GFP group. MI, myocardial infarction; EF, ejection fraction; FS, fractional shortening; LVDD, left ventricular diastolic diameter; LVSD, left ventricular systolic diameter
Lefty1 Inhibits TGF-β1- and MI-Induced Smad2 and ERK1/2 Activation
Taken together, the above results demonstrated that Lefty1 could attenuate fibrosis in vitro and in vivo. Hence, we further investigated the mechanisms behind the anti-fibrotic effects of Lefty1. Previous studies demonstrated Lefty1 exerted its anti-tumor or anti-EMT (epithelial–mesenchymal transition) effects by blocking both TGF-β/Smad and MAPK signaling cascade. Therefore, we first investigated whether Lefty1 could ameliorate post-infarction fibrosis by blocking TGF-β signal pathway in vitro. The WB analysis of Smad2/3, p-Smad2, p-Smad3, ERK1/2, p-ERK1/2, p38 MAPK, p-p38 MAPK, JNK, and p-JNK was conducted. Results showed that in TGF-β1-treated CFBs, the expression levels of p-Smad2/3 and MAPK signaling molecules were significantly increased (Fig. 4a and b). Compared with AdGFP mice, AdLefty1 injection significantly decreased the expression level of p-Smad2 and p-ERK1/2, while diminished expression level of p-smad2 and p-ERK1/2 could be reversed by silencing Lefty1, as demonstrated by Fig. 4b. Moreover, consistent results were presented in experimental MI mice. At 4 weeks after MI, the expression levels of p-Smad2/3 and MAPK signaling molecules were dramatically increased compared with sham-operated mice, while only p-Smad2 and p-ERK1/2 levels were markedly decreased in rAAV9-Lefty1 mice compared with rAAV9-GFP mice (Fig. 4c). In summary, the inhibition of p-Smad2 or p-ERK1/2 pathway may be the central mechanism of Lefty1 to attenuate TGF-β1 or MI-induced fibrosis.
Lefty1 inhibits the phosphorylation of Smad2 and ERK1/2 induced by TGF-β1 or MI. a Cultured CFBs were infected with AdLefty1 vectors, followed by stimulation with TGF-β1 (10 ng/ml) for 48 h. Proteins in CFBs were detected by Western blot analysis (left) and quantification (right) using anti-p-Smad2, anti-p-Smad3, anti-t-Smad2/3, anti-p-ERK1/2, anti-t-ERK1/2, anti-p-JNK1/2, anti-t-JNK1/2, anti-p-p38 MAPK, anti-t-p38 MAPK, and GAPDH (n = 3 batches of cells per group). b Cultured CFBs were infected with AdshLefty1 vectors, followed by stimulation with TGF-β1 (10 ng/ml) for 48 h. Proteins in CFBs were detected by Western blot analysis (left) and quantification (right) using anti-p-Smad2, anti-p-Smad3, anti-t-Smad2/3, anti-p-ERK1/2, anti-t-ERK1/2, anti-p-JNK1/2, anti-t-JNK1/2, anti-p-p38 MAPK, anti-t-p38 MAPK, and GAPDH (n = 3 batches of cells per group). *P < 0.05 vs. PBS group; #P < 0.05 vs. rAAV9-GFP group. c Western blot analysis of LV samples from infarct areas for p-Smad2, p-Smad3, t-Smad2/3, p-ERK1/2, t-ERK1/2, p-JNK1/2, t-JNK1/2, p-p38 MAPK, t-p38 MAPK, and GAPDH (n = 7 mice per group). *P < 0.05 vs. sham operation group; #P < 0.05 vs. rAAV9-GFP group. GAPDH was used as loading control. p-Smad2, phosphorylated small mother against decapentaplegic 2; p-Smad3, phosphorylated small mother against decapentaplegic 3; t-Smad2/3, total small mother against decapentaplegic 2/3; p-ERK1/2, phosphorylated extracellular signal-regulated kinase 1/2; t-ERK1/2, total phosphorylated extracellular signal-regulated kinase 1/2; p-JNK, phosphorylated c-Jun N-terminal kinase; t-JNK, total c-Jun N-terminal kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase
Discussion
Cardiac fibrosis could be like a double-edged sword post-MI: initially, it is integral to promoting infarct zones healing and scar formation; however, excessive and perpetual fibrosis gradually leads to dilated heart chambers and impaired cardiac function. The TGF-β superfamily is a pluralistic group of pleiotropic multifunctional proteins. In mammals, the TGF-β superfamily consists of 33 members, including TGF-β, activin, nodal, bone morphogenetic protein (BMP), and growth differentiation factor (GDF). They are involved in a variety of physiological activities, such as growth, proliferation, differentiation, and apoptosis [14]. In the process of myocardial fibrosis and ventricular remodeling, these proteins exhibit diverse and divergent effects. For instance, TGF-β1 mediates the transformation of fibroblasts into myofibroblasts and potently stimulates the synthesis of ECM, which are central roles of TGF-β1 in the pathogenesis of fibrosis [15]. Activin A promotes the proliferation and differentiation of CFBs and can be used as a predictor of left ventricular remodeling and mortality in patients with ST segment elevation myocardial infarction [16, 17]. BMP4 not only induces CFBs to synthesis more collagen but also promotes myocardial apoptosis [18, 19]. By contrast, some members have also been reported to show cardioprotective effects. BMP7 and 10 have been reported to reduce collagen deposition along with inhibition of EMT [20, 21]. The increase of GDF15 expression was accompanied by the decrease of the myocardial collagen area and the reduction of the mRNA level of type I and type III collagen [22]. The interrelationship between TGF-β superfamily and post-MI fibrosis is intricate and thus should be fully studied.
Lefty, a novel member of the TGF-β superfamily, is involved in the differentiation of stem cells and the establishment of laterality in the embryo [23]. Previous studies have demonstrated that Lefty1 acts as a potent inhibitor of the TGF-β signaling pathway, as evidenced by blocking the proliferation and differentiation of fibroblasts and inhibiting collagen synthesis in non-cardiac tissues [9, 10, 24, 25]. Given the promising anti-fibrotic effects of Lefty1 on other organs fibrosis, we, therefore, have a strong interest in the role of Lefty1 on MI-induced CF. In the present study, we found an elevated level of Lefty1 in experimental MI mice, and TGF-β1 stimulated neonatal rat fibroblasts. To elucidate whether Lefty1 could inhibit CFBs, we used adenoviral vectors to transfer the Lefty1 gene into the neonatal CFBs. The results showed that the overexpression of Lefty1 significantly inhibited TGF-β1-mediated CFBs’ proliferation, differentiation, and reduced collagen deposition. Contrarily, the inhibition of fibroblast proliferation and differentiation, as well as collagen secretory capacities, was abrogated after low expression of Lefty1. We further constructed mice MI model to verify the roles of Lefty1 again and confirmed that Lefty1 also possesses antifibrotic properties in vivo, as evidenced by smaller infarct size, decreased collagen deposition and α-SMA mRNA level in the infarct border zone, as well as the maintenance of cardiac systolic and diastolic function in rAAV9 mice compared with control mice, which could be partly attributable to decreased CFB proliferation, differentiation, and collagen synthesis activities. In the pathogenesis of post-MI fibrosis, in addition to the excessive proliferation and differentiation of fibroblasts, the overdeposition of collagen, the perpetual myocardial apoptosis is an important player involved. Reduced myocardial apoptosis is beneficial for maintaining cardiac function. However, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was not performed in our study to verify whether myocardial apoptosis was reduced, which was a limitation of the present study and needed to be further validated. Despite this, we are the first to report that Lefty1 could mitigate CF after MI in vivo and in vitro, which was consistent with previous studies of Lefty1.
Considering that Lefty1 acts as an inhibitor of TGF-β signaling by suppressing Smad2/3 phosphorylation after TβRI activation and MAPK signaling pathway [24, 26], we probed canonical Smad- and non-Smad dependent signal pathway molecules to clarify how Lefty1 alleviate myocardial fibrosis in vitro and in vivo. Previous studies have established that the activation of TGF-β1/Smad2 signaling is involved in the pathogenesis of myocardial fibrosis [27,28,29,30]. In our research, TGF-β1 treatment, as well as coronary ligation, markedly increased the expression of p-Smad2 and p-Smad3. Lefty1 overexpression significantly suppressed the level of p-Smad2, while the level of p-Smad3 remained unchanged, suggesting that Lefty1 alleviated cardiac fibrosis by partly interfering with Smad2 signaling pathway, which is consistent with previous results. As for the non-canonical pathway, we discovered the upregulation of MAPK signaling pathway molecules in both TGF-β1-treated CFBs and MI mice, whereas only phospho-extracellular signal-regulated kinase 1/2 (p-ERK1/2) level significantly decreased in response to Lefty1 overexpression, suggesting that the ERK1/2 signaling pathway was also a contributor to the anti-fibrotic effect of Lefty1. We did not observe significant changes in protein levels of phosphorylated active form of JNK1/2 and p38 MAPK after Lefty1 overexpression. Our result was consistent with previous evidence demonstrating the ERK1/2 pathway has frequently been found to be associated with cardiac fibrosis in various studies [31,32,33,34]. However, we only investigated the TGF-β1/Smad2/3 and MAPK signaling pathways; the effects on other signaling pathways and crosstalk between these pathways require further study.
In summary, we determined the protective roles of Lefty1 in alleviating post-MI fibroblasts proliferation, differentiation, and secretion as well as reducing infarct size and preserving cardiac function via partly suppressing p-Smad2 and p-ERK1/2 signaling pathways. Our findings highlight the importance of Lefty1 in the prevention of post-infarction fibrosis and may help identify potential targets for therapeutic intervention of cardiac fibrosis.
Abbreviations
- TGF-β1:
-
Transforming growth factor-β1
- MI:
-
Myocardial infarction
- CFB:
-
Cardiac fibroblast
- CF:
-
Cardiac fibrosis
- ECM:
-
Extracellular matrix
- HF:
-
Heart failure
- ALK:
-
Activin receptor–like kinase
- Smad:
-
Small mother against decapentaplegic
- MAPK:
-
Mitogen-activated protein kinase
- PI3K:
-
Phosphoinositide 3-kinase
- ERK:
-
Extracellular signal-regulated kinase
- JNK:
-
c-Jun N-terminal kinase
- EMT:
-
Epithelial-mesenchymal transition
- UUO:
-
Unilateral ureteral obstruction
- rAAV9:
-
Recombinant adeno-associated virus serotype 9
- GFP:
-
Green fluorescent protein
- PBS:
-
Phosphate buffered saline
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- α-SMA:
-
α-smooth muscle actin
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OD:
-
Optical density
- LVSD:
-
LV end-systolic diameter
- LVDD:
-
End-diastolic diameter
- EF:
-
Ejection fraction
- FS:
-
Fractional shortening
- BMP:
-
Bone morphogenetic protein
- GDF:
-
Growth differentiation factor
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
References
Talman, V., & Ruskoaho, H. (2016). Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell and Tissue Research, 365, 563–581.
Dobaczewski, M., Chen, W., & Frangogiannis, N. G. (2011). Transforming growth factor (TGF)-β signaling in cardiac remodeling. Journal of Molecular and Cellular Cardiology, 51, 600–606.
Heldin, C. H., Miyazono, K., & ten Dijke, P. (1997). TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature, 390, 465–471.
Euler-Taimor, G., & Heger, J. (2006). The complex pattern of SMAD signaling in the cardiovascular system. Cardiovascular Research, 69, 15–25.
Liu, G., Ma, C., Yang, H., & Zhang, P. Y. (2017). Transforming growth factor β and its role in heart disease. Experimental and Therapeutic Medicine, 13, 2123–2128.
Zhang, Y. E. (2017). Non-Smad signaling pathways of the TGF-β family. Cold Spring Harbor Perspectives in Biology, 9, a022129.
Kosaki, K., Bassi, M. T., Kosaki, R., Lewin, M., Belmont, J., Schauer, G., & Casey, B. (1999). Characterization and mutation analysis of human LEFTY A and LEFTY B, homologues of murine genes implicated in left-right axis development. American Journal of Human Genetics, 64, 712–721.
Mason, J. M., Xu, H. P., Rao, S. K., Leask, A., Barcia, M., Shan, J., Stephenson, R., & Tabibzadeh, S. (2002). Lefty contributes to the remodeling of extracellular matrix by inhibition of connective tissue growth factor and collagen mRNA expression and increased proteolytic activity in a fibrosarcoma model. The Journal of Biological Chemistry, 277, 407–415.
Xu, C., Xu, M., Wang, W., & Zhang, J. (2016). Lefty1 alleviates renal tubulointerstitial injury in mice with unilateral ureteral obstruction. Molecular Medicine Reports, 13, 901–908.
Zhang, L., Liu, X., Liang, J., Wu, J., Tan, D., & Hu, W. (2020). Lefty-1 inhibits renal epithelial-mesenchymal transition by antagonizing the TGF-β/Smad signaling pathway. Journal of Molecular Histology, 51, 77–87.
Fei, W., Kijima, D., Hashimoto, M., Hashimura, M., Oguri, Y., Kajita, S., Matsumoto, T., Yokoi, A., & Saegusa, M. (2017). A functional role of LEFTY during progesterone therapy for endometrial carcinoma. Cell Communication and Signaling: CCS, 15, 56.
Chen, Y. H., Wang, Q., Li, C. Y., Hou, J. W., Chen, X. M., Zhou, Q., Chen, J., Wang, Y. P., & Li, Y. G. (2017). Haplodeficiency of activin receptor-like kinase 4 alleviates myocardial infarction-induced cardiac fibrosis and preserves cardiac function. Journal of Molecular and Cellular Cardiology, 105, 1–11.
Ni, L., Scott Jr., L., Campbell, H. M., Pan, X., Alsina, K. M., Reynolds, J., Philippen, L. E., Hulsurkar, M., Lagor, W. R., Li, N., & Wehrens, X. H. T. (2019). Atrial-specific gene delivery using an adeno-associated viral vector. Circulation Research, 124, 256–262.
Morikawa, M., Derynck, R., & Miyazono, K. (2016). TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harbor Perspectives in Biology, 8, a021873.
Hanna, A., & Frangogiannis, N. G. (2019). The role of the TGF-β superfamily in myocardial infarction. Frontiers in Cardiovascular Medicine, 6, 140.
Hu, J., Wang, X., Wei, S. M., Tang, Y. H., Zhou, Q., & Huang, C. X. (2016). Activin A stimulates the proliferation and differentiation of cardiac fibroblasts via the ERK1/2 and p38-MAPK pathways. European Journal of Pharmacology, 789, 319–327.
Lin, J. F., Hsu, S. Y., Teng, M. S., Wu, S., Hsieh, C. A., Jang, S. J., Liu, C. J., Huang, H. L., & Ko, Y. L. (2016). Activin a predicts left ventricular remodeling and mortality in patients with ST-elevation myocardial infarction. Acta Cardiologica Sinica, 32, 420–427.
Sun, B., Huo, R., Sheng, Y., Li, Y., Xie, X., Chen, C., Liu, H. B., Li, N., Li, C. B., Guo, W. T., Zhu, J. X., Yang, B. F., & Dong, D. L. (2013). Bone morphogenetic protein-4 mediates cardiac hypertrophy, apoptosis, and fibrosis in experimentally pathological cardiac hypertrophy. Hypertension, 61, 352–360.
Pachori, A. S., Custer, L., Hansen, D., Clapp, S., Kemppa, E., & Klingensmith, J. (2010). Bone morphogenetic protein 4 mediates myocardial ischemic injury through JNK-dependent signaling pathway. Journal of Molecular and Cellular Cardiology, 48, 1255–1265.
Merino, D., Villar, A. V., García, R., Tramullas, M., Ruiz, L., Ribas, C., Cabezudo, S., Nistal, J. F., & Hurlé, M. A. (2016). BMP-7 attenuates left ventricular remodelling under pressure overload and facilitates reverse remodelling and functional recovery. Cardiovascular Research, 110, 331–345.
Zeisberg, E. M., Tarnavski, O., Zeisberg, M., Dorfman, A. L., McMullen, J. R., Gustafsson, E., Chandraker, A., Yuan, X., Pu, W. T., Roberts, A. B., Neilson, E. G., Sayegh, M. H., Izumo, S., & Kalluri, R. (2007). Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature Medicine, 13, 952–961.
Ren, Q., Lin, P., Wang, Q., Zhang, B., & Feng, L. (2019). Chronic peripheral ghrelin injection exerts antifibrotic by increasing growth differentiation factor 15 in rat hearts with myocardial fibrosis induced by isoproterenol. Physiological Research, 69, 439–450.
Meno, C., Saijoh, Y., Fujii, H., Ikeda, M., Yokoyama, T., Yokoyama, M., Toyoda, Y., & Hamada, H. (1996). Left-right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature, 381, 151–155.
Zhang, L., Zhang, J., Xu, C., Zhou, X., Wang, W., Zheng, R., Hu, W., & Wu, P. (2015). Lefty-1 alleviates TGF-β1-induced fibroblast-myofibroblast transdifferentiation in NRK-49F cells. Drug Design, Development and Therapy, 9, 4669–4678.
Ma, H., Qiao, S., Wang, Z., Geng, S., Zhao, Y., Hou, X., Tian, W., Chen, X., & Yao, L. (2017). Microencapsulation of Lefty-secreting engineered cells for pulmonary fibrosis therapy in mice. American Journal of Physiology. Lung Cellular and Molecular Physiology, 312, L741–l747.
Ulloa, L., & Tabibzadeh, S. (2001). Lefty inhibits receptor-regulated Smad phosphorylation induced by the activated transforming growth factor-beta receptor. The Journal of Biological Chemistry, 276, 21397–21404.
Gao, L., Wang, L. Y., Liu, Z. Q., Jiang, D., Wu, S. Y., Guo, Y. Q., Tao, H. M., Sun, M., You, L. N., Qin, S., Cheng, X. C., Xie, J. S., Chang, G. L., & Zhang, D. Y. (2020). TNAP inhibition attenuates cardiac fibrosis induced by myocardial infarction through deactivating TGF-β1/Smads and activating P53 signaling pathways. Cell Death & Disease, 11, 44.
Chen, L. L., Yin, H., & Huang, J. (2007). Inhibition of TGF-beta1 signaling by eNOS gene transfer improves ventricular remodeling after myocardial infarction through angiogenesis and reduction of apoptosis. Cardiovascular Pathology, 16, 221–230.
Huang, W., Rubinstein, J., Prieto, A. R., & Wang, D. H. (2010). Enhanced postmyocardial infarction fibrosis via stimulation of the transforming growth factor-beta-Smad2 signaling pathway: role of transient receptor potential vanilloid type 1 channels. Journal of Hypertension, 28, 367–376.
Liu, H., Liu, A., Shi, C., & Li, B. (2016). Curcumin suppresses transforming growth factor-β1-induced cardiac fibroblast differentiation via inhibition of Smad-2 and p38 MAPK signaling pathways. Experimental and Therapeutic Medicine, 11, 998–1004.
Wu, H., Li, G. N., Xie, J., Li, R., Chen, Q. H., Chen, J. Z., Wei, Z. H., Kang, L. N., & Xu, B. (2016). Resveratrol ameliorates myocardial fibrosis by inhibiting ROS/ERK/TGF-β/periostin pathway in STZ-induced diabetic mice. BMC Cardiovascular Disorders, 16, 5.
Wang, Y., Guo, Z., Gao, Y., Liang, P., Shan, Y., & He, J. (2019). Angiotensin II receptor blocker LCZ696 attenuates cardiac remodeling through the inhibition of the ERK signaling pathway in mice with pregnancy-associated cardiomyopathy. Cell & Bioscience, 9, 86.
Luo, S., Hieu, T. B., Ma, F., Yu, Y., Cao, Z., Wang, M., Wu, W., Mao, Y., Rose, P., Law, B. Y., & Zhu, Y. Z. (2017). ZYZ-168 alleviates cardiac fibrosis after myocardial infarction through inhibition of ERK1/2-dependent ROCK1 activation. Scientific Reports, 7, 43242.
Pan, Z., Zhao, W., Zhang, X., Wang, B., Wang, J., Sun, X., Liu, X., Feng, S., Yang, B., & Lu, Y. (2011). Scutellarin alleviates interstitial fibrosis and cardiac dysfunction of infarct rats by inhibiting TGFβ1 expression and activation of p38-MAPK and ERK1/2. British Journal of Pharmacology, 162, 688–700.
Funding
This study was supported by Grants from the National Natural Science Foundation of China (No. 81700271) and S&T Program of Hebei (No. H2018105054).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All experimental procedures were conducted in compliance with both the Animal Care and Use Committee of Capital Medical University and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (the 8th Edition, NRC 2011). This article does not contain any studies with human participants performed by any of the authors.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Cy., Zhang, Jr., Li, Xx. et al. Lefty1 Ameliorates Post-infarction Fibrosis by Suppressing p-Smad2 and p-ERK1/2 Signaling Pathways. J. of Cardiovasc. Trans. Res. 14, 636–646 (2021). https://doi.org/10.1007/s12265-020-10089-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-020-10089-2