Abstract
Circadian rhythm is manifested by the behavioral and physiological changes from day to night, which is controlled by the pacemaker and its regulator. The former is located at the suprachiasmatic nuclei (SCN) in the anterior hypothalamus, while the latter is composed of clock genes present in all tissues. Circadian desynchronization influences normal patterns of day-night rhythms such as sleep and alertness cycles, rest and activity cycles. Parkinson’s disease (PD) exhibits diurnal fluctuations. Circadian dysfunction has been observed in PD patients and animal models, which may result in negative consequences to the homeostasis and even exacerbate the disease progression. Therefore, circadian therapies, including light stimulation, physical activity, dietary and social schedules, may be helpful for PD patients. However, the cellular and molecular mechanisms that underlie the circadian dysfunction in PD remain elusive. Further research on circadian patterns is needed. This article summarizes the existing research on the circadian rhythms in PD, focusing on the clinical symptom variations, molecular changes, as well as the available treatment options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, affecting about 315 per 100,000 people [1]. The patients often suffer from motor symptoms including rigidity, bradykinesia, tremor, and impaired balance, and also non-motor symptoms such as sleep disturbance, pain, cognitive deficits, depression. Pathologically, it is featured by the loss of dopaminergic neurons in the substantia nigra and the formation of Lewy bodies. According to the Braak staging theory, other brain regions are also affected in PD, which may contribute to the development of some non-motor symptoms [2, 3]. Recently, increasing attention has been paid to non-motor symptoms, which pose much stress on the life quality of patients and their caregivers. Particularly, several studies have found that motor activity and responsiveness to anti-PD drugs vary through out the day. Likewise, sleep-wake cycles, visual performance and autonomic dysfunction show diurnal fluctuations. Moreover, the secretion pattern of endocrine hormone is also affected. All these imply that the circadian rhythm is disrupted in PD.
Increasing evidence has shown that there are reciprocal interactions between DA system and circadian rhythm. On the one hand, circadian genes can modulate the dopamine synthesis. First, tyrosine hydroxylase, the rate-limiting enzyme for dopamine synthesis, is regulated by the circadian locomotor cycle kaput (Clock) gene [4]. It can modulate the transcription of tyrosine hydroxylase, dopamine activity transporter and D1 receptor via targeting the E-Box element, which is located in the promoter regions of these DA-related genes [5]. Second, the circadian genes may also affect the dopaminergic activity in the ventral tegmental area at the posttranscriptional level [6]. On the other hand, dopamine is thought to regulate some Clock genes in a receptor-dependent manner, though the D1 receptor may play a different role from the D2 subtype [7]. To be specific, dopamine can upregulate the transcriptional activity of Clock/Bmal complex through enhancing the recruitment and phosphorylation of the transcriptional coactivator cAMP-responsive element-binding proteins [8]. In the dorsal striatum, Hood et al. reported that the rhythm of Per2 expression could be modified by D2 receptor activation, while the Per2 rhythm in the suprachiasmatic nuclei (SCN) remained unaffected [9]. Consistently, Imbesi et al. found that D2 receptor agonists inhibited the expression of Clock and Per1 genes, while D1 receptor agonists enhanced the expression of Per1, Clock, NPAS2 and Bmal1 [7]. Besides, Yujnovsky et al. found a drastic decrease of Per1 gene transcription in the retinas of D2R (dopamin D2 receptor)-null mice [8]. Dopamine also resets the rhythm during the prenatal stage in the circadian pacemaker SCN [10, 11]. Moreover, the melatonin, which is secreted from the pineal gland, acts as a stimulator of the fetal SCN [12] and is also controlled by dopamine receptor signaling [13].
Consequently, it is reasonable to link the symptoms fluctuation in PD patients with the dysregulation of circadian rhythm (Fig. 1). Therefore, to better understand the biorhythm dysfunction in PD, we will review literatures on the circadian rhythm of PD, and explore its possible mechanisms.
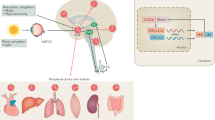
A simplified scheme of the disturbed circadian system in PD. Reduced time cues are obtained from external zeitgebers in PD patients, due to the impaired visual acuity and disabled motor ability. Meanwhile, the internal circadian clock outputs are damaged in SCN, where the α-synuclein is accumulated. As a result, disrupted rhythm of clock genes in SCN impairs its modulation of other central and peripheral clocks, which might have negative consequences on behavioral and physiological functions. SCN, suprachiasmatic nuclei; SN, substantia nigra.
Sleep Disorders
The sleep-wake cycle is controlled by the circadian clock [14]. In a large survey on non-motor symptoms of PD, about 64% patients have sleep problems, only next to the psychiatric disorders [15]. The sleep disorder can be categorized into insomnia (difficulty in falling or staying asleep), excessive daytime somnolence (EDS), rapid eye movement sleep behavioral disorder (RBD), etc. Sleep fragmentation and reduced sleep efficiency have a bad impact on the life quality of patients, and may accelerate the development of PD dementia.
Insomnia is one of the most common sleep disorders in patients with PD. Several investigations have shown that about 50% of PD patients have insomnia complaints [16, 17]. Both motor and non-motor symptoms may be responsible for the insomnia. For instance, the overnight emergence of motor symptoms like tremor, rigidity and dyskinesia may result in awakenings and being unable to fall back to sleep again [18]. Pain, nocturia and mood disorders are important factors influencing insomnia in general population. The nocturia inevitably affects the sleep maintenance and is associated with PD severity and duration. Kurtis et al. reported that depression is also correlated with nocturnal sleep scores [19]. The depression severity may affect sleep initiation and maintenance difficulties [20]. Taking dopaminergic drugs, especially L-dopa in the late day, may influence sleep at night owing to the relatively higher levels of dopamine in the plasma [21].
EDS is more frequent in the PD patients compared to the age-matched controls. In a multicenter, cross-sectional study, researchers found 87% of PD patients had complaints about EDS, while other reports showed that the prevalence ranged from 20% to 50%. Although these studies are not similar, they all suggest that EDS is quite common among sleep disorders [19]. Elevated Epworth Sleep Scale (ESS) scores, male gender, long duration and severity of the disease are the major risks for EDS [22]. Several studies indicated that dopaminergic load acts as a correlative factor for a higher ESS score and increased daytime sleepiness in PD [23, 24]. In addition, Yi PL et al. proved that IL-1β in the hypothalamus mediates the EDS in the rotenone rat model and that the TNF–NF-κB signaling is involved in another hemiparkinsonian rat model [25, 26]. These results suggest that the accumulation of inflammatory cytokines may play an important role in regulating sleep cycles.
RBD is characterized by the absence of muscle atonia during REM sleep as well as dreaming enacting. The prevalence of RBD in PD is about 15%–60% [27]. A longitudinal study by Claassen et al. indicated that RBD often precedes the clinical manifestation of PD by 15–50 years [28]. Moreover, the presence of RBD not only correlates with the high incidence of neurodegenerative disorders [29] such as PD, but also stands for a worse prognosis with reduced sympathetic activity and increased cognitive impairment [30,31,32]. But the pathophysiology of RBD in PD is not fully understood. RBD is possibly due to the specific degeneration of descending REM-on excitatory glutamatergic neurons localized in the pontine sublateraldorsal nucleus and the REM-on inhibitory GABA or glycinergic neurons in the ventral medullary reticular formation [33]. Besides, α-synuclein aggregates are found in the submandibular gland in the majority of patients with idiopathic RBD, which imply that RBD may be also associated with the ongoing synucleinopathy [34].
In MPTP-induced monkey model of PD, the disturbance of REM sleep and the appearance of EDS precede the emergence of typical motor signs. And the sleep–wake architecture is dramatically disrupted with increased number of arousals and reduced sleep efficacy [35]. In line with this, chin muscle disturbance is also found in REM sleep in MPTP marmoset model, which mimics RBD in the premotor phase of PD [36].
Motor fluctuation
Rest-activity rhythm is recorded by an actigraphy in patients with PD. As Van Hilten et al. reported, normal motor activity reached the lowest level at night, and gradually increased during the daytime. But the rhythm is modified in PD patients. In the mild and moderate stage patients, the pattern of motor activity remains unaltered, despite that the mean level of motor activity is lower than that in healthy elderly subjects. Meanwhile, in the advanced stage patients, the indices show the absence of diurnal changes [37, 38]. Some studies have also reported that PD patients show lower activity levels and higher rest levels, which are in strong correlation with the disease severity [39, 40]. Furthermore, some patients often complain that L-dopa seems to have little improvement effect on motor symptoms later in the day than in the morning, whereas some even have no responses in the evening at all. However, the underlying mechanisms are still unclear. The motor response to repeated L-dopa administration was evaluated in PD patients by Bonuccelli et al. The results showed that there were progressive daytime worsening motor scores both in stable and wearing-off patients, but not in de novo patients. It is possible that the phenomenon was caused by the tolerance to the repeated doses of L-dopa, because the plasma 3-O-methyldopa level increased after repeated L-dopa administration [41]. Another study using continuous L-dopa infusions also reported diurnal worsening of motor fluctuations in PD. The concentration of plasma large neutral amino acids was higher in the evening than in the morning, which suggested the contribution of peripheral pharmacokinetic mechanisms to the weaker response to repeated doses of L-dopa [42]. Piccini et al. evaluated the motor performance hourly in patients receiving different drugs. Only the L-dopa-treated group showed a progressive daytime worsening, accompanied by an increase in plasma 3-O-methyldopa level, however, this phenomenon was not found in the bromocriptine and de novo groups [43].
In an unilaterally 6-OHDA-lesioned rat model, the total amount of locomotor activity was reduced but the phase remained unchanged [44]. In a study examining the circadian rhythm in wild-type α-synuclein transgenic mice, the wheel-running test demonstrated an impaired locomotor activity with a lower nighttime activity [45]. Monville et al. injected L-dopa into PD rat models three times a day but found no significant influence on the formation of dyskinesia at night [46]. However, there is no direct evidence to clarify whether the worsening movement symptom in the late day is dependent on the pharmacokinetics of the drugs or not.
In light of these studies, although the results of these studies are not consistent, they still prove that the decremental response in the evening does not occur in the de novo patients. This indicates that the disease stage may be a major contributor while the pharmacokinetic factors also affect the motor fluctuation.
Temperature Imbalance
The circadian rhythm of core body temperature is mainly under the control of SCN [47]. It has been reported that the mesor of body temperature is significantly lower in PD than in the healthy controls, and the gap between the mesor and nadir temperature is reduced, which may be related with the severity of PD [48]. Another study confirmed the impaired thermoregulation of endogenous opioid system in PD by evaluating the effect of naloxone on the body temperature in the postmenopausal women. Meanwhile, this study also provided evidence that the core body temperature with PD is lower than that of controls [49]. Suzuki et al. revealed that PD patients with depression had lower amplitudes of core body temperature and higher minimum rectal temperature, indicating that the characteristics of core body temperature may serve as a biomarker for PD depression [50]. Chronic rotenone-treated rats also showed lower amplitudes and instability phase of body temperature, as compared to the controls. The magnitude of the alterations was positively correlated with the degree of movement disorders [51].
Interestingly, Rango et al. using proton magnetic resonance spectroscopy found that PD patients exhibit a slightly increased temperature in the visual cortex and the centrum semiovale at rest [52]. Sumida et al. also examined the temperature of the intraventricular cerebrospinal fluid in PD patients by diffusion-weighted imaging thermometry. They found that male PD patients had significantly higher cerebrospinal fluid temperature values than the male controls, but this was not observed in the women [53]. Most importantly, the increased brain temperature may further impair the mitochondrial energetics, forming a vicious cycle during PD progression.
Autonomic System Dysfunctions
The changes of heart rate and blood pressure have a 24-h period, depending on the endogenous circadian rhythms and sleep-activity rhythm. The cardiovascular autonomic regulation has been reported impaired in PD.
A study by Ejaz et al. confirmed the presence of reversal of circadian rhythm in 93% PD patients using the method of 24 h ambulatory blood pressure monitoring (ABPM) [54]. They also found that all of the PD patients had postprandial hypotension and nocturnal hypertension. Schmidt et al. observed a similar pathological nocturnal blood pressure regulation [55]. Usually, the mean arterial pressure decreases by 10%–20% during sleep, compared to the waking time. But in most PD patients, this nocturnal fall of blood pressure is lost independent of the orthostatic hypotension. In an ABPM study using a large sample size of 111 PD patients, Berganzo et al. found abnormal ambulatory blood pressure rhythm in PD. Moreover, the impaired blood pressure pattern was correlated with the dose of dopaminergic drugs and the prevalence of autonomic disorders [56]. In other words, PD patients are also at a high risk of cardiovascular diseases if they lack recognition of the hypertension component of orthostatic hypotension. As a result, ABPM should be taken into account for the diagnosis of nocturnal hypertension, and appropriate management of antihypertensive and dopaminergic treatments in PD patients should be considered.
The heart rate variability (HRV) analysis is a widely accepted method for indirect evaluation of activity variability of the autonomic nervous system. Kallio et al. investigated 50 untreated PD patients with different initial symptoms. Hypokinesia or rigidity onset PD patients had an obvious absence of HRV than those with tremor onset and suffered higher risk of autonomic nervous system disturbances [57]. Devos et al. recorded HRV of 30 patients in different PD stages through Holter electrocardiographic monitoring. They found no sympathovagal balance dysfunction in mild untreated PD patients, but a loss of HRV and distinct sympathetic morning peak with decreased nocturnal vagal parameters in moderate and severe L-dopa-treated patients. They speculated that the evolutive HRV decrease is correlated with the disease severity [58]. However, Harnod et al. reported that the impaired HRV is more likely to be associated with the motor symptom duration of PD, but not with the disease severity or patient age. Meanwhile, PD patients are more likely to have a worse cardiac parasympathetic dysregulation than sympathetic dysregulation [59]. Recently, an investigation was conducted to evaluate whether the other non-symptoms such as RBD contribute to the cardiovascular dysautonomia. The results showed that both sympathetic and parasympathetic values are higher at night, implying that RBD might be susceptible to cardiovascular diseases, and that these two indices had a specificity of 100% for distinguishing RBD patients from those all PD individuals [60].
In the 6-OHDA–lesioned rat model, the amplitude of the heart rate was significantly decreased and the L-dopa replacement therapy rescued the abolished circadian rhythms, especially for the heart rate rhythm [61]. A recent study demonstrated that neurogenic orthostatic hypotension occurred as a result of the progressive noradrenergic denervation from sympathetic terminals upon standing [62].
In conclusion, the rhythmic loss in cardiovascular autonomic system is a problem in PD that should no longer be ignored, because it could increase the risk of cardio-cerebral vascular incidents, affecting the patients’ life quality and life spans.
Visual Impairment
The retina is a sensory organ as well as an endogenous circadian clock. As a circadian clock, the retina can modulate a plenty of circadian rhythms, such as rod and cone balance, visual sensitivity and electroretinogram b-wave amplitude, and dopamine synthesis. In mammals, the retinal clock and its outputs may have an impact on the trophic situation in eyes [63]. Dysfunction of the retinal clock may result in a poorer susceptibility of photoreceptor and a lower survival rate of the retinal neurons. Dopamine, as the major catecholamine in the retina of vertebrates, plays a key role in the adaptation to light [64].
Struck et al. tested 43 eyes in the PD patients for the fluctuation of contrast sensitivity at 2-h intervals. At the beginning of the morning, contrast sensitivity in PD does not differ from the normal controls. But it gets worse in the late phase at 2:30 PM in PD while remaining unaltered throughout the day in controls [65]. This suggests that the contrast sensitivity abnormality in PD may be related to dopamine deficiency.
Dopamine not only has an influence on light adaptation in the retina, but also modulates the function of the intrinsically photosensitive retinal ganglion cells by regulating the expression of the photopigment melanopsin [66]. In the retina, dopaminergic amacrine cells express clock genes, which could adapt the retina to the environment. Using the PERIOD2::LUCIFERASE fusion protein knock-in model, researchers reported that the dopamine regulates the Per proteins and plays a significant role in resetting the retinal rhythms by D1 receptor [67]. By activating the D1 receptor, dopamine may modulate the phase and amplitude of the circadian genes. Thus, loss of dopaminergic neurons in PD is related with the abnormal regulations of melanopsin-based photocurrent, which may further affect the light-adaptive or circadian modulation.
Hormonal Disruption
It has been shown that the melatonin synthesis and the corticosteroid secretion are directly or indirectly regulated by SCN. Increasingly, the output of melatonin and cortisol can be used as well-accepted markers of the central clockwork to reflect the endogenous rhythmicity. Nowadays, these markers have been studied in PD.
Melatonin
The melatonin is a natural hormone mainly produced in the mammalian pineal gland during the dark phase. SCN receives information from zeitgebers, transforms it into the photoperiodic information, and then delivers it to the pineal gland by neuronal pathways. It is well-known that melatonin is involved in the regulation of the sleep-wake cycle [68]. However, two-thirds of the PD patients suffer from sleep disturbance. Therefore, several studies have been carried out to examine the serum melatonin levels. Fertl et al. found that the melatonin secretion patterns in PD did not change, but the phase was advanced compared to the aged-matched subjects [69]. They further found that the advanced phase in PD patients was possibly affected by a central nervous dopaminergic effect caused by L-dopa treatment, but not the disease itself [70]. Bordet et al. investigated the circadian melatonin pattern at different stages of PD. They found that dopaminergic treatments could render a significant phase advance in plasma melatonin levels. The amount of melatonin secretion during the daytime is increased in PD with L-dopa-related motor complications. However, during the nighttime, it decreases significantly in these patients [71].
The melatonin secretion pattern in PD has not received much attention until recent years. Bolitho et al. found that the dopaminergic treatment increases the secretion of melatonin and induces a delayed sleep onset relative to the melatonin secretion onset [72]. In other words, dopamine may result in the uncoupling between melatonin synthesis and sleep-wake circle, which may account for part of the sleep disturbances in L-dopa-treated PD patients. Videnovic et al. found that in L-dopa-treated PD patients, the amplitude of melatonin rhythm and 24-h circulating melatonin levels are apparently lower [73]. Similarly, Breen et al. reported that in early-stage PD, almost half of the newly diagnosed PD patients have sleep complaints. They showed reduced melatonin production with arrhythmic expression. In addition, the cortisol level is elevated and the brain and muscle arnt-like protein-1 (Bmal1) expression is altered in PD patients when compared with controls [74]. Further, the reduced melatonin output in PD patients is significantly related to the degeneration of hypothalamic gray matter and the disease severity [75]. Melatonin, with antioxidant and anti-inflammatory properties, is known to have beneficial effects against brain mitochondrial dysfunction with age [76], and may play a neuroprotective role in PD. Therefore, the impaired rhythm of melatonin secretion may be a result and also a cause of PD progression.
Cortisol
Cortisol secretion is dominated by the hypothalamic–pituitary–adrenal (HPA) axis, which receives the circadian flow from the hypothalamic paraventricular nucleus. So the secretory rhythm of cortisol could be regarded as a sensitive marker of circadian function, which is also impaired in PD patients.
Hartmann et al. measured a 24-h pulsatile cortisol release profile in plasma. They found that PD patients secrete significantly more cortisol per burst into plasma and the diurnal mean cortisol secretion rate is significantly higher but tends to be flattened than the age-matched controls. Furthermore, the concomitant L-dopa therapy may not contribute to this endocrine abnormality, as dopamine does not play a major role in modulating the HPA system activity [77]. The hypercortisolism has also been found in patients with early-stage PD. Breen et al. reported that there was an elevated total serum cortisol level and half of the patients had arrhythmic cortisol profiles but with no phase shifting [74]. This phenomenon has also been verified with the saliva biopsy in PD patients. Moreover, the diurnal cortisol concentration is not affected by L-dopa treatment, or the duration or severity of the disease [78]. Likewise, in an early study of MPTP-treated dogs, a significant increase in 24 h plasma cortisol concentration was observed but the expression rhythm disappeared. This implied that the HPA axis is modulated by the circadian rhythm and dopamine function [79]. Aziz et al. examined other neuroendocrine hormones in the serum, such as growth hormone, thyrotropin and prolactin, in 8 recently-diagnosed PD patients for 24 h, but no significant alteration was found in both mean levels or secretion patterns [80].
Meanwhile, some evidence could support the disorders of hormone release rhythm in PD. Langston et al. found the lewy body formation in every hypothalamus in 30 PD patients, and the lateral and posterior hypothalamic nuclei and tuberomamillary nucleus were most frequently involved in the highest average lewy body counts [81]. Several studies have proved the deficient dopamine transmission and reduced monoamine storage capacity in the hypothalamus of PD patients [82,83,84]. Likewise, an in vivo 11C-raclopride PET study indicated a reduction in the hypothalamic D2 receptor in PD compared with healthy age-matched controls [85]. In other words, the degeneration in hypothalamic nuclei may lead to the abnormalities in endocrine system. The above results provide information that the hypothalamus dysfunction contributes to the individual difference of non-motor syndromes among PD patients.
Clock Gene Difference
The physiological and behavioral rhythms are generated by an endogenous biological clock-SCN, and regulated by a series of clock genes [86]. There are several circadian genes known as key clock genes, such as Clock, Bmal, Period (Per1, Per2, Per3) and Cryptochrome (Cry1, Cry2). An increasing body of evidence supports that these clock genes are not only expressed in central circadian pacemakers, but also in many peripheral cells and tissues [87, 88]. Recently, several studies have demonstrated the desynchronized oscillatory in PD patients at the molecular level.
A study using peripheral leukocytes in the whole blood revealed that the mRNA expression of Bmal1 was significantly lower in PD patients during the evening, while Per1 expression showed no difference. And the relative Bmal1 level had a correlation with motor severity and sleep quality [89]. In addition, this group continued to check other key circadian genes in PD, and the results showed that the expression pattern of Bmal2, but not Clock or Dec1, was altered compared to controls [90]. Recently, Cai and his coworkers also tested genetic polymorphisms in circadian disruptions and their susceptibility to PD pathogenesis. They recruited 125 tag single-nucleotide polymorphisms of 1,394 PD patients and 1,342 controls for 8 key clock genes. Finally they observed a decrease of Bmal expression in PD and reported that the Bmal variant had a suitable association with the risk for the tremor dominant subtype, while the Per1 variant was with the postural instability and gait difficulties dominant subtype [91]. This suggests that disruption of the clock genes may not only alter the circadian periodicity and exacerbate the disease progression, but also play an etiological role in PD.
Modifications of these clock genes have also been confirmed in the 6-OHDA animal models, in which a reduction of the daily striatal Per2 expression has been reported [9]. However, expression of Per2 within the SCN was not altered in an α-synuclein overexpressing transgenic line [45]. In a rotenone-induced PD model, the daily pulse of Per2, Cry1, and Bmal1 was decreased in SCN. Melatonin administration restored the phase of Per1 but had no effect on other clock genes, indicating the differential sensitivity of clock genes towards melatonin [92].
Lin et al. proposed a mechanism that may account for the clock gene alterations in PD. As we know, histone acetylation, DNA methylation, non-coding RNA, and so on, play an important role in regulating the expression of clock genes, and ultimately, circadian phenotypes [93]. Abnormal CpG methylation has been observed in neurodegenerative disorders, including PD [94,95,96]. In PD, the methylation status in the NPAS2 (the paralog of Clock) promoter is decreased and the NPAS2 expression is increased. This, in turn, would activate the expression of Rorα and Rev-erbα, which serve as the main regulators of Bmal1 expression. Above all, the epigenetic alterations in NPSA2 expression may be the primary cause of changes in Bmal1 and Bmal2 in the leukocytes of PD patients [96]. Curtis AM et al. identified the importance of Bmal1 in modulating the inflammatory response, by regulating miR-155 and some proinflammatory cytokines including TNF-α [97].
In addition, the regular expression of clock genes is closely related to other circadian functions. Several studies show that the molecular regulators of the circadian clock have different influences on sleep patterns. For example, in NPAS2-deficient mice, non-REM sleep is reduced and more sleep time is required following sleep deprivation [98]. But the absence of Cry1, 2 is characterized by hypersomnolence [99]. Researchers also demonstrated that locomotor activity in both constant darkness and light–dark cycles was impaired and total activity levels were reduced in Bmal1 knockout mice [100, 101]. Similarly, Per1 mutants displayed significantly shorter circadian period and were unable to maintain the precision and stability [102]. Interestingly, deletion of Bmal1 from the vascular smooth muscle cells impaired vessel contractility and consequently, decreased mean arterial pressure through peripheral inputs [103]. In addition, deletion of Bmal1 also induced the attenuated rhythm of the body temperature [101]. Some clock genes also modulate the visual information processing to light. In the absence of Rev-erbα, mouse retinas modified scotopic threshold responses and increased pupillary constriction, thus enhancing the sensitivity to the light [104]. As to the relationship between melatonin and the daily changes of clock genes, Kandalepas PC et al. clarified that melatonin could induce phase advance of Per1 and Per2 at dusk through activation of protein kinase C [105]. In sum, the evidence clearly implicates that clock genes interact with other systems to maintain the balance of overall circadian rhythms.
Circadian Therapy
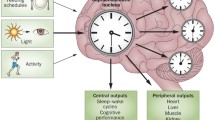
Increasing evidence suggests that circadian oscillation is disturbed in PD and that circadian regulation may become a new target for therapeutic intervention. Circadian therapy is aimed at regulating the biorhythm by changing the external zeitgebers. And the main external zeitgebers, such as light, physical activity, dietary and social schedules [86], could exert a synchronizing effect on internal biological periodicities.
Light therapy has previously been used as a method of psychiatric therapy, mainly for seasonal affective disorder. Many promising initiations have extended light treatment to a wider disease spectrum [106]. Dopamine is a chemical messenger for light adaptation [64], and exposure to light could increase retinal dopamine activity [107]. There is a sign that, circadian light entrainment is increasingly applied to PD patients attempting to reset the biologic clock. Paus et al. conducted a pilot study to evaluate changes of clinical symptoms in 36 PD patients receiving half an hour bright light therapy (BLT) in the morning for 15 days with the illuminance of 7500 lx. Different from the placebo group, BLT resulted in a marked improvement in Parkinson’s Disease Rating Scale part I, II and IV scores and depression [108]. In another study, Willis et al. also arranged 12 PD patients to BLT once daily at an intensity of 1000 to 1500 lx, before the usual time of sleep onset for 1 h. Two weeks later, amelioration was observed in bradykinesia and rigidity in most patients, as well as elevated mood, improved sleep and reduced demands for drugs [109]. Later, this group carried out a retrospective and open-label study in 129 PD patients to assess the systematic application of BLT. The results revealed that BLT facilitated motor performance recovery of PD patients, and decreased the dosage of dopamine replacement drugs. Meanwhile, improvements in mood and sleep quality were also observed in patients with other neurological disorders [110]. These studies have demonstrated the enabling effect of BLT on motor and non-motor manifestations in PD. Future research should still focus on early diagnosed or more severe PD patients to delay the progress or relieve the burden. Moreover, due to the advantages of non-invasion, low cost and convenient usage, BLT is a good option worth considering.
On the other hand, physical exercise, as a non-photic time cue of the circadian clock, could also delay the disease progression in some way. Yamanaka et al. proposed a hypothesis that physical exercise could enhance the sleep-wake cycle, which was independent of the circadian pacemaker [111]. Another study indicated that morning and evening exercise differentially regulate the autonomic nervous system [112]. More evidence was provided in animal models. For example, voluntary wheel-running could affect physiological circadian rhythms and delay the phase of peripheral Per2 expression [113]. Also, a meta-analysis showed that physical exercises including Argentine Tango and Tai Ji Quan may be appropriate choices for postural instability therapy in PD [114,115,116]. However, few exploratory studies have examined the effect of physical exercise on circadian systems in PD patients.
Questions to Be Answered
Although many studies are aimed to clarify the relationship between circadian dysfunction and PD, it is still difficult to determine whether circadian disruption is a causal factor for PD pathogenesis or a consequence of PD progression. First, it is certain that circadian disruption could exacerbate some pathological events of PD. For example, neuroinflammation is controlled by the intrinsic circadian clock in the microglia [117], and Bmal −/− could induce robust neuroinflammation responses. The amount of autophagy-related proteins in the brain displayed a 24 h rhythm, which could be blunted by sleep fragmentation [118]. As we know, α-synuclein aggregate, the main pathology of PD, is mostly degraded via autophagy. Mitochondrion is the target of most neurotoxins of PD and its dysfunction is associated with PD-related genes, such as PINK1, DJ-1, and PARKIN. One recent research showed that the function of mitochondrial respiration displayed in a diurnal manner, which was regulated by the clock proteins [119]. Second, the circadian system may also be disrupted by PD progression. SCN is found with the α-synuclein deposit, which may cause injury to the key cells of SCN. Moreover, the circadian dysfunction may be secondary to dopamine depletion, which is often observed in neurotoxin-induced PD models. In addition, several circadian manifestations in PD patients are often more severe in the late stage than in the early stage, which suggests that they are a consequence of the disease or the long-term drug medication. Therefore, in our opinion, the disrupted biorhythm and PD interact as both cause and effect.
Conclusion
PD is a multisystem disease and a growing body of evidence suggests the circadian rhythm disturbance. From this review, we could understand extensively about the fluctuation of sleep-wake cycle, motor disability, autonomic nervous system, etc. Besides the dopamine replacement treatments, strengthening the circadian functions, such as setting up a good life style, physical activity and melatonin therapy, may be a potential approach to improving the life quality of PD patients and slowing the disease progression.
References
Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 2014, 29: 1583–1590.
Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci 2010, 1184: 15–54.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003, 24: 197–211.
McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A 2005, 102: 9377–9381.
Kawarai T, Kawakami H, Yamamura Y, Nakamura S. Structure and organization of the gene encoding human dopamine transporter. Gene 1997, 195: 11–18.
Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry 2010, 68: 503–511.
Imbesi M, Yildiz S, Dirim Arslan A, Sharma R, Manev H, Uz T. Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience 2009, 158: 537–544.
Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc Natl Acad Sci U S A 2006, 103: 6386–6391.
Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, et al. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci 2010, 30: 14046–14058.
Kovacikova Z, Sladek M, Bendova Z, Illnerova H, Sumova A. Expression of clock and clock-driven genes in the rat suprachiasmatic nucleus during late fetal and early postnatal development. J Biol Rhythms 2006, 21: 140–148.
Seron-Ferre M, Mendez N, Abarzua-Catalan L, Vilches N, Valenzuela FJ, Reynolds HE, et al. Circadian rhythms in the fetus. Mol Cell Endocrinol 2012, 349: 68–75.
Torres-Farfan C, Rocco V, Monso C, Valenzuela FJ, Campino C, Germain A, et al. Maternal melatonin effects on clock gene expression in a nonhuman primate fetus. Endocrinology 2006, 147: 4618–4626.
Gonzalez S, Moreno-Delgado D, Moreno E, Perez-Capote K, Franco R, Mallol J, et al. Circadian-related heteromerization of adrenergic and dopamine D(4) receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biol 2012, 10: e1001347.
Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Brain Res Rev 2005, 49: 429–454.
Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 2009, 24: 1641–1649.
Lee MA, Prentice WM, Hildreth AJ, Walker RW. Measuring symptom load in Idiopathic Parkinson’s disease. Parkinsonism Relat Disord 2007, 13: 284–289.
Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord 2007, 22: 1623–1629.
van Hilten B, Hoff JI, Middelkoop HA, van der Velde EA, Kerkhof GA, Wauquier A, et al. Sleep disruption in Parkinson’s disease. Assessment by continuous activity monitoring. Arch Neurol 1994, 51: 922–928.
Kurtis MM, Rodriguez-Blazquez C, Martinez-Martin P, Group E. Relationship between sleep disorders and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 2013, 19: 1152–1155.
Happe S, Schrodl B, Faltl M, Muller C, Auff E, Zeitlhofer J. Sleep disorders and depression in patients with Parkinson’s disease. Acta Neurol Scand 2001, 104: 275–280.
Chahine LM, Daley J, Horn S, Duda JE, Colcher A, Hurtig H, et al. Association between dopaminergic medications and nocturnal sleep in early-stage Parkinson’s disease. Parkinsonism Relat Disord 2013, 19: 859–863.
Suzuki K, Miyamoto M, Miyamoto T, Iwanami M, Hirata K. Sleep disturbances associated with Parkinson’s disease. Parkinsons Dis 2011, 2011: 219056.
Tan EK, Lum SY, Fook-Chong SM, Teoh ML, Yih Y, Tan L, et al. Evaluation of somnolence in Parkinson’s disease: comparison with age- and sex-matched controls. Neurology 2002, 58: 465–468.
Tholfsen LK, Larsen JP, Schulz J, Tysnes OB, Gjerstad MD. Development of excessive daytime sleepiness in early Parkinson disease. Neurology 2015, 85: 162–168.
Yi PL, Tsai CH, Lu MK, Liu HJ, Chen YC, Chang FC. Interleukin-1beta mediates sleep alteration in rats with rotenone-induced parkinsonism. Sleep 2007, 30: 413–425.
Lu CY, Yi PL, Tsai CH, Cheng CH, Chang HH, Hsiao YT, et al. TNF-NF-kappaB signaling mediates excessive somnolence in hemiparkinsonian rats. Behav Brain Res 2010, 208: 484–496.
Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson’s disease. Exp Neurol 2013, 243: 45–56.
Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010, 75: 494–499.
Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009, 72: 1296–1300.
Sorensen GL, Mehlsen J, Jennum P. Reduced sympathetic activity in idiopathic rapid-eye-movement sleep behavior disorder and Parkinson’s disease. Auton Neurosci 2013, 179: 138–141.
Vendette M, Gagnon JF, Decary A, Massicotte-Marquez J, Postuma RB, Doyon J, et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology 2007, 69: 1843–1849.
Postuma RB, Bertrand JA, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S, et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: a prospective study. Mov Disord 2012, 27: 720–726.
Luppi PH, Clement O, Valencia Garcia S, Brischoux F, Fort P. New aspects in the pathophysiology of rapid eye movement sleep behavior disorder: the potential role of glutamate, gamma-aminobutyric acid, and glycine. Sleep Med 2013, 14: 714–718.
Vilas D, Iranzo A, Tolosa E, Aldecoa I, Berenguer J, Vilaseca I, et al. Assessment of alpha-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol 2016, 15: 708–718.
Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, et al. Sleep disorders in Parkinson’s disease: the contribution of the MPTP non-human primate model. Exp Neurol 2009, 219: 574–582.
Verhave PS, Jongsma MJ, Van den Berg RM, Vis JC, Vanwersch RA, Smit AB, et al. REM sleep behavior disorder in the marmoset MPTP model of early Parkinson disease. Sleep 2011, 34: 1119–1125.
van Hilten JJ, Hoogland G, van der Velde EA, Middelkoop HA, Kerkhof GA, Roos RA. Diurnal effects of motor activity and fatigue in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1993, 56: 874–877.
van Hilten JJ, Middelkoop HA, Kerkhof GA, Roos RA. A new approach in the assessment of motor activity in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1991, 54: 976–979.
Niwa F, Kuriyama N, Nakagawa M, Imanishi J. Circadian rhythm of rest activity and autonomic nervous system activity at different stages in Parkinson’s disease. Auton Neurosci 2011, 165: 195–200.
Pan W, Kwak S, Li F, Wu C, Chen Y, Yamamoto Y, et al. Actigraphy monitoring of symptoms in patients with Parkinson’s disease. Physiol Behav 2013, 119: 156–160.
Bonuccelli U, Del Dotto P, Lucetti C, Petrozzi L, Bernardini S, Gambaccini G, et al. Diurnal motor variations to repeated doses of levodopa in Parkinson’s disease. Clin Neuropharmacol 2000, 23: 28–33.
Nutt JG, Carter JH, Lea ES, Woodward WR. Motor fluctuations during continuous levodopa infusions in patients with Parkinson’s disease. Mov Disord 1997, 12: 285–292.
Piccini P, Del Dotto P, Pardini C, D’Antonio P, Rossi G, Bonuccelli U. Diurnal worsening in Parkinson patients treated with levodopa. Riv Neurol 1991, 61: 219–224.
Baier PC, Branisa P, Koch R, Schindehutte J, Paulus W, Trenkwalder C. Circadian distribution of motor-activity in unilaterally 6-hydroxy-dopamine lesioned rats. Exp Brain Res 2006, 169: 283–288.
Kudo T, Loh DH, Truong D, Wu Y, Colwell CS. Circadian dysfunction in a mouse model of Parkinson’s disease. Exp Neurol 2011, 232: 66–75.
Monville C, Torres EM, Pekarik V, Lane EL, Dunnett SB. Genetic, temporal and diurnal influences on L-dopa-induced dyskinesia in the 6-OHDA model. Brain Res Bull 2009, 78: 248–253.
Tong J, Qin LQ, Wang DJ. Mechanism of pineal and suprachiasmatic regulation on circadian rhythm of body temperature in rats. Space Med Med Eng (Beijing) 2000, 13: 101–103.
Zhong G, Bolitho S, Grunstein R, Naismith SL, Lewis SJ. The relationship between thermoregulation and REM sleep behaviour disorder in Parkinson’s disease. PLoS One 2013, 8: e72661.
Cagnacci A, Bonuccelli U, Melis GB, Soldani R, Piccini P, Napolitano A, et al. Effect of naloxone on body temperature in postmenopausal women with Parkinson’s disease. Life Sci 1990, 46: 1241–1247.
Suzuki K, Miyamoto T, Miyamoto M, Kaji Y, Takekawa H, Hirata K. Circadian variation of core body temperature in Parkinson disease patients with depression: a potential biological marker for depression in Parkinson disease. Neuropsychobiology 2007, 56: 172–179.
Lax P, Esquiva G, Esteve-Rudd J, Otalora BB, Madrid JA, Cuenca N. Circadian dysfunction in a rotenone-induced parkinsonian rodent model. Chronobiol Int 2012, 29: 147–156.
Rango M, Arighi A, Bonifati C, Bresolin N. Increased brain temperature in Parkinson’s disease. Neuroreport 2012, 23: 129–133.
Sumida K, Sato N, Ota M, Sakai K, Nippashi Y, Sone D, et al. Intraventricular cerebrospinal fluid temperature analysis using MR diffusion-weighted imaging thermometry in Parkinson’s disease patients, multiple system atrophy patients, and healthy subjects. Brain Behav 2015, 5: e00340.
Ejaz AA, Sekhon IS, Munjal S. Characteristic findings on 24-h ambulatory blood pressure monitoring in a series of patients with Parkinson’s disease. Eur J Intern Med 2006, 17: 417–420.
Schmidt C, Berg D, Herting, Prieur S, Junghanns S, Schweitzer K, et al. Loss of nocturnal blood pressure fall in various extrapyramidal syndromes. Mov Disord 2009, 24: 2136–2142.
Berganzo K, Diez-Arrola B, Tijero B, Somme J, Lezcano E, Llorens V, et al. Nocturnal hypertension and dysautonomia in patients with Parkinson’s disease: are they related? J Neurol 2013, 260: 1752–1756.
Kallio M, Haapaniemi T, Turkka J, Suominen K, Tolonen U, Sotaniemi K, et al. Heart rate variability in patients with untreated Parkinson’s disease. Eur J Neurol 2000, 7: 667–672.
Devos D, Kroumova M, Bordet R, Vodougnon H, Guieu JD, Libersa C, et al. Heart rate variability and Parkinson’s disease severity. J Neural Transm (Vienna) 2003, 110: 997–1011.
Harnod D, Wen SH, Chen SY, Harnod T. The association of heart rate variability with parkinsonian motor symptom duration. Yonsei Med J 2014, 55: 1297–1302.
Salsone M, Vescio B, Fratto A, Sturniolo M, Arabia G, Gambardella A, et al. Cardiac sympathetic index identifies patients with Parkinson’s disease and REM behavior disorder. Parkinsonism Relat Disord 2016, 26: 62–66.
Boulamery A, Simon N, Vidal J, Bruguerolle B. Effects of L-dopa on circadian rhythms of 6-OHDA striatal lesioned rats: a radiotelemetric study. Chronobiol Int 2010, 27: 251–264.
McDonald C, Newton JL, Burn DJ. Orthostatic hypotension and cognitive impairment in Parkinson’s disease: Causation or association? Mov Disord 2016, 31: 937–946.
McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res 2014, 39: 58–76.
Witkovsky P. Dopamine and retinal function. Doc Ophthalmol 2004, 108: 17–40.
Struck LK, Rodnitzky RL, Dobson JK. Circadian fluctuations of contrast sensitivity in Parkinson’s disease. Neurology 1990, 40: 467–470.
Van Hook MJ, Wong KY, Berson DM. Dopaminergic modulation of ganglion-cell photoreceptors in rat. Eur J Neurosci 2012, 35: 507–518.
Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol 2008, 6: e249.
Garfinkel D, Laudon M, Zisapel N. Improvement of sleep quality by controlled-release melatonin in benzodiazepine-treated elderly insomniacs. Arch Gerontol Geriatr 1997, 24: 223–231.
Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in Parkinson’s disease. J Neural Transm Park Dis Dement Sect 1991, 3: 41–47.
Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in de novo parkinsonian patients: evidence for phase-shifting properties of L-dopa. J Neural Transm Park Dis Dement Sect 1993, 5: 227–234.
Bordet R, Devos D, Brique S, Touitou Y, Guieu JD, Libersa C, et al. Study of circadian melatonin secretion pattern at different stages of Parkinson’s disease. Clin Neuropharmacol 2003, 26: 65–72.
Bolitho SJ, Naismith SL, Rajaratnam SM, Grunstein RR, Hodges JR, Terpening Z, et al. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med 2014, 15: 342–347.
Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol 2014, 71: 463–469.
Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol 2014, 71: 589–595.
Breen DP, Nombela C, Vuono R, Jones PS, Fisher K, Burn DJ, et al. Hypothalamic volume loss is associated with reduced melatonin output in Parkinson’s disease. Mov Disord 2016.
Bogaerts V, Theuns J, van Broeckhoven C. Genetic findings in Parkinson’s disease and translation into treatment: a leading role for mitochondria? Genes Brain Behav 2008, 7: 129–151.
Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I. Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol Aging 1997, 18: 285–289.
Tornhage CJ, Skogar O, Borg A, Larsson B, Robertsson L, Andersson L, et al. Short- and long-term effects of tactile massage on salivary cortisol concentrations in Parkinson’s disease: a randomised controlled pilot study. BMC Complement Altern Med 2013, 13: 357.
Mizobuchi M, Hineno T, Kakimoto Y, Hiratani K. Increase of plasma adrenocorticotrophin and cortisol in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated dogs. Brain Res 1993, 612: 319–321.
Aziz NA, Pijl H, Frolich M, Roelfsema F, Roos RA. Diurnal secretion profiles of growth hormone, thyrotrophin and prolactin in Parkinson’s disease. J Neuroendocrinol 2011, 23: 519–524.
Langston JW, Forno LS. The hypothalamus in Parkinson disease. Ann Neurol 1978, 3: 129–133.
Javoy-Agid F, Ruberg M, Pique L, Bertagna X, Taquet H, Studler JM, et al. Biochemistry of the hypothalamus in Parkinson’s disease. Neurology 1984, 34: 672–675.
Shannak K, Rajput A, Rozdilsky B, Kish S, Gilbert J, Hornykiewicz O. Noradrenaline, dopamine and serotonin levels and metabolism in the human hypothalamus: observations in Parkinson’s disease and normal subjects. Brain Res 1994, 639: 33–41.
Moore RY, Whone AL, Brooks DJ. Extrastriatal monoamine neuron function in Parkinson’s disease: an 18F-dopa PET study. Neurobiol Dis 2008, 29: 381–390.
Politis M, Piccini P, Pavese N, Koh SB, Brooks DJ. Evidence of dopamine dysfunction in the hypothalamus of patients with Parkinson’s disease: an in vivo 11C-raclopride PET study. Exp Neurol 2008, 214: 112–116.
Videnovic A, Lazar AS, Barker RA, Overeem S. ‘The clocks that time us’–circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 2014, 10: 683–693.
Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T. Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol 2004, 5: 18.
Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci U S A 2005, 102: 3111–3116.
Cai Y, Liu S, Sothern RB, Xu S, Chan P. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson’s disease. Eur J Neurol 2010, 17: 550–554.
Ding H, Liu S, Yuan Y, Lin Q, Chan P, Cai Y. Decreased expression of Bmal2 in patients with Parkinson’s disease. Neurosci Lett 2011, 499: 186–188.
Gu Z, Wang B, Zhang YB, Ding H, Zhang Y, Yu J, et al. Association of ARNTL and PER1 genes with Parkinson’s disease: a case-control study of Han Chinese. Sci Rep 2015, 5: 15891.
Mattam U, Jagota A. Daily rhythms of serotonin metabolism and the expression of clock genes in suprachiasmatic nucleus of rotenone-induced Parkinson’s disease male Wistar rat model and effect of melatonin administration. Biogerontology 2015, 16: 109–123.
Liu C, Chung M. Genetics and epigenetics of circadian rhythms and their potential roles in neuropsychiatric disorders. Neurosci Bull 2015, 31: 141–159.
Liu HC, Hu CJ, Tang YC, Chang JG. A pilot study for circadian gene disturbance in dementia patients. Neurosci Lett 2008, 435: 229–233.
West RL, Lee JM, Maroun LE. Hypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patient. J Mol Neurosci 1995, 6: 141–146.
Lin Q, Ding H, Zheng Z, Gu Z, Ma J, Chen L, et al. Promoter methylation analysis of seven clock genes in Parkinson’s disease. Neurosci Lett 2012, 507: 147–150.
Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci U S A 2015, 112: 7231–7236.
Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, et al. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 2003, 301: 379–383.
Franken P, Dudley CA, Estill SJ, Barakat M, Thomason R, O’Hara BF, et al. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proc Natl Acad Sci U S A 2006, 103: 7118–7123.
Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103: 1009–1017.
Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 2005, 28: 395–409.
Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 2001, 105: 683–694.
Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, et al. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest 2015, 125: 324–336.
Ait-Hmyed Hakkari O, Acar N, Savier E, Spinnhirny P, Bennis M, Felder-Schmittbuhl MP, et al. Rev-Erbalpha modulates retinal visual processing and behavioral responses to light. FASEB J 2016, 30: 3690-3701.
Kandalepas PC, Mitchell JW, Gillette MU. Melatonin signal transduction pathways require E-box-mediated transcription of Per1 and Per2 to reset the SCN clock at dusk. PLoS One 2016, 11: e0157824.
Terman M. Evolving applications of light therapy. Sleep Med Rev 2007, 11: 497–507.
Witkovsky P, Veisenberger E, Haycock JW, Akopian A, Garcia-Espana A, Meller E. Activity-dependent phosphorylation of tyrosine hydroxylase in dopaminergic neurons of the rat retina. J Neurosci 2004, 24: 4242–4249.
Paus S, Schmitz-Hubsch T, Wullner U, Vogel A, Klockgether T, Abele M. Bright light therapy in Parkinson’s disease: a pilot study. Mov Disord 2007, 22: 1495–1498.
Willis GL, Turner EJ. Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol Int 2007, 24: 521–537.
Willis GL, Moore C, Armstrong SM. A historical justification for and retrospective analysis of the systematic application of light therapy in Parkinson’s disease. Rev Neurosci 2012, 23: 199–226.
Yamanaka Y, Hashimoto S, Masubuchi S, Natsubori A, Nishide SY, Honma S, et al. Differential regulation of circadian melatonin rhythm and sleep-wake cycle by bright lights and nonphotic time cues in humans. Am J Physiol Regul Integr Comp Physiol 2014, 307: R546–557.
Yamanaka Y, Hashimoto S, Takasu NN, Tanahashi Y, Nishide SY, Honma S, et al. Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am J Physiol Regul Integr Comp Physiol 2015, 309: R1112–1121.
Yasumoto Y, Nakao R, Oishi K. Free access to a running-wheel advances the phase of behavioral and physiological circadian rhythms and peripheral molecular clocks in mice. PLoS One 2015, 10: e0116476.
Klamroth S, Steib S, Devan S, Pfeifer K. Effects of Exercise Therapy on Postural Instability in Parkinson Disease: A Meta-analysis. J Neurol Phys Ther 2016, 40: 3–14.
Rios Romenets S, Anang J, Fereshtehnejad SM, Pelletier A, Postuma R. Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: a randomized control study. Complement Ther Med 2015, 23: 175–184.
Li F, Harmer P. Economic evaluation of a Tai Ji Quan intervention to reduce falls in people with Parkinson disease, Oregon, 2008–2011. Prev Chronic Dis 2015, 12: E120.
Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun 2015, 45: 171–179.
He Y, Cornelissen-Guillaume GG, He J, Kastin AJ, Harrison LM, Pan W. Circadian rhythm of autophagy proteins in hippocampus is blunted by sleep fragmentation. Chronobiol Int 2016, 33: 553–560.
Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, et al. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci U S A 2016, 113: E1673–1682.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (81471299), Jiangsu Provincial Special Program of Medical Science (BL2014042), Suzhou Clinical Key Disease Diagnosis and Treatment Technology Foundation (LCZX201304), the Plans for Graduate Research and Innovation in Colleges and Universities of Jiangsu Province, China (KYZZ15_0334) and Suzhou Medical Key Discipline Project, the Priority Academic Program Development of Jiangsu Higher Education Institutions, China (PAPD) and Suzhou Clinical Research Center of Neurological Disease (Szzx201503).
Author information
Authors and Affiliations
Corresponding author
Additional information
Siyue Li and Yali Wang contributed equally to this review.
Rights and permissions
About this article
Cite this article
Li, S., Wang, Y., Wang, F. et al. A New Perspective for Parkinson’s Disease: Circadian Rhythm. Neurosci. Bull. 33, 62–72 (2017). https://doi.org/10.1007/s12264-016-0089-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-016-0089-7