Abstract
This study aimed to evaluate the procurement, efficacy, and applications of different acellular matrices (AMs) on rat esophagi. Sixteen rats were used. Following tissue harvesting from donor rats, AMs were obtained by detergent-enzymatic treatment (DET) from the esophagus, jejunum, and trachea. Fifteen rats were allocated into groups defined by implemented AM types: esophageal (EAM), intestinal (IAM), and tracheal (TAM). According to groups, 5 × 5 mm esophageal wall defect repair was performed with AMs. Samples were examined for hyperemia, edema, inflammatory cells, and neovascularization by scoring. The existence of muscle cells was investigated with immunohistochemical assessment. The procurement of AM in vitro and its implementation on the damaged esophageal wall was achieved successfully. There was a significant difference between groups regarding hyperemia, inflammatory cells, and neovascularization (p < 0.05). Hyperemia and cell infiltration were higher, and neovascularization was poorer in TAM than in others (p < 0.05). The neovascularization was significantly higher in the EAM group (p < 0.05). Increased edema was detected in the IAM group than others, albeit with no significant difference. Staining with Masson’s trichrome and desmin antibodies, showing increased inflammatory response on the matrix site, was not detected to regenerate muscle cells. Our DET approaches provided that AMs with potential for regeneration have been obtained from different tissues. Grafting by TAM caused higher grades of inflammation with poor angiogenesis and existing chondrocytes. Decreased inflammatory response of EAM was thought to be due to the native tissue-derived matrix. These results suggest that promoting self-regeneration with native tissue-derived AM can be improved and more convenient in damaged tissue repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reconstruction of esophageal lesions represents a burden to children and pediatric surgeons. Unlike the rest of the gastrointestinal (GI) tract, the esophagus has little redundancy [1]. Therefore, the lack of autologous tissue for esophageal reconstruction is a significant challenge that requires additional supplementation for optimal repair [1,2,3]. Currently, esophageal replacement procedures are performed as surgical interposition of either jejunal/colonic grafts or gastric pull-ups [4, 5]. Even though these conventional surgical techniques have been efficient, they are associated with a high incidence of morbidity and complications [4, 6], necessitating novel alternatives for esophageal substitution.
Recent decades have presented implications for tissue engineering in esophageal repair as promising alternative strategies proceeding on two primary subjects: cell-seeding and scaffolds [4, 5, 7,8,9]; cell-seeded scaffolds and acellular matrix (AM) are the two constituents approved for tissue engineering. AM serves as a framework to repopulate host cells, and the AM derived from the decellularization of native tissues has been recently suggested as the optimal scaffold choice [1, 10, 11].
Several esophageal tissue repair studies have been carried out with various AMs of esophagus-origin and other tissues such as the small intestinal and gastric mucosae and dura mater [1, 9, 12,13,14,15]. However, no previous study has addressed the efficiency of tracheal AM regarding the esophageal tissue. Furthermore, although some studies have been conducted on AMs derived from different organs, no study has investigated the efficacy of three simultaneously used naturally-derived AMs in esophageal tissue repair. Thus, our study aimed to (i) obtain suitable and standardized decellularized matrices from the esophagus, intestine, and trachea, (ii) evaluate the homogenous and heterogeneous matrices’ histological compositions, together with their compatibilities with the esophageal wall, (iii) assess the early clinical outcomes of tissue regeneration after implementation of AMs in a rat esophagus model with an intact mucosa and partially excised muscle layer.
Material and Methods
The Committee for Institutional Animal Care and Use in Uludag University, Bursa, Turkey, approved all the procedures (decision # 2021–03/12, date: 16.03.2021), and the study was conducted following the principles outlined in the Declaration of Helsinki.
Animal Model
Except for one donor subject, 15 Wistar albino rats weighing between 200 and 250 g were randomly assigned into three groups. The esophageal patch repair (EPR) procedure was performed by an esophageal AM (EAM) in the EAM group (EAMG) (n = 5), an intestinal AM (IAM) in the IAM group (IAMG) (n = 5), and a tracheal AM (TAM) in the TAM group (TAMG) (n = 5). The rats were fed orally with liquid nutrition and water.
Tissue Procurement for the Source of Acellular Matrix
Under general anesthesia, the esophageal, tracheal, and small intestinal tissues were harvested from the donor rat following a midline incision extending from the cervical region to the abdomen (Fig. 1). The tissue samples were cut 1–3 cm in length and trimmed of excess mesentery tissue. Then they were kept in phosphate buffer saline (PBS) solution with antibiotics (penicillin 100 U/ml, streptomycin 100 μg/ml, and amphotericin-B 0.25 μg/ml) at + 4 °C until the experimental procedure.
Decellularization Procedures
Detergent-enzymatic treatment (DET) was performed as described previously [5, 16, 17]. First, the samples were washed thoroughly with deionized water at + 4 °C for 24 h. Tissues were then perfused with 4% sodium deoxycholate (SDC) containing 1% Triton X-100 at room temperature for 4 h. Next, tissues were treated with 2000 kU DNase-I (Roche) for the esophagus and intestine and 50 kU DNase- I (Roche) for trachea in 1 M NaCl at room temperature for 24 h to remove the cellular components from the matrix, as previously described in the literature [5, 16, 17]. In addition, the tracheal AMs were rinsed with ddH2O at + 4 °C for 41 h [17]. Subsequently, AMs were kept in the PBS solution containing 5% antibiotic and antimycotic at + 4 °C until implantation. The protocol’s all stages were performed on the shaker. The detergent extraction steps are summarized in Table 1. The macroscopic views of AMs are shown in Fig. 2.
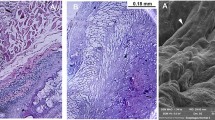
Macroscopic view of the decellularized esophageal matrix (a), acellular jejunal matrix (b), and acellular tracheal matrix (c). HE staining showed that the DET led to efficient decellularization since no remaining cells were observed in the decellularized tissues (esophagi, b; jejunum, d; and trachea, f), preserved tracheal chondrocytes (f)
Histological Examination of Acellular Matrices
The samples were fixed in 10% neutral-buffered formalin for 24 h. The fixed tissues were embedded in paraffin wax and sectioned in 3-μm thickness. The sections were stained with hematoxylin–eosin (HE) to evaluate decellularization efficiency in the pre-implanted matrices (Fig. 2). All slides were observed via light microscopy (CX-41 Olympus, Model U-D03).
Surgical Procedure
Anesthetized animals were shaved and cleaned with a povidone-iodine solution. All surgical procedures were performed under the guidance of light microscopy. The muscular layer of the esophageal wall was partially excised, as previously reported [18]; a portion of the esophageal muscle 5 × 5 mm in size was excised to establish an esophageal wall defect with intact mucosa. The acellular matrices in accordance with each group were patched into the created defects, and they were fixed with interrupted 8–0 polypropylene (Prolene, 2775G, Ethicon, USA) sutures (Fig. 3). No antibiotics or analgesia were applied to rats after surgery.
Histological Evaluation of the Specimens
Rats were euthanized on the 21st post-grafting day. Tissue samples were collected for histologic analysis, fixed in formalin, embedded, sectioned, stained with HE to evaluate inflammatory cells, and stained with Masson’s trichrome (MT) to indicate the implanted AM;s area and histoarchitecture of esophageal layers. Blind observers examined the slides. The degrees of hyperemia, edema, and vascularization were scored in a semi-quantitative manner (0: no staining; 1 + : < 25% inflammatory cells positive; 2 + : 26–75% inflammatory cells positive; 3 + : > 75% inflammatory cells positive), and the staining intensity was interpreted as none, low, moderate or high.
The immunohistochemical (IHC) staining by the streptavidin-peroxidase (HRP/DAB) method with antibody desmin (Thermo Scientific, PA1-37,556, USA; dilution 1:300) was used to visualize the muscle cells and collagen fibers. The positive and negative outcomes were included.
Statistical Analysis
The results were presented as the median (minimum–maximum) values or frequency and percentage. The Kruskal–Wallis test was used to compare groups, and the Bonferroni test was the test for multiple comparisons. A p-value less of than 0.05 was considered statistically significant. Statistical analyses were performed with IBM SPSS Statistics for Windows Ver. 23.0 (IBM Corp., Armonk, NY; released 2015).
Results
The AMs were successfully obtained from different tissues by DET (Fig. 2a, c, and e). The HE-stained sections of decellularized esophagus, trachea, and intestine showed the absence of cell nuclei and muscle fibers, indicating that no intact cells remained within the obtained AMs (Fig. 2b, d, and e).
One week after EPR, two of the 15 rats were lost, and the remaining 13 survived. Macroscopically, all esophagi had a typically repaired morphologic appearance with no stenosis, dilatation, and graft detachment (Fig. 4a–c). Three weeks after AM placement, regenerated tissues were integrated with the suture materials and patch surfaces, and no hyperplastic changes were observed in the proximal and distal parts of the grafts, except in the TAM group (Fig. 4c).
Macroscopic evaluation of integration for implemented AMs to the esophagus; the overlying layer was well-integrated with the native esophagus without borderline hypertrophy in EAM (a); there was excellent regeneration with minimal hypertrophy in IAM (b); and the TAM surface showed increased inflammation, deleted wound margin, hypertrophy, and severe adhesion that did not allow removal of liver tissue (c). Bullet symbol, implanted acellular matrices; filled pentagon, adhesive liver tissue
Light microscopic examination of luminal sections revealed that all AM grafts were located on the intact mucosa, within the muscular layer’s cleft, covered with connective tissue cells and apparent neovascularization (Fig. 5a–c). Histologic examination of the EPR areas showed regenerated connective tissues on the AM sites. In addition, reactive host responses were identified in the surroundings of AMs, including giant inflammatory cells, edema, and neovascularization with no pathologic alterations. Also, the existing chondrocytes in the heterogeneous matrix were observed to cause an increased polymorphonuclear cell count.
HE staining demonstrated increased inflammatory cell infiltration (bullet symbol), neovascularization (filled star), and suture material (section sign) around the matrices. There was minimal inflammatory cell infiltration in EAM, while it considerably increased in TAM (HE × 40 original magnification). MT staining identified AMs and esophageal layers in all sections (g, h, j). There was homogeneous connective tissue regeneration on the EAM surfaces (g), less regenerated connective tissue with predominant edema in IAM (h), and heterogeneous least connective tissue on account of chondrocytes on TAM surface (f) (MT × 40 original magnification). Immunohistochemical staining (anti-desmin antibody) clearly showed esophageal muscle layers around the matrices (k, m, o; desmin × 40 original magnification). No muscle cell was observed on any matrices (l, n, p desmin × 100 original magnification). HE, hematoxylin eosin staining; MT, Masson’s tricrome staining; L, esophageal lumen; section sign suture material; filled triangle, muscle layer; filled star, vascularization; bullet, giant cell, polimorph nuclei infiltration; filled pentagon, adhesive liver tissue
Histological examination of the HE-stained AMs revealed hyperemia, edema, connective tissue formation, and polymorphonuclear cell infiltrations on the grafted areas. In particular, giant cells were found more prominently with connective tissue morphology around the suture material, different than the others (Fig. 5d–f). Also, HE staining revealed increased neovascularization on EAMG compared to the others (Fig. 5d).
MT staining revealed that the connective tissues on the graft materials were prominently blue-stained in all groups. In addition, while MT staining showed the regenerated connective tissues in EAMG and IAMG, it also indicated chondrocytes, expressing less connective tissue in TAMs’ regenerated surfaces (Fig. 5g, h, and j).
IHC staining revealed preserved matrix components and no muscle cells on the matrix surface. The IHC staining for desmin did not show the esophageal muscular layer’s regeneration at all matrix graft sites 3 weeks after the implantation (Fig. 5k, m, and o). There was no muscle cell on regenerated matrix tissue in all groups. The margins of the native esophageal muscle layer could be observed around the defect in all groups; however, it was prominently thickened in TAMG (Fig. 5k–m).
The statistical scoring results of the inflammation components (hyperemia, edema, inflammatory cell infiltration, and neovascularization) are summarized in Table 2. The statistical comparisons between the groups reveal p-values of 0.012, 0.781, 0.040, and 0.011 for hyperemia, inflammatory cell, edema, and neovascularization, respectively) (Table 2). Thus, there were significant differences among the three groups regarding hyperemia, inflammatory cells, and neovascularization (p < 0.05).
The hyperemia score and the inflammatory cellular infiltration were lower, whereas the neovascularization score was the highest in the EAMG (Table 2). Both median values of the hyperemia score and the polymorphonuclear cell infiltration were significantly higher in TAMG than in others (2.50 and 3.00, respectively) (Table 2). Even though enhanced edema was identified at histopathological assessment in two subjects with implanted IAM, there were no statistically significant differences among the groups regarding edema (p > 0.05).
The groups’ pairwise comparisons are presented in Table 3. The median values of hyperemia, inflammatory cell infiltration, and neovascularization score in EAMG and IAMG were quite close, and there were no statistically significant differences between the two groups (p = 1.00, p = 0.272, and p = 0.066, respectively) (Table 3).
Pairwise comparisons of EAMG versus TAMG revealed statistically significant differences regarding total inflammatory scores in implanted matrices (p = 0.015, p = 0.040, and p = 0.013, respectively) (Table 3). On the other hand, pairwise comparisons revealed no statistically significant differences between EAMG and IAMG and between IAMG and TAMG in (Table 3). The histopathological scoring results are shown as a boxplot graph (Fig. 6).
Discussion
This study evaluated the possibility of obtaining in vitro implantable AMs, composed of homologous and heterologous tissues, and the outcomes of their patching to the esophagus in a rat model. To our knowledge, this is the first study with a simultaneous assessment of various AMs used for EPR in a rat model.
Acellular Matrices in General
Several studies have pointed out that the AMs can stimulate the ingrowth of host cells, vascular networks, matrix formation, and tissue-specific macro and micro-architecture [1, 5, 10, 19, 20]. Likewise, in our study, the histologic analysis of EPR sites in all groups provided evidence of integration processes. In addition, increased neovascularization was observed 3 weeks after EPR in AMs in our study, suggesting the presence of constructive matrix remodeling process rather than a scar tissue or foreign body response, as in Okuyama et al.’s study about the patch esophagoplasty [6].
Many studies have revealed promising results with seeded matrix, stem cell niche, and differentiated cells to regenerate the muscular layer of the esophagus in tissue engineering. Overall, these studies have emphasized that AM grafts without cell seeding had no potential to develop muscle cell regeneration [8, 10, 21, 22]. Although mentioned studies have demonstrated muscular proliferation on grafted sites, our study was conducted without cell cultures on AMs, and no desmin positive cells were observed on implanted AM sites.
Esophageal Acellular Matrix
Our study showed homogeneous connective tissue and less inflammatory process in the EAM than in the other AMs. Although the inflammation events in all AM groups were similar, only the EAM group provided statistically significant results [4, 8, 10, 21, 23]. The studies with EAM published in the medical literature confirmed regenerative tissue development, improved vascularization, and increased inflammatory process, similar to our study [10, 21, 22, 24]. In addition, Bhrany et al. reported an effective decellularization procedure for the EAM and defined the EAM as a usable graft that keeps in collagen, a basement membrane for restoring, maintaining, or improving esophageal function [25]. However, their study’s rat-derived EAMs were introduced into subcutaneous pockets of the rats, not into esophageal tissue. In our study, we patched the EAM only to the muscular layer of the rat esophagus.
Intestinal Acellular Matrix
Lopes et al. used IAM grafts to repair esophageal defects on the rat model’s cervical and abdominal segments [26]. They reported that invasion of patches by migrating mononuclear inflammatory cells, mainly lymphocytes, and neovascularization were identified in the early postoperative course. They concluded that the graft sites were remodeled into host-like tissues and functioned well [26]. In another published study, Catry et al. reported no muscle regeneration in the absence of seeded mesenchymal stem cells and emphasized that the cell-seeded IAM model is vital for muscle regeneration [21]. In the present study, mild inflammation and edema were observed with increased fibroblast and neovascular ingrowth around the IAM on the third week after grafting. In addition, no muscle cell was encountered along with the patch. These findings represented similar properties of IAM integration to Lopes et al.’s and Catry et al.’s studies [21, 26].
Tracheal Acellular Matrix
Preliminary studies in animal models have shown that maintenance of the TAM depends partially on the chondrocytes, which play essential roles in synthesizing and secreting cartilage matrices and collagen fibers [27,28,29]. The presence of an intense proteoglycan–collagen matrix and absence of vascularization would protect chondrocytes from host antigens. Although Liu et al. concluded that tracheal grafts treated with DET could be used in immunosuppressant-free allotransplantation in 2000, no further study has supported the use of TAM for regeneration of the esophagus to date [30]. Our study confirmed the efficacy of the procedure, despite the existing chondrocytes, increased inflammatory response, less vascularization, and heterogeneous connective tissue regeneration. We believe that the expectation to provide an intact esophageal lumen with a reinforced wall by chondrocytes needs to be studied further.
Limitation of the Study
This study’s primary limitation was the lack of ultrastructural analysis. The other limitations were our experiment’s short duration and its relatively small number of animals. In addition, the absence of another experimental group with entirely damaged esophageal wall layers did not enable further comparisons.
Conclusions
The DET to obtain AM provided scaffolding for mucosal support and repair of esophageal wall avoiding fistula and histological regeneration in experimental animals. Further research is required to enhance this model in larger numbers of animals and more prominent defects in the esophageal wall.
References
Totonelli G, Maghsoudlou P, Fishman JM, Orlando G, Ansari T, Sibbons P et al (2012) Esophageal tissue engineering: a new approach for esophageal replacement. World J Gastroenterol 18(47):6900–6907
Badylak S, Meurling S, Chen M, Spievack A, Simmons-Byrd A (2000) Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg 35(7):1097–1103
Udelsman B, Mathisen DJ, Ott HC (2018) Bioprosthetics and repair of complex aerodigestive defects. Ann Cardiothorac Surg 7(2):284–292
Arakelian L, Kanai N, Dua K, Durand M, Cattan P, Ohki T (2018) Esophageal tissue engineering: from bench to bedside. Ann N Y Acad Sci 1434(1):156–163
Totonelli G, Maghsoudlou P, Georgiades F, Garriboli M, Koshy K, Turmaine M et al (2013) Detergent enzymatic treatment for the development of a natural acellular matrix for oesophageal regeneration. Pediatr Surg Int 29(1):87–95
Okuyama H, Umeda S, Takama Y, Terasawa T, Nakayama Y (2018) Patch esophagoplasty using an in-body-tissue-engineered collagenous connective tissue membrane. J Pediatr Surg 53(2):223–226
Saxena AK (2014) Esophagus tissue engineering: designing and crafting the components for the “hybrid construct” approach. Eur J Pediatr Surg 24(3):246–262
Arakelian L, Caille C, Faivre L, Corte L, Bruneval P, Shamdani S et al (2019) A clinical-grade acellular matrix for esophageal replacement. J Tissue Eng Regen Med 13(12):2191–2203
Urita Y, Komuro H, Chen G, Shinya M, Kaneko S, Kaneko M et al (2007) Regeneration of the esophagus using gastric acellular matrix: an experimental study in a rat model. Pediatr Surg Int 23(1):21–26
Luc G, Charles G, Gronnier C, Cabau M, Kalisky C, Meulle M et al (2018) Decellularized and matured esophageal scaffold for circumferential esophagus replacement: proof of concept in a pig model. Biomaterials 175:1–18
Hou R, Wang X, Wei Q, Feng P, Mou X, Zhu Y et al (2019) Biological properties of a bionic scaffold for esophageal tissue engineering research. Colloids Surf B Biointerfaces 179:208–217
Tan JY, Chua CK, Leong KF, Chian KS, Leong WS, Tan LP (2012) Esophageal tissue engineering: an in-depth review on scaffold design. Biotechnol Bioeng 109(1):1–15
Badylak SF (2004) Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol 12(3–4):367–377
Adair-Kirk TL, Senior RM (2008) Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol 40(6–7):1101–1110
Grikscheit T, Ochoa ER, Srinivasan A, Gaissert H, Vacanti JP (2003) Tissue-engineered esophagus: experimental substitution by onlay patch or interposition. J Thorac Cardiovasc Surg 126(2):537–544
Totonelli G, Maghsoudlou P, Garriboli M, Riegler J, Orlando G, Burns AJ et al (2012) A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials 33(12):3401–3410
Conconi MT, De Coppi P, Di Liddo R, Vigolo S, Zanon GF, Parnigotto PP et al (2005) Tracheal matrices, obtained by a detergent-enzymatic method, support in vitro the adhesion of chondrocytes and tracheal epithelial cells. Transpl Int 18(6):727–734
Wen YC, Liang XL, Liang JH (2019) Experimental study of the tissue repair of neo-esophagus after esophageal muscularis resection. ASAIO J 65(8):902–907
Saxena AK, Kofler K, Ainodhofer H, Hollwarth ME (2009) Esophagus tissue engineering: hybrid approach with esophageal epithelium and unidirectional smooth muscle tissue component generation in vitro. J Gastrointest Surg 13(6):1037–1043
Taylor DA, Sampaio LC, Ferdous Z, Gobin AS, Taite LJ (2018) Decellularized matrices in regenerative medicine. Acta Biomater 74:74–89
Catry J, Luong-Nguyen M, Arakelian L, Poghosyan T, Bruneval P, Domet T et al (2017) Circumferential esophageal replacement by a tissue-engineered substitute using mesenchymal stem cells: an experimental study in mini pigs. Cell Transplant 26(12):1831–1839
Marzaro M, Vigolo S, Oselladore B, Conconi MT, Ribatti D, Giuliani S et al (2006) In vitro and in vivo proposal of an artificial esophagus. J Biomed Mater Res A 77(4):795–801
Poghosyan T, Catry J, Luong-Nguyen M, Bruneval P, Domet T, Arakelian L et al (2016) Esophageal tissue engineering: current status and perspectives. J Visc Surg 153(1):21–29
Ozeki M, Narita Y, Kagami H, Ohmiya N, Itoh A, Hirooka Y et al (2006) Evaluation of decellularized esophagus as a scaffold for cultured esophageal epithelial cells. J Biomed Mater Res A 79(4):771–778
Bhrany AD, Beckstead BL, Lang TC, Farwell DG, Giachelli CM, Ratner BD (2006) Development of an esophagus acellular matrix tissue scaffold. Tissue Eng 12(2):319–330
Lopes MF, Cabrita A, Ilharco J, Pessa P, Patricio J (2006) Grafts of porcine intestinal submucosa for repair of cervical and abdominal esophageal defects in the rat. J Invest Surg 19(2):105–111
Ma R, Li M, Luo J, Yu H, Sun Y, Cheng S et al (2013) Structural integrity, ECM components and immunogenicity of decellularized laryngeal scaffold with preserved cartilage. Biomaterials 34(7):1790–1798
Haykal S, Soleas JP, Salna M, Hofer SO, Waddell TK (2012) Evaluation of the structural integrity and extracellular matrix components of tracheal allografts following cyclical decellularization techniques: comparison of three protocols. Tissue Eng Part C Methods 18(8):614–623
Bhosale AM, Richardson JB (2008) Articular cartilage: structure, injuries and review of management. Br Med Bull 87:77–95
Liu Y, Nakamura T, Yamamoto Y, Matsumoto K, Sekine T, Ueda H et al (2000) A new tracheal bioartificial organ: evaluation of a tracheal allograft with minimal antigenicity after treatment by detergent. ASAIO J 46(5):536–539
Acknowledgements
The authors would like to thank Professor Musa Ozgur Ozyigit, Professor, for excellent laboratory work in the Department of Pathology in Uludag University Veterinary Faculty, Assoc. Professor Guven Ozkaya, for statistical analysis in the Department of Biostatistics at Uludag University, Faculty of Medicine, and Faruk Kucukyıldız, the Veterinary Doctor from the Department of Experimental Animals Research at Uludag University, Faculty of Medicine, for the technical help. Finally, special thanks to Huseyin Erbil Tapan, the Mechanical Engineer, for his invaluable support.
Author information
Authors and Affiliations
Contributions
Conceptualization: Esra Ozcakir; methodology: Esra Ozcakir, Mete Kaya; formal analysis and investigation: Esra Ozcakir, Mete Kaya, Fatih Celik, Zehra Avci, Sabire Guler. Writing — original draft preparation: Esra Ozcakir. Writing — review and editing: Esra Ozcakir, Mete Kaya. Supervision: Mete Kaya.
Corresponding author
Ethics declarations
Ethics Approval
The Ethics Committee for Institutional Animal Care and Use Committee of Uludag University, Bursa, Turkey, approved this study (number: 2021–03/12, date: 16.03.2021). The study was carried out following the principles outlined in the Declaration of Helsinki. In addition, all institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ozcakir, E., Celik, F., Guler, S. et al. The Use of Acellular Matrices Obtained by the Esophagus, Intestine, and Trachea for Esophageal Wall Repair: an Experimental Study on a Rat Model. Indian J Surg 85, 748–757 (2023). https://doi.org/10.1007/s12262-022-03542-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-022-03542-w