Abstract
In pathologies of the esophagus such as esophageal atresia, cancers and caustic injuries, methods for full thickness esophageal replacement require the sacrifice of healthy intra-abdominal organs such as the stomach and the colon. These methods are associated with high morbidity, mortality and poor functional results. The reconstruction of an esophageal segment by tissue engineering (TE) could answer this problem. For esophageal TE, this approach has been explored mainly by a combination of matrices and cells. In this chapter, we will discuss the studies on full organ esophageal decellularization, including the animal models, the methods of decellularization and recellularization.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 History
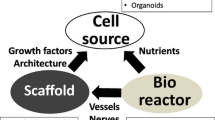
The esophagus is a tubular hollow organ composed of four layers (innermost mucosa, submucosa, muscularis propria and adventitia) and different cell types including epithelial, glandular and muscle cells (Poghosyan et al. 2011; Kuo and Urma 2006). It has been shown previously that extracellular matrix (ECM) can induce the recruitment and differentiation of cells in their relative compartments through its biochemical and biomechanical properties (Reing et al. 2009). Decellularized organs have the advantage of preserving these complex properties (Crapo et al. 2011), and very early after the development of decellularization methods, research groups started working on decellularized tissues for esophageal TE.
Decellularized ECM of other organs such as skin (Bozuk et al. 2006), urinary bladder (Badylak et al. 2005) or small intestinal submucosa (SIS) (Badylak et al. 2011) have been tried in animal models and humans for several types of esophageal repair. These matrices were shown to be efficient for superficial lesions or partial defects (Badylak et al. 2011). However, no success has been reported for full thickness circumferential esophageal replacement with non-esophageal matrices, with or without cells. Therefore, researchers turned to organ specific ECM for esophageal TE.
The first report on decellularized esophagus dates back to 2005 in a rat model (Ozeki et al. 2006). Porcine esophagi have also been decellularized in several studies with success (Koch et al. 2012; Totonelli et al. 2013; Luc et al. 2018; Arakelian et al. 2019).
We will discuss the challenges faced for a clinical use of these ECM in humans (Fig. 2.1).
2.2 Material and Methods
2.2.1 Animal Models
Full esophageal decellularization has been mainly carried out on rat (Ozeki et al. 2006; Urbani et al. 2018) and porcine esophagi (Luc et al. 2018; Arakelian et al. 2019). Even though rat esophagi have served as an important proof of concept, protocols developed on this small animal model cannot be directly applied to esophagi corresponding to human size. Porcine esophagus has the advantage of a highly similar structure and size compared to the human one (Ziegler et al. 2016). For this reason, porcine esophagus seems to be a relevant model to develop decellularization methods that can be used for a human esophageal decellularization.
2.2.2 Decellularization
The decellularization of the esophagus has been mainly carried out using detergents including SDS, DEOX, triton X-100 or Chaps (Mallis et al. 2019). The detergent is used for rupturing the cell membranes and eliminating cell content. Calcium chelator EDTA has also been added to facilitate cell detachment and improve the decellularization (Arakelian et al. 2019). The nature, concentration and treatment period can highly affect the quality of the final product. For smaller esophageal models, lower concentrations or mild detergents can be used for decellularization. However, for larger models, higher concentrations of stronger detergents such as SDS and DEOX were needed and the treatment period was extended to several days.
Decellularization protocols showed that even though in some experiments in the rat esophagi, DNA can be eliminated by cycles of detergent treatment (Mallis et al. 2019), in larger animal esophagi, the detergent alone does not remove DNA and the cell nuclei (Arakelian et al. 2019). Therefore, esophageal decellularization protocols include a DNase treatment. In the two recent decellularization studies in porcine model, one treated the decellularized matrix 12 h with 2000 Kunitz units of DNase-I (Sigma-Aldrich) (Luc et al. 2018), whereas the other team privileged a shorter 3 h treatment with 100 u/ml clinical grade DNase (Pulmozyme) (Arakelian et al. 2019).
At the end of decellularization, an efficient rinsing method should be developed in order to fully remove these detergents to avoid cytotoxicity. In small animal models, abundant rinsing with water or PBS was reported to be sufficient to remove these detergents. In bigger models, the rinsing cycles were much longer or it could be necessary to use an absorbing resin which significantly improved detergent removal and reduced cytotoxicity (Arakelian et al. 2019).
In the first attempts of decellularization, mechanical treatment, along with enzymatic and detergent treatment, was achieved by placing the esophagi under constant agitation (Ozeki et al. 2006) or by perfusing the organ using a speed roller pump (Totonelli et al. 2013). These methods increased detergent and enzyme infiltration within the esophagus and improved decellularization compared to static conditions. However, these technics worked better for smaller rat esophagi compared to larger and thicker porcine ones. Furthermore, these are open systems which require a high level of manual manipulation and an increased risk of contamination. The recent decellularization protocols included the use of bioreactors for liquid perfusion (Luc et al. 2018) or perfusion and axial rotation (Arakelian et al. 2019). These closed systems increased the efficiency of decellularization and reduced manual handling which may be an advantage for future clinical applications.
2.2.3 Sterilization
The esophagus is an organ which is in constant exchange with extracorporeal, non-sterile environment. It is therefore important to use a sterilization method to prevent bacterial and fungal growth throughout the decellularization or at the end of the process. For decontamination, a team used sodium azide (Luc et al. 2018), a molecule which can be highly toxic (Chang and Lamm 2003) and not recommended for clinical use. Others privileged the use of antibiotics (ATB) for an initial decontamination. For this purpose, a mix of ATB (gentamycin, clindamycin vancomycin and amphotericin B), previously used for vascular graft applications, was validated for esophageal decontamination (Arakelian et al. 2019). Due to the high concentration of the ATB, it is important to efficiently remove them at the end of the decellularization to avoid toxicity, while preserving the sterility of the decellularized matrix.
Another option is a final sterilization with chemical or physical treatments. Chemical treatments can include ethylene oxide or peracetic acid (PAA). The difficulty with these treatments is that these products may remain in the decellularized tissue and induce cytotoxicity (Lucas et al. 2017). Furthermore, it has been shown that PAA can prevent vascularization of soft tissues after implantation in vivo (Scheffler et al. 2008). Physical sterilization includes treatment with gamma rays. Even though this treatment efficiently removes bacterial, fungal and viral contaminations, it can compromise the biomechanical properties of the decellularized matrices (Witt et al. 2016).
2.2.4 Characterization of the Decellularized Matrix
As for other decellularized organs, the recommended criteria to define a complete decellularization are to validate the absence of residual cells, the elimination of DNA (less than 50 µg/mg of dry mass) and to make sure that no residual DNA fragments exceeding 200 bp remains in the tissue (Crapo et al. 2011). However, these recommendations can vary slightly according to the nature and the origin of the tissue. Furthermore, the general structure, the bioactive molecules and the biomechanical properties should also be maintained after decellularization.
2.2.5 DNA Quantification
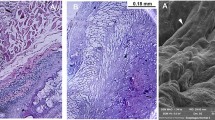
In decellularized esophagi, DNA was extracted from the matrix and was then quantified. In all these studies, an efficient elimination of DNA was demonstrated (Luc et al. 2018; Arakelian et al. 2019). For DNA fragment size, an electrophoresis of the extracted DNA on agarose gel was carried out which showed that no large DNA fragments (more than 200 bp) was visible. The elimination of nuclei was also shown by DAPI staining (Luc et al. 2018; Arakelian et al. 2019; Mallis et al. 2019).
2.2.6 General Structure and Composition
For the demonstration of cell removal, histology (HES staining) remains the standard method of validation (Luc et al. 2018; Arakelian et al. 2019). Furthermore, it is important to show that the components of the ECM such as collagens, elastin fibers, glycosaminoglycans (GAGs) and other molecules are preserved after decellularization. In two studies of rat and porcine esophageal decellularization, collagen has been quantified using a hydroxyproline assay kit (Mallis et al. 2019) or stained with picrosirius red and analyzed by histochemistry (Arakelian et al. 2019). These studies showed that most of the collagen was preserved, despite some loss of structure. Elastin fibers have been stained with orcein after esophageal decellularization, and it was shown that they were highly preserved after decellularization. Finally, GAG quantification with dimethylmethylene blue assay (DMMB) or staining with toluidine blue (Arakelian et al. 2019) showed that there was a major loss of these molecules after decellularization. However, immunostaining with specific antibodies showed that the loss of GAGs was mainly related to chondroitin sulfates, whereas the heparan sulfates and dermatan sulfates were preserved (Arakelian et al. 2019). These last two categories of GAGs are the main ones involved in the biomechanical properties of the matrix, as well as the binding and the delivery of hormones and growth factors (Kjellén and Lindahl 2018). It is important to mention that the extent of loss of these molecules highly depends on the nature and concentration of the detergent, as well as the duration of the treatment (Mallis et al. 2019). It is therefore important to develop a protocol which allows an efficient decellularization without a major loss of structural molecules.
2.2.7 Biomechanical Properties
The biomechanical properties of the decellularized esophagi have been evaluated and compared to the native esophagi. The two methods that have been used to evaluate the biomechanical properties are burst pressure test and tensile strength. In the decellularized esophagi, porosity was detected which prevented the decellularized esophagi from reaching a burst point (Luc et al. 2018). Tensile tests showed that in the transversal orientation, the decellularized and native esophagi had similar properties. On the other hand, in the longitudinal orientation, the decellularized esophagi were stiffer than the native one (Luc et al. 2018; Arakelian et al. 2019). As for in vivo implantation, decellularized esophagi were easily handled for surgical procedures and were resistant to sutures.
2.2.8 Immunogenicity and Biocompatibility
One of the main purposes of decellularization is to reduce the immune reaction of the host to avoid graft rejection and fibrosis. To study these properties in decellularized esophagi, an in vitro assay was developed based on the proliferation of lymphocytes stained with fluorescent molecule and analyzed by flow cytometry (Arakelian et al. 2019).This assay showed that the decellularized esophagi did not induce lymphocyte proliferation and indicated the absence of an acute immunogenicity.
However, the immune reaction is a complex mechanism and true immunogenicity should be evaluated in vivo. In another study, this reaction was evaluated by a subcutaneous implantation of the matrix in non-immunosuppressed Winstar rats (Luc et al. 2018). After 14 days, an induction of inflammatory response with infiltration of mononuclear cells was shown.
2.2.9 Cytotoxicity
As the products used for decellularization such as detergents and a high dose of ATB are toxic for cells, it is important to make sure that they are efficiently removed after decellularization. It is therefore necessary to develop assays to answer these questions efficiently. In decellularized esophagi, the main assays used so far were based on the evaluation of cell viability, by direct or indirect methods (Iso 10993-5-2009). In the direct method, mesenchymal stromal cells (MSCs) were seeded on the decellularized esophagi and the viability and metabolic activity were evaluated by neutral red assay and MTT assay, respectively (Luc et al. 2018). In the indirect method, the decellularized esophagi were incubated with cell culture medium and the supernatant was then used for Balb/3T3 cell culture. The viability of these cells was evaluated by flow cytometry after annexin V and 7AAD staining (Arakelian et al. 2019).The difficulty with a direct MTT assay is that the resulting dye is absorbed by the matrix, and it is difficult to have accurate and reproducible results. The indirect method allows to overcome this difficulty and to evaluate the release of toxic substances by the matrix. Both methods can be used for short term cytotoxicity evaluation. However, the presence of detergents and toxic substances should be further evaluated by mass spectrometry and long-term cytotoxicity should also be evaluated in vivo.
2.3 Cell Seeding
2.3.1 Cell Types and Origin
Cell seeding on decellularized esophagi has been explored in order to functionalize these matrices and to evaluate the potential of cells to accelerate tissue regeneration. For in vivo applications, it is essential to question the cell types and their origin (autologous or allogeneic), as this choice conditions the desired mechanism of action. The first choice is to use differentiated cells, organ-specific or not, such as epithelial cells (Ozeki et al. 2006; Urbani et al. 2018; Asnaghi et al. 2009; Barron et al. 2016; Jensen et al. 2018; Poghosyan et al. 2015; Nakase et al. 2008), smooth muscle cells (Barron et al. 2016; Poghosyan et al. 2015; Takeoka et al. 2019) and endothelial cells (Takeoka et al. 2019). The functionalization of the decellularized esophagus by these cells can be induced either by a direct colonization of the ECM by the seeded cells or by paracrine effects. It has been shown that some cells can indeed secrete factors that can attract the host cells and accelerate tissue regeneration (Marzaro et al. 2006; Xiuunl et al. 2009).
The other option is to use non-differentiated cells. To date, no clear stem cell niche, able to give rise to all the cell types, has been identified in the adult esophagus (Seery 2002). Regarding stem cells, another possibility is to seed the matrix with MSCs either originating from adipose tissue or bone marrow (Hass et al. 2011).These cells promote the recruitment of patient cells in situ through paracrine effects, accelerate re-vascularization and reduce inflammatory and scarring processes (Luc et al. 2018; Arakelian et al. 2019; Asnaghi et al. 2009; Jensen et al. 2018; Poghosyan et al. 2015; Takeoka et al. 2019; Tan et al. 2013; Francesca et al. 2018; Catry et al. 2017). It was shown that bone marrow MSC seeded on a non-esophageal extracellular matrix accelerated muscle regeneration and re-epithelialisation in a patch esophagoplasty and a full thickness esophageal replacement models (Tan et al. 2013; Catry et al. 2017).
Beyond these mechanistic aspects, the origin of cells can lead to significant constraints. Indeed, autologous cells will require a sample of the patient, isolation, amplification and then the constitution of the substitute; while the use of allogeneic cells will reduce the production time, but raises the question of immunological rejection. Thanks to their immunomodulatory properties, MSCs are an interesting source for the recellularization of decellularized esophagi.
2.3.2 Seeding Methods
The decellularized esophagus is a cylindrical hollow tube with an inner and outer surface. The challenge is therefore to decide which layer should be seeded and how to distribute the cells evenly on the matrix.
Cell density, as well as the duration of cell culture in-vitro are further important parameters to ensure the colonization of the matrix by the cells and their infiltration. Five teams showed very variable culture times, ranging from 7 to 21 days (Ozeki et al. 2006; Luc et al. 2018; Arakelian et al. 2019; Urbani et al. 2018). The number of seeded cells varies from one study to another from 1.105 to several millions per cm2 (Ozeki et al. 2006; Luc et al. 2018; Arakelian et al. 2019; Urbani et al. 2018). These parameters could be different according to cell types and their capacity to adhere and proliferate.
Some tubular esophageal substitutes were seeded under static conditions. Cells were deposited on the outer surface or were injected inside the lumen using a pipette (Catry et al. 2017; Poghosyan et al. 2013). However, in most studies, axial rotation was applied to homogenize cell distribution on the matrix. This rotation was achieved either manually at regular time intervals (Urbani et al. 2018; Barron et al. 2016; Jensen et al. 2018) or using a continuous rotation system (Ozeki et al. 2006; Arakelian et al. 2019; Urbani et al. 2018; Asnaghi et al. 2009; Francesca et al. 2018). These systems include: (1) an axial rotary bioreactor with partial liquid immersion of the substitute (Asnaghi et al. 2009), (2) an axial rotating stirrer with a filter plug tube (Arakelian et al. 2019), (3) a rotating bioreactor with a full liquid immersion of the matrix (Francesca et al. 2018) or (4) a Waverotor bioreactor (Thermonics, Tokyo, Japan) (Ozeki et al. 2006). The advantage of using a bioreactor for cell seeding is that it allows a homogeneous cell distribution, as well as reducing manual intervention and a better control of oxygenation, pH and cellular metabolism. These parameters are important for the reproducibility of cell seeding and for a future clinical application under GMP conditions. Urbani et al. clearly demonstrated the benefits of a dynamic culture (Urbani et al. 2018). However, the animal model used being the rat, the transposition to a human-sized esophagus remains to be demonstrated. Cell sheet technology is another option of cell seeding on the decellularized esophagi. This method has been explored using MSCs. To summarize, MSCs were cultured in a dish at a very high confluence and the cell sheet was rolled around a decelluarized esophagus (Luc et al. 2018). Cell sheet seeding can be improved using thermoresponsive polymers such as pNIPAM which allow a full cell sheet detachment upon changing the temperature. This method has already been validated in a clinical trial for superficial lesions using epithelial cells (Yamaguchi et al. 2017) and could be used for seeding of decellularized esophagi.
2.4 Clinical Applications
Commercialized non-esophageal decellularized natural ECM have previously been tested in clinical trials for treating esophageal leaks with decellularized skin or superficial esophageal lesions with SIS patches to prevent stenosis (Bozuk et al. 2006; Badylak et al. 2011). However, these methods have never been successfully applied for full thickness circumferential replacement humans.
For the clinical application of decellularized esophagi, it is important to consider the regulatory aspect which will be applied. In Europe, for example, if the matrix is to be used alone, without cell seeding, it could be considered as an implantable medical device “IMD” or as “human cells, tissues, and cellular and tissue-based product (HCT/P)”. One of the main determining criteria for choosing between these two categories is the origin of the decellularized matrix and the nature of the protocol. A final decontamination is mandatory for IMDs. A human matrix can be treated both as an IMD and a HCT/P, whereas a porcine decellularized esophagus can only be treated as an IMD. In both categories, it is necessary to show the sterility of the matrix and both can involve an initial decontamination with antibiotics and a final sterilization using gamma rays or chemicals such as ethylene oxide. For IMD, the quality controls should be carried out to obtain a CE marking and the matrix can be produced by pharmaceutical companies. A HCT/P, however, should be produced in special accredited facilities such as human tissue banks. In both categories, a long-term conservation method should be validated which could include the preparation of a frozen matrix bank.
If the decellularized esophageal matrix is to be seeded with cells before implantation, the final product is considered as an advanced therapy medicinal product (ATMP), corresponding to a new category of regulations (https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products-overview). This means that on top of evaluating the biological properties and the sterility of the matrix, the nature of the cells and the culture conditions on the matrix before and after in vivo implantation should be evaluated. The cells should be isolated and cultured in a clinical grade cell culture media, and the optimal cell density as well as in vitro maturation time should be clearly defined. Once implanted in the animal, the possible migration of the cells within different organs, as well as their tumorigenic potential, should be carefully evaluated. Unlike MD and tissue products, ATMPs need to be produced in authorized special facilities such as platforms or pharmaceutical industries.
For all the three categories, a pre-clinical trial in a big animal model is necessary to show the efficiency of the matrix in esophageal replacement.
One of the challenges for in vivo esophageal replacement is the method of vascularization. As the esophagus is composed of microvessels coming from the aorta and the surrounding organs, it is necessary to find a vascularization method to prevent organ necrosis. The option that has been tested in previous esophageal tissue engineering studies has been a maturation step in the omentum (Luc et al. 2018; Poghosyan et al. 2015). These studies showed that a tubular substitute composed of SIS for esophageal replacement was successfully vascularized by this option. This method has also been used successfully for the vascularization of a rat decellularized esophagus and a porcine one. However, long-term efficiency after organ replacement should be evaluated in vivo.
References
Arakelian L, Caille C, Faivre L, Corté L, Bruneval P, Shamdani S et al (2019) A clinical-grade acellular matrix for esophageal replacement. J Tissue Eng Regen Med. https://doi.org/10.1002/term.2983
Asnaghi MA, Jungebluth P, Raimondi MT, Dickinson SC, Rees LEN, Go T et al (2009) A double-chamber rotating bioreactor for the development of tissue-engineered hollow organs: From concept to clinical trial. Biomaterials 30(29):5260–5269
Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO et al (2005) Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res
Badylak SF, Hoppo T, Nieponice A, Gilbert TW, Davison JM, Jobe BA (2011) Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A [Internet] 17(11–12):1643–50. Available from: http://www.liebertonline.com/doi/abs/https://doi.org/10.1089/ten.tea.2010.0739
Barron MR, Blanco EW, Aho JM, Chakroff J, Johnson J, Cassivi SD et al (2016) Full-thickness oesophageal regeneration in pig using a polyurethane mucosal cell seeded graft. J Tissue Eng Regen Med 12(1):175–185
Bozuk MI, Fearing NM, Leggett PL (2006) Use of decellularized human skin to repair esophageal anastomotic leak in humans. JSLS 10:83–85
Catry J, Luong-Nguyen M, Arakelian L, Poghosyan T, Bruneval P, Domet T et al (2017) Circumferential esophageal replacement by a tissue-engineered substitute using mesenchymal stem cells. Cell Transplant [Internet] 26(12):1831–1839. Available from: http://journals.sagepub.com/doi/https://doi.org/10.1177/0963689717741498
Chang S, Lamm SH (2003) Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol
Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243
Hass R, Kasper C, Böhm S, Jacobs R (2011) Different populations and sources of human mesenchymal stem cells ( MSC ): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9(1):12
Jensen T, Wanczyk H, Sharma I, Mitchell A, Sayej WN, Finck C (2018) Polyurethane scaffolds seeded with autologous cells can regenerate long esophageal gaps: an esophageal atresia treatment model. J Pediatr Surg
Kjellén L, Lindahl U (2018) Specificity of glycosaminoglycan–protein interactions. Curr Opin Struct Biol 50:101–108
Koch H, Graneist C, Emmrich F, Till H, Metzger R, Aupperle H et al (2012) Xenogenic esophagus scaffolds fixed with several agents: Comparative in vivo study of rejection and inflammation. J Biomed Biotechnol
Kuo B, Urma D (2006) Esophagus—anatomy and development. GI Motil online [Internet], pp 1–20. Available from: http://www.nature.com/gimo/contents/pt1/full/gimo6.html
La Francesca S, Aho JM, Barron MR, Blanco EW, Soliman S, Kalenjian L et al (2018) Long-term regeneration and remodeling of the pig esophagus after circumferential resection using a retrievable synthetic scaffold carrying autologous cells. Sci Rep [Internet] 8(1):4123. Available from: http://www.nature.com/articles/s41598-018-22401-x
Luc G, Charles G, Gronnier C, Cabau M, Kalisky C, Meulle M et al (2018) Decellularized and matured esophageal scaffold for circumferential esophagus replacement: proof of concept in a pig model. Biomaterials 175:1–18
Lucas AD, Merritt K, Hitchins VM, Woods TO, Mcnamee SG, Lyle DB et al (2017) Residual ethylene oxide in medical devices and device material residual ethylene oxide in medical devices and device material
Mallis P, Chachlaki P, Katsimpoulas M, Stavropoulos-Giokas C, Michalopoulos E (2019) Optimization of decellularization procedure in rat esophagus for possible development of a tissue engineered construct. Bioengineering
Marzaro M, Vigolo S, Oselladore B, Conconi MT, Ribatti D, Giuliani S et al (2006) In vitro and in vivo proposal of an artificial esophagus. J Biomed Mater Res A 77(4):795–801
Nakase Y, Nakamura T, Kin S, Nakashima S, Yoshikawa T, Kuriu Y et al (2008) Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J Thorac Cardiovasc Surg 136(4):850–859
Ozeki M, Narita Y, Kagami H, Ohmiya N, Itoh A, Hirooka Y et al (2006) Evaluation of decellularized esophagus as a scaffold for cultured esophageal epithelial cells. J Biomed Mater Res Part A
Poghosyan T, Gaujoux S, Chirica M, Munoz-Bongrand N, Sarfati E, Cattan P (2011) Functional disorders and quality of life after esophagectomy and gastric tube reconstruction for cancer. J Visc Surg [Internet] 148:e327—e335. Available from: http://ac.els-cdn.com.gate2.inist.fr/S1878788611001093/1-s2.0-S1878788611001093-main.pdf?_tid=102588d6-8322-11e7-8a8b-00000aacb35f&acdnat=1502956978_4c9f951fb448fd427801b6ae238e4cf9
Poghosyan T, Gaujoux S, Vanneaux V, Bruneval P, Domet T, Lecourt S et al (2013) In vitro development and characterization of a tissue-engineered Conduit\nResembling esophageal wall using human and pig skeletal myoblast, Oral\nEpithelial Cells, and biologic scaffolds. Tissue Eng Part a 19(19–20):2242–2252
Poghosyan T, Sfeir R, Michaud L, Bruneval P, Domet T, Vanneaux V et al (2015) Circumferential esophageal replacement using a tube-shaped tissue-engineered substitute: an experimental study in minipigs. Surg (United States) [Internet] 158(1):266–277. https://doi.org/10.1016/j.surg.2015.01.020
Reing JE, Zhang L, Myers-irvin J, Ph D, Cordero KE, Freytes DO et al (2009) Degradation products of extracellular matrix affect cell migration and proliferation. 15(3)
Scheffler SU, Cmd JG, Cmd JK, Przybilla D (2008) Remodeling of ACL allografts is inhibited by peracetic acid sterilization. Clin Orthop Relat Res 466:1810–1818
Seery JP (2002) Stem cells of the oesophageal epithelium. J Cell Sci 115:783–1789
Takeoka Y, Matsumoto K, Taniguchi D, Tsuchiya T, Machino R, Moriyama M et al (2019) Regeneration of esophagus using a scaffoldfree biomimetic structure created with biothree-dimensional printing. PLoS ONE 14(3):1–12
Tan B, Wei RQ, Tan MY, Luo JC, Deng L, Chen XH et al (2013) Tissue engineered esophagus by mesenchymal stem cell seeding for esophageal repair in a canine model. J Surg Res 182(1):40–48
Totonelli G, Maghsoudlou P, Georgiades F, Garriboli M, Koshy K, Turmaine M et al (2013) Detergent enzymatic treatment for the development of a natural acellular matrix for oesophageal regeneration. Pediatr Surg Int
Urbani L, Camilli C, Phylactopoulos D, Crowley C, Natarajan D, Scottoni F et al (2018) Multi-stage bioengineering of a layered oesophagus with in vitro expanded muscle and epithelial adult progenitors. Nat Commun
Witt TA, Marler RJ, Pislaru SV, Robert D (2016) Low-dose gamma irradiation of decellularized heart valves results in tissue injury in vitro and in vivo. Ann Thorac Surg 101(2):667–674
Xiuunl I, Xiaoz UO, Weiz HI, Pengy A, Huiix IE, Zhiingy A (2009) Grafts of porcine small intestinal submucosa with cultured autologous oral mucosal epithelial cells for esophageal repair in a canine model. Exp Biol Med (Maywood) 234(4):453–461
Yamaguchi N, Isomoto H, Kobayashi S, Kanai N, Kanetaka K, Sakai Y et al (2017) Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep 7(1)
Ziegler A, Gonzalez L, Blikslager A (2016) Large animal models: the key to translational discovery in digestive disease research. Cmgh [Internet] 2(6):716–24. https://doi.org/10.1016/j.jcmgh.2016.09.003
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Arakelian, L., Godefroy, W., Faivre, L., Cattan, P. (2021). Esophagus Decellularization. In: Kajbafzadeh, AM. (eds) Decellularization Methods of Tissue and Whole Organ in Tissue Engineering. Advances in Experimental Medicine and Biology, vol 1345. Springer, Cham. https://doi.org/10.1007/978-3-030-82735-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-82735-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82734-2
Online ISBN: 978-3-030-82735-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)