Abstract
The effects of blood glucose and hemoglobin A1C [HbA1C] levels before one anastomosis gastric bypass surgery (OAGB) on post-operative fasting blood sugar (FBS), HbA1C, and body composition (BC) are not adequately studied. This study assessed optimized pre-operative hyperglycemia management on BC and glycemic factors after OAGB. This single-center study included 373 patients with type 2 diabetes who underwent OAGB (294 women), with 6, 12, and 24 months post-operative follow-up. The National Obesity Surgery Database provided data. To investigate the trend of changes in FBS, HbA1C, and BC data between two groups of controlled and uncontrolled diabetes during the study, the generalized estimating equations were used. Of total participants, 27.3% (n = 102) had controlled diabetes before surgery. After adjusting for age, sex, anti-hyperglycemic medication, and biliopancreatic limb length, patients with controlled diabetes pre-surgery showed significantly lower FBS (β coefficient: − 28.92; 95%CI: − 33.98 to − 23.85) and HbA1C (β coefficient: − 1.06; 95%CI: − 1.26 to − 0.86) than those with uncontrolled diabetes post-operative follow-up time points. However, there was no significant difference in total percentage weight loss, visceral fat, muscle and fat mass (P > 0.05) between groups after surgery. Pre-operative diabetes control significantly affects post-OAGB diabetes status, but not body composition values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morbid obesity and its closely associated comorbidity, type 2 diabetes mellitus (T2DM), are two of the most challenging current health issues globally [1]. Despite the partial effectiveness of lifestyle changes, such as a decrease in calorie intake, along with more physical activity and pharmaceutical intervention, weight loss surgery is a proven successful and durable treatment for patients with diabetes and morbid obesity [1, 2]. Although laparoscopic Roux-en-Y gastric bypass (RYGB) is currently the gold-standard treatment for these patients, one anastomosis gastric bypass-mini gastric bypass (OAGB-MGB) has been demonstrated as an effective alternative to RYGB in treating patients with T2DM and morbid obesity [3]. Reasons for the antidiabetic effects of gastric bypass surgeries include the following: decrease in caloric intake, durable long-term weight loss, accompanied by a change in neuro-hormonal signals, reductions in anti-incretin effects due to duodenal bypass, and upregulation of cell membrane insulin receptors [3].
However, it is not yet clear what effect pre-operative glycemic status has on diabetes after OAGB-MGB surgery, since previous studies have mainly focused on the importance of pre-RYGB hemoglobin A1c (HbA1c) levels on post-surgery diabetes remission [4, 5] or the comparison of the glycemic status in two groups of pre-diabetic and diabetic patients after OAGB-MGB [6]. Despite multiple positive effects of OAGB-MGB, an unbalanced pattern of muscle mass distribution (lower muscle mass) has been observed following the surgery, and this reduction was higher in diabetics than in non-diabetics at 1 year post-surgery. Furthermore, people with diabetes have been observed to lose less visceral fat than non-diabetic patients [7]. However, limited data are available on the effect of pre-OAGB-MGB glycemic status on the post-operative body composition. The purpose of the present study is therefore to assess the effect of pre-operative controlled or uncontrolled T2DM on post-OAGB-MGB body composition parameters and glycemic factors, with a follow-up period of 2 years. A secondary aim is to assess the effect of pre-operative glycemic status on the percentage of excess weight loss (%EWL) and total weight loss (%TWL) after surgery.
Patients and Methods
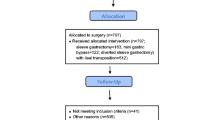
This single-center cohort study was conducted on all patients with type 2 diabetes (n = 373) and morbid obesity who had been referred to the obesity clinic of Hazrat-e Rasool General Hospital (Center of Excellence of the European Branch of the International Federation for Surgery of Obesity), and who had undergone OAGB-MGB between October 2010 and May 2018. All patients were included on historical study. This study is reported in accordance with STROCSS guidelines [8]. Diabetes was diagnosed either on the basis of American Diabetes Association criteria [9], or for those patients who had already been treated with insulin or diabetes medication. All patients were at least 18 years old and had a pre-operative BMI ≥ 35 kg/m2. Patients with chronic renal failure or revisional OAGB-MGB, women who were lactating or pregnant after surgery, those taking steroid immunosuppressive drugs (corticosteroids), or with pre-operative history of alcohol addiction and/or substance abuse, were excluded (Fig. 1). Eligible patients were classified according to pre-operative hemoglobin A1c (HbA1c) and fasting blood sugar (FBS) as controlled (HbA1c ≤ 7% and FBS ≤ 130 mg/dl) or uncontrolled diabetes (HbA1c > 7% or FBS > 130 mg/dl) [10, 11]. The study was conducted according to the Declaration of Helsinki and approved by the Research Ethics Committee (Ethics number: IR.IUMS.REC.13970134).
Data Collection
All pre-operative and post-operative (6, 12, and 24 months after the surgery) data, including demographic, anthropometric, biochemical, and body composition analysis values, were extracted from the National Obesity Surgery Database (http://obesitysurgery.ir/) which is the only national database of bariatric surgery. Data were recorded in the mentioned database by trained staff. Weight loss was expressed as percentage of %EWL and %TWL, calculated as previously described [12]. The details of pre-and post-operative management of each patient after attending the obesity clinic are as follows.
Pre-operative management
-
Identical pre-operative evaluations, including psychological assessment, nutrition and exercise counseling, measurement of biochemical factors, and body composition features were performed for all patients.
Post-operative management
-
All patients were discharged with proton pump inhibitors (40 mg of pantoprazole twice a day for at least 6 months) and preventive anticoagulation medication (subcutaneous heparin; 5000 units bd for 5 days). Likewise, from the first post-operative day, metformin was reintroduced. However, 50% of pre-operative Neutral Protamine Hagedorn (NPH) or long-acting analog insulin was also given. Adjustments to or withdrawal from metformin or insulin dosages were related to serum glucose levels, which were routinely measured by the medical team in periodic visits. Insulin was discontinued if FBS was <130 mg/dl, while metformin therapy was withdrawn if FBS was <100 mg/dl. In addition, the same protocol for nutrition and supplement therapy was performed for all patients. A clear liquid diet was started on the first post-operative day. A full liquid diet was implemented from days 2–10 after surgery. Afterwards, a pureed, then soft diet was recommended for ten consecutive days, respectively. Each patient received standardized nutritional education about consuming adequate protein (1.1–1.5 g/ideal body weight) and daily intake of multivitamin and mineral supplements. Furthermore, intramuscular neurobion (including 1000 μg of vitamin B12, 100 mg of vitamin B1, and 100 mg of vitamin B6), oral vitamin D3 (50000IU), and calcium citrate (500 mg) were administered post-surgery every month, every 2 weeks, and daily for all patients. If micronutrient deficiencies were observed, additional supplementation was prescribed [13].
Surgical Technique
All OAGB-MGB were performed by the same surgical teams. The operation involves 15–18-cm-long gastric pouch and 120–200-cm side-to-side loop (45 mm) gastrojejunostomy from the Treitz ligament. Longitudinal gastrojejunostomy was performed on the posterior aspect of the pouch, and the enterostomy site was sewn with 2–0 polydioxanone [PDS].
Statistical Analysis
All eligible patients with T2DM were entered into the study whose characteristics were available at the National Obesity Surgery Database (http://iranobesitysurgery.com/). Prior to analysis, data screening and cleaning were applied to detect any data-entry errors. SPSS statistical software Ver.22.0 (Armonk, NY: IBM Corp) was used for statistical analysis of the data. Continuous data were presented as mean (standard deviation: [SD]), and categorical data were presented as frequencies and percentages. The independent sample t test was used to compare differences between two studied groups. The chi-square test is used to analyze the differences between categorical variables. To investigate the trend of changes in FBS, HbA1c, and body composition analysis data in two groups of pre-operative controlled and uncontrolled diabetes, the generalized estimating equations (GEE) were used. In the GEE analysis, FBS, HbA1c, and body composition parameters were the response variables, while diabetes status (pre-operative controlled or uncontrolled diabetes) and time were the main factor and covariate, respectively. Age, sex, biliopancreatic limb length, and anti-hyperglycemic medication were considered as confounding factors, considering the literature review, and significance levels of less than 0.2 in the univariate analysis. P < 0.05 was considered as statistically significant.
Results
Baseline Characteristics
Overall, 373 patients (294 women and 79 men) met the initial selection criteria. At baseline, mean (SD) patient age, weight, height, and body mass index (BMI) were 46.21 SD 9.53 years, 120.30 SD 22.43 kg, 162.45 SD 9.39 cm, and 45.48 SD 6.89 kg/m2, respectively. Of total participants, 27.3% (n = 102) had controlled and 72.7% (n = 271) had uncontrolled diabetes. Furthermore, at baseline, 21.7% (n = 81), 13.9% (n = 52), and 66.8% (n = 249) used insulin, glibenclamide, and metformin to control blood glucose levels, respectively. In the controlled diabetes group, 4.9% (n = 5), 7.8% (n = 8), and 56.9% (n = 58) were treated by insulin, glibenclamide and metformin, respectively. In the uncontrolled diabetes group, however, the values were 28% (n = 76), 16.2% (n = 44), and 70.5% (n = 191). Pearson chi-square test showed that uncontrolled T2DM group used of significantly higher anti-hyperglycemic medications before surgery than controlled group (P < 0.001 for insulin; P = 0.013 for metformin; and P = 0.037 for glibenclamide). However, there were no differences observed in age, gender, education, marital status, and alcohol consumption between controlled and uncontrolled T2DM cases (Table 1). All participants had undergone OAGB-MGB with mean (± SD) biliopancreatic limb length of 185.37 SD 18.50 cm in controlled diabetes group and 183.36 SD 14.97 uncontrolled T2DM group. No significant difference was observed between groups (P = 0.30).
Patient Characteristics at Various Time Points
The trends of weight, BMI, %EWL, %TWL, biochemical parameters, and body composition features at various time points are presented in Table 2. Superior %EWL and %TWL were seen in the controlled diabetes group 2 years after surgery (%EWL: 80.33 SD 19.70 and %TWL: 35.19 SD 9.42 in controlled diabetes group vs. %EWL: 76.24 SD 22.13 and %TWL: 32.75 SD 9.58 in uncontrolled diabetes group). However, the comparison of the means of %EWL and %TWL at 2 years post-operatively between groups was not statistically significant (P = 0.24 and P = 0.12; respectively). Additionally, a notable improvement in serum levels of glycemic factors, lipid profiles, aspartate amino transferase (AST), alanine aminotransferase (ALT), and uric acid was observed in the two groups 2 years after surgery. Likewise, body fat reserves, fat free mass, and muscle mass were reduced in both groups over 2 years post-operation.
Multivariable Risk Factor Analysis
GEE linear models were used to assess the effect of pre-operative glycemic status on biochemical (FBS and HbA1c) and body composition parameters (fat mass, muscle mass, and visceral fat), as well as BMI, %EWL, and %TWL values after OAGB-MGB (Table 3).
According to the β coefficients, as adjusted correlation coefficients for predicting independent variables, those patients with controlled diabetes before surgery tended to have lower FBS and HbA1c after surgery (all P < 0.001). However, there were no significant differences in %EWL, %TWL, BMI, fat mass, muscle mass, and visceral fat levels between the two groups of controlled or uncontrolled diabetes (P > 0.05). As shown in Table 3, sex, age, biliopancreatic limb length, and metformin use did not significantly predict FBS and HbA1c changes during the study; however, pre-operative use of glibenclamide or insulin had a significant negative effect on post-operative FBS and HbA1c. Furthermore, the efficacy of pre-OAGB-MGB glycemic status on later FBS, HbA1c, BMI, %EWL, % TWL, and body composition parameters was significantly associated with follow-up time (all P < 0.001).
Discussion
The current study investigated the changes in glycemic factors, body composition, BMI, %EWL, and %TWL in patients who underwent OAGB-MGB with pre-operative controlled or uncontrolled T2DM. Both study groups (controlled or uncontrolled T2DM) were comparable regarding their baseline pre-operative characteristics including age, gender, education, marital status, and alcohol consumption, and no significant difference was observed between them. However, there were significant differences between both groups with respect to patients’ baseline anti-hyperglycemic medication. The main results indicate that pre-operative FBS level ≤ 130 mg/dl or HbA1c level ≤ 7% was significantly associated with decreased FBS and HbA1c after surgery, compared to patients with pre-operative FBS level > 130 mg/dl or HbA1c level > 7%, during 2 years of follow-up. These findings corroborate previous studies regarding RYGB [4, 5]. However, no study of these effects (good vs. poor preoperative glycemic control on post-operative FBS, HbA1c levels and body composition parameters) in OAGB-MGB had been previously investigated over the same time period. A historical study had revealed that careful management of HbA1c levels in patients with T2DM undergoing RYGB could be more effective in diabetes remission a year after surgery [4]. Likewise, the association of pre-RYGB HbA1c improvements with better post-operative glycemic control had previously been reported [5]. It is noteworthy that in this context, the present model did not show that age, sex, and biliopancreatic limb length use to be significant predictors of FBS and HbA1c levels over the 2 years post-OAGB-MGB. However, pre-operative use of glibenclamide or insulin had a significant negative effect on post-operative FBS and HbA1c. Regarding the sex, age, and biliopancreatic limb length, the finding is in concordance with a previous study on gastric cancer patients with diabetes who underwent RY gastrojejunostomy [14]. On the other hand, in another prior paper, age was identified as a predictive factor of HbA1c, although no effect was found by sex [4]. In terms of age, these inconsistencies may be due to differences in study duration (1-year vs. 2-year follow-up) and the mean age of participants (54 vs. 46). Additionally, unlike the present study, a significant difference in age was observed between the two groups in the above-mentioned study [4]. Conversely, from the point of view of the association between pre-operative hyperglycemia management with glibenclamide or insulin and post-operative FBS and HbA1c, the present study’s findings are consistent with previous studies which showed patients who received less insulin before surgery were less likely to achieve long-term T2DM remissions after bariatric surgeries [15]. It seems that better pre-operative beta-cell function in insulin production is associated with better post-operative glycemic control. As a whole, however, controlling blood sugar before surgery is very important for post-operative glycemic controlled, metabolic surgery for this group of patients and should not be delayed [16]. Moreover, in the present research, no significant difference was found in BMI, %EWL, and %TWL of the controlled diabetic group, compared to the age-, sex-, biliopancreatic limb length, and anti-hyperglycemic medication-adjusted uncontrolled diabetic group following OAGB-MGB. However, the association between optimal HbA1c management during the pre-bariatric surgery period and better post-operative weight loss had been observed previously [5]. These heterogeneities may point to differences in the duration of follow-up (2 years in the present study vs. 3 years in the mentioned study [5]) and statistical analysis method (GEE vs. unpaired Student t-test). Furthermore, no potential confounding variables were controlled [5], whereas the present results were obtained after adjusting several covariates. In addition, the current study found that the group with poor pre-operative glycemic control had no significant difference in post-operative body composition (fat mass, muscle mass, and visceral fat), in comparison with the optimal pre-operative blood glucose-and HbA1c control group. No similar evidence was observed in the field; however, many other studies unrelated to weight loss surgeries have suggested a link between elevated levels of HbA1c and lower lean body mass [17]. Additionally, in a cross-sectional observational study on sedentary ambulatory patients with T2DM, no significant difference was found in the muscle mass between the control and non-control groups; however, uncontrolled HbA1c and FBS were associated with higher subcutaneous and total body fat, respectively [18]. The lack of significant differences in body composition components between the two groups in the present study may be due to the regular follow-up of both groups of patients at the obesity clinic, with regular body composition evaluation at months 3, 6, 12, 18, and 24 by the specialized nutrition and sports medicine team. It is possible that early administration of appropriate dietary recommendations, paired with tailored exercise training, maybe an underlying explanation for such similarity between these two groups. The main strength of the present study was that the effect of glycemic control status before OAGB-MGB on post-operative FBS, HbA1c, and body composition parameters was evaluated for the first time. However, the single-center sample selection can be considered as a limitation. It is worth noting, however, that the database is nationwide and records patient characteristics referred from all over the country. Therefore, it seems reasonable to infer that the results may be generalizable with minimal bias. Additionally, the average duration of diabetes (years) was not recorded in the database. Likewise, historical data of patients were recorded, which could be another limitation. However, data collection and recording were carried out by trained staff, and all data were additionally re-assessed by the authors.
Conclusion
Based on current findings, and in the absence of a previous definitive study on a one anastomosis gastric bypass-mini gastric bypass population, it can be seen that careful management of fasting blood sugar and hemoglobin A1c levels in patients with type 2 diabetes mellitus undergoing one anastomosis gastric bypass-mini gastric bypass (regardless of patient age, gender, anti-hyperglycemic medication and even biliopancreatic limb length) can exert beneficial effects on post-operative diabetes control. However, baseline pre-OAGB-MGB glycemic control was not predictive of percentage of excess weight loss, total weight loss, and body composition parameter improvement after surgery. Further large-scale studies are needed, with a suggested additional focus on the effects of pre-operative diabetes duration.
Data availability
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly and they were assured raw data would remain confidential. So supporting data is not available.
Code Availability
Not applicable.
References
Lee W-J, Almulaifi A, Chong K, Yao W-C, Tsou JJ, Ser K-H, Lee Y-C, Chen S-C, Chen J-C (2016) Bariatric versus diabetes surgery after five years of follow up. Asian J Surg 39:96–102. https://doi.org/10.1016/j.asjsur.2015.04.001
Busetto L (2015) Timing of bariatric surgery in people with obesity and diabetes. Ann Transl Med 3. https://doi.org/10.3978/j.issn.2305-5839.2015.03.62
Abou Ghazaleh R, Bruzzi M, Bertrand K, M’harzi L, Zinzindohoue F, Douard R, Berger A, Czernichow S, Carette C, Chevallier J-M (2017) Is mini-gastric bypass a rational approach for type-2 diabetes? Curr Atheroscler Rep 19:1–6. https://doi.org/10.1007/s11883-017-0689-3
English TM, Malkani S, Kinney RL, Omer A, Dziewietin MB, Perugini R (2015) Predicting remission of diabetes after RYGB surgery following intensive management to optimize preoperative glucose control. Obes surg 25:1–6. https://doi.org/10.1007/s11695-014-1339-2
Perna M, Romagnuolo J, Morgan K, Byrne TK, Baker M (2012) Preoperative hemoglobin A1c and postoperative glucose control in outcomes after gastric bypass for obesity. Surg Obes Relat Dis 8:685–690. https://doi.org/10.1016/j.soard.2011.08.002
Carbajo M, Jiménez J, Castro MJ, Ortiz-Solórzano J, Arango A (2014) Outcomes in weight loss, fasting blood glucose and glycosylated hemoglobin in a sample of 415 obese patients, included in the database of the European accreditation council for excellence centers for bariatric surgery with laparoscopic one anastomosis gastric bypass. Nutr Hosp 30:1032–1038. https://doi.org/10.3305/nh.2014.30.5.7720
Garciacaballero M, Reyes-Ortiz A, García M, Martínez-Moreno J, Toval J, García A, Mínguez A, Osorio D, Mata J, Miralles F (2014) Changes of body composition in patients with BMI 23–50 after tailored one anastomosis gastric bypass (BAGUA): influence of diabetes and metabolic syndrome. Obes surg 24:2040–2047. https://doi.org/10.1007/s11695-014-1288-9
Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G, Bashashati M, Millham FH, Orgill DP, Noureldin A (2019) STROCSS 2019 Guideline: strengthening the reporting of cohort studies in surgery. Int J Surg 72:156–165. https://doi.org/10.1016/j.ijsu.2019.11.002
Association AD (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33:S62–S69. https://doi.org/10.2337/dc14-S081
Kassaian SE, Goodarzynejad H, Boroumand MA, Salarifar M, Masoudkabir F, Mohajeri-Tehrani MR, Pourhoseini H, Sadeghian S, Ramezanpour N, Alidoosti M (2012) Glycosylated hemoglobin (HbA1c) levels and clinical outcomes in diabetic patients following coronary artery stenting. Cardiovasc Diabetol 11:1–10. https://doi.org/10.1186/1475-2840-11-82
Monnier L, Colette C (2009) Target for glycemic control: concentrating on glucose. Diabetes Care 32:S199–S204. https://doi.org/10.2337/dc09-S310
Ansar H, Zamaninour N, Pazouki A, Kabir A (2019) Weight loss after one anastomosis gastric bypass-mini gastric bypass (OAGB-MGB): patient-related perioperative predictive factors. Obes Surg 1–8. https://doi.org/10.1007/s11695-019-04270-z
Zamaninour N, Pazouki A, Kermansaravi M, Seifollahi A, Kabir A (2020) Changes in body composition and biochemical parameters following laparoscopic one anastomosis gastric bypass: 1-year follow-up. Obes Surg 1–7. https://doi.org/10.1007/s11695-020-04901-w
Kim JW, Kim KY, Lee SC, Yang DH, Kim BC (2015) The effect of long Roux-en-Y gastrojejunostomy in gastric cancer patients with type 2 diabetes and body mass index< 35 kg/m2: preliminary results. Ann Surg Treat Res 88:215. https://doi.org/10.4174/astr.2015.88.4.215
Dicker D, Golan R, Aron-Wisnewsky J, Zucker J-D, Sokolowska N, Comaneshter DS, Yahalom R, Vinker S, Clément K, Rudich A (2019) Prediction of long-term diabetes remission after RYGB, sleeve gastrectomy, and adjustable gastric banding using DiaRem and advanced-DiaRem scores. Obes Surg 29:796–804. https://doi.org/10.1007/s11695-018-3583-3
Samuel N, Mustafa A, Hawkins H, Wei N, Boyle M, De Alwis N, Small P, Mahawar K and Carr W (2021) Influence of pre-operative HbA1c on bariatric surgery outcomes—the Sunderland (UK) Experience. Obes Surg 1–6.
Wierzbicka E, Swiercz A, Pludowski P, Jaworski M, Szalecki M (2018) Skeletal status, body composition, and glycaemic control in adolescents with type 1 diabetes mellitus. J Diabetes Res 2018. https://doi.org/10.1155/2018/8121634
Solanki JD, Makwana AH, Mehta HB, Kamdar P, Gokhale PA, Shah CJ (2016) Effect of current glycemic control on qualitative body composition in sedentary ambulatory Type 2 diabetics. Niger Med J 57:5. https://doi.org/10.4103/0300-1652.180562
Acknowledgements
The authors extend their sincere thanks to all participants and the National Obesity Surgery Database team, who prepared us very useful data.
Author information
Authors and Affiliations
Contributions
Negar Zamaninour: conceptualization, methodology, formal analysis, writing original draft. Hastimansooreh Ansar: conceptualization, assistance in writing original draft. Abdolreza Pazouki: conceptualization, acquisition of data and supervision. Mohadeseh Hassan zadeh: assistance in writing original draft. Ali Kabir: conceptualization, formal analysis, supervision, review and editing.
Corresponding author
Ethics declarations
Ethics and Consent Statements
This study was conducted in accordance with the Helsinki declaration and approved by the Research Ethics committee (Ethics Number: IR.IUMS.REC.13970134) of Iran University of Medical Sciences, Tehran, Iran. Written informed consent form was received from all patients at the time of first registry in our database for any possible anonymous usage from their data.
Consent for Publication
All authors are in agreement with publication of the manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zamaninour, N., Ansar, H., Pazouki, A. et al. Effect of Optimized Pre-operative Glycemic Status on Diabetes and Body Composition After One Anastomosis Gastric Bypass in 373 Patients. Indian J Surg (2022). https://doi.org/10.1007/s12262-022-03419-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12262-022-03419-y