Abstract
Rectoanal intussusception (RAI) treated using laparoscopic ventral rectopexy (LVR) may give rise to rectorectal intussusception (RRI) during defecation postoperatively. However, only a few studies have analyzed the results of LVR using pelvic floor imaging, which is important when interpreting postoperative symptoms in patients with RAI. Thus, this study was designed to find the preoperative variables that may help predict the postoperative occurrence of RRI and to determine whether RRI may have negative effects on bowel symptoms after LVR for RAI. Consecutive patients treated between 2012 and 2017 were included. Defecatory function was evaluated using the Constipation Scoring System (CSS) and the Fecal Incontinence Severity Index (FISI). Defecography was performed before and 6 months after LVR. Of the 66 patients with RAI preoperatively, 34 had mixed obstructed defecation (OD) and fecal incontinence (FI), 18 had OD alone, and 12 had FI alone. Twelve months after surgery, a reduction of at least 50% was observed in the CSS score of 25 patients (52%) with OD and in the FISI of 37 incontinent patients (87%). Postoperatively, RAI was replaced with RRI in 21 and posterior RAI in 2 patients. These anatomical changes were found in patients who had a greater anorectal angle at rest preoperatively. However, the improvement in bowel symptoms was unrelated to the anatomical changes. Improvement in bowel symptoms after LVR for RAI was unrelated to the postoperative occurrence of RRI or posterior RAI, which were found in patients who had a vertical rectum at rest preoperatively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ideal surgical treatment for rectoanal intussusception (RAI) should correct the anatomical abnormality and derived symptoms, which range from obstructed defecation (OD) to fecal incontinence (FI). Various surgical procedures have been used to treat RAI. Posterior rectopexy improves continence, although associated constipation tended to worsen after surgery [1]. Perineal procedures, including the internal Delorme operation [2] or stapled transanal rectal resection [3], alleviate OD, but they are associated with recurrent prolapse, de novo urgency, and incontinence due to alteration in rectal compliance.
In 2004, D’Hoore et al. proposed using laparoscopic ventral rectopexy (LVR) for the treatment of external rectal prolapse (ERP) [4]. This anterior approach, which limits rectal mobilization without lateral dissection, reduced the incidence of postoperative constipation, as compared with posterior rectopexy [5]. Other authors have reproducibly demonstrated the safety of this minimally invasive abdominal procedure and its ability to provide a long-term cure for ERP and RAI with little surgical morbidity [6]. However, few studies have analyzed the results of LVR using pelvic floor imaging, which is important when interpreting postoperative symptoms in patients with RAI. Our preliminary study demonstrated the findings of evacuation proctography after LVR, where RAI was eliminated in all patients but nearly one-third of patients developed new-onset rectorectal intussusception (RRI) [7]. The present study aimed to find a predictive factor for postoperative occurrence of RRI and to determine whether RRI may have negative effects on bowel symptoms after LVR for RAI.
Material and Methods
Study Participants
Data on all patients who underwent LVR for RAI between May 2012 and September 2017 were prospectively entered into a pelvic floor database. The diagnosis of RAI was suggested on the basis of a history of OD and/or FI, anal discomfort, and clinical examination and was confirmed using evacuation proctography. No patient underwent a colonic transmit study. Proctograms were evaluated using the criteria proposed by Shorvon et al. [8]. Anorectal function was evaluated using 2 different scores: the Constipation Scoring System (CSS) [9] and the Fecal Incontinence Severity Index (FISI) [10]. Indications for surgery were RAI at the time of proctography with symptoms of OD or FI, with failed standard medical management. No patient underwent biofeedback treatment, because of a lack of well-trained physiotherapists in the clinic. Informed consent was obtained from all patients. This study was approved by the Ethical Committee of Kameda Medical Center (Approval number: 18–158).
Surgical Technique
The surgical procedure for LVR was carried out as described by D’Hoore et al. [4]. Dissection was conducted exclusively anterior to the rectum, preserving the lateral ligaments, and the rectovaginal septum was carefully dissected down to the pelvic floor. The distal extent, which is usually 2–3 cm from the anal verge, was confirmed though digital rectal examination. The dissection performed using this procedure spares the hypogastric nerves and parasympathetic nerves from the lateral ligament and avoids mobilization of the mesorectum.
A strip of polypropylene (3 × 20 cm2) mesh was introduced, provisionally attached to the anterior rectum, and sutured as distally as possible to the rectal wall using 6 interrupted, nonabsorbable sutures (2–0 Tycron, Covidien, Japan). The posterior wall of the vagina was fixed to the mesh with 2 additional sutures of the same type. A modified technique for the introduction of the mesh was used. Namely, a nylon thread with straight needle was passed by the perineal operator through the posterior wall of the vagina at the distal extent of the dissection, which was caught in the abdominal cavity, extracted from one of the trocars and fixed at the end of the mesh extracorporeally. The mesh was then introduced and pulled toward the pelvic floor using the nylon thread. The mesh was then attached to the rectum and vagina as described above and secured tension-free to the sacral promontory by using a protack device (Autosuture, Tyco Healthcare, Mansfield, Massachusetts, USA). In male patients, the deepest part of the rectovesical pouch was dissected down to the pelvic floor. The mesh was introduced and sutured as distally as possible to the anterior rectum. The mesh was peritonealized by suturing the free edge of the previously divided peritoneum over the mesh to avoid small bowel adhesions. The nylon thread was cut transvaginally at the end of surgery.
Evacuation Proctography
A standard proctography technique was used as previously described [7]. RAI was diagnosed when the apex of the rectal intussusception impinged on the internal anal orifice or was intra-anal, based on the images taken during maximal straining defecation. Anterior or posterior intussusception descent was measured as previously reported [11]. RRI was differentiated from RAI if the apex remained intrarectal and did not impinge on the internal anal orifice [8]. The presence of the rectocele was classified as grade 1 (< 2 cm in depth), grade 2 (2–4 cm in depth), or grade 3 (> 4 cm in depth) [12]. The size was calculated in standard manner in the anterior- posterior dimension by measuring the distance between the actual most ventral part of the anterior rectal wall and an extrapolated line indicating the expected position of the rectal wall [13]. Enterocele or sigmoidocele was diagnosed when the extension of the loop of the bowel was located between the vagina and rectum. Pelvic floor descent during defecation was estimated by observing the degree of the anorectal junction in relation to the inferior margin of the ischial tuberosity. The anorectal angle was defined as the angle between the axis of the anal canal and the posterior wall of the distal half of the rectum. The “rectal axis” was defined as the angle between the horizontal and the center of the rectum as defined by Shorvon [8]. The anorectal angle and the “rectal axis” were measured on the images taken at rest.
Follow-Up
Patients were followed-up 3, 6, and 12 months after the procedure. FISI and CSS scores were reported at those visits. The questionnaires were handed out to patients by a nurse staff member and self-administered in the outpatient clinic. If patients did not report to the clinic for a check-up, they were asked to report both FISI and CSS scores via a phone. Evacuation proctography was performed 6 months after the procedure.
Statistical Analysis
Quantitative data were expressed as the median and range. Analysis was performed using the Wilcoxon’s signed-rank test for paired data (two-sided p test). Univariate associations between continuous variables were analyzed using Pearson’s correlation coefficients. A stepwise multiple regression analysis was used to establish which preoperative variables best predicted the postoperative occurrence of rectal intussusception. The data were analyzed by using R software packages (V.3.3.2; R Development Core Team). p < 0.05 was considered to indicate statistical significance.
Results
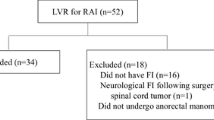
The 66 patients whose data are included in this study all underwent LVR for symptomatic RAI. The median age of patients was 74 (48–93) years and 59/66 (89%) were female. Among the patients, 34 (52%) had mixed OD and FI, 18 (27%) had OD alone, 12 (18%) had FI alone, and 2 (3%) had sensation of vaginal bulge. The median duration of follow-up was 36 (12–71) months (Table 1). The median vaginal delivery was 2 (0–5). A total of 36 patients (55%) had undergone previous pelvic surgery. The median operation time was 179 min (range; 107-335 min) and blood loss was 5 ml (range; 0–70 ml). There was no conversion to open surgery. One patient underwent reoperation 22 months after the first surgery because there was no postoperative improvement in her symptoms and possible mesh detachment. The repeated surgery revealed that mesh was fixed at the sacral hollow instead of the sacral promontory. Re-LVR using another mesh at the sacral promontory was performed, but there was no improvement in the patient’s symptoms. The median length of the postoperative stay was 1 day (range; 1–7 days). There was no postoperative mortality or major morbidity. No patient was readmitted for medical or surgical complications.
Three patients had intraoperative complications; one with ureter injury, the other two with vaginal injury. Those were repaired intraoperatively. Postoperative complications occurred in two patients; both developed port-site infection. There were no major postoperative complications and mesh-related complications during follow-up.
Obstructed Defecation
Fifty-two patients (79%) presented with OD preoperatively, 18 with pure OD and 34 with mixed OD/FI. In 48 patients who filled the CSS score 12 months after surgery, 25 patients (52%) showed reduction of at least 50% in their CSS scores, while 23 (48%) showed persistent OD. Overall, CSS scores were significantly reduced at 3 months [median preop 12 vs. postop 9, p < 0.001 (Table 2)] and remained significantly reduced 6 or 12 months after surgery (Table 2). The proportion of patients who had a reduction of at least 50% in their CSS scores was 33% at 3 months, 43% at 6 months and 52% at 12 months. Among the remaining 14 patients without constipation preoperatively, one developed de novo constipation.
Fecal Incontinence
Forty-six patients (70%) presented with FI preoperatively, 12 with pure FI and 34 with mixed OD/FI. In 43 patients who filled the FISI score 12 months after surgery, 37 patients (86%) showed reductions of at least 50% in the FISI scores, while 6 patients (14%) showed persistent FI. Overall, FISI scores were significantly reduced at 3 months [median preop 30 vs. postop 14, p < 0.0001 (Table 2)] and remained significantly reduced at 6 and 12 months.
The proportion of patients who had a reduction of at least 50% in their FISI scores was 57% at 3 months, 71% at 6 months, and 86% at 12 months after surgery.
Evacuation Proctography
Preoperative evacuation proctography showed a median anterior descent and posterior intussusception descent were 22 mm (range, 7–42 mm) and 20 mm (range, 0–44 mm), respectively. Proctography performed 6 months after surgery revealed that 2 patients had posterior RAI (Table 3). In 21 patients, the RAI was replaced by RRI (Fig. 1). Enterocele disappeared in all 15 patients. Grade 1, 2, and 3 rectocele were found in 6/17/3 patients before and 27/2/0 patients after surgery, respectively; the rectocele size was significantly reduced [median preop 27 mm vs postop 10 mm, p < 0.0001]. Pelvic floor descent was significantly reduced postoperatively. [median preop 24 mm vs postop 22 mm, p = 0.007] (Table 3). Preoperative variables including anorectal angle, the “rectal axis,” pelvic floor descent, and the presence of enterocele were not associated with significant improvements (at least 50% reduction in scores) of either OD or FI symptoms at each postoperative time (Tables 4 and 5).
Pearson’s Correlation Coefficients
The correlations between preoperative variables and postoperative occurrence of rectal intussusception are shown in Table 6. Preoperative anorectal angle and the “rectal axis” were significantly associated with the occurrence of rectal intussusception including RRA and posterior RAI, respectively, i.e., the greater the angle, the greater the incidence of rectal intussusception (Table 6).
Regression Analysis
The results of stepwise multiple regression analysis done using preoperative variables are shown in Table 7. The preoperative anorectal angle at rest was significantly associated with the postoperative occurrence of rectal intussusception (Table 7).
Postoperative Occurrence of Rectal Intussusception and Symptoms
There were no significant differences in the proportion of patients who had a significant improvement of OD at each postoperative time between patients with RRI and without rectal intussusception (Table 4). Additionally, there were no significant differences in the percentage of patients who had a significant improvement in FI at each postoperative time between those with RRI and those without rectal intussusception (Table 5). Posterior RAI occurred in 2 patients postoperatively, which was too small a number to compare within the groups, but both patients improved in terms of their OD or FI at 12 months.
Discussion
Summary of Results
This study demonstrated that improvements in defecation symptoms and anatomical correction were found after LVR in almost all patients with RAI. The improvement in symptoms was unrelated to the postoperative occurrence of RRI or posterior RAI, which was found in patients who had a greater anorectal angle at rest preoperatively.
New-Onset of RRI and Posterior PAI
Our previous study reported the significance of evacuation proctography after LVR, which achieved successful anatomical correction of RAI [7]. This was confirmed in all patients in this study except two. Similarly, enterocele was eliminated in all affected patients, and the size of rectocele or pelvic floor descent was reduced. Replaced RRI appeared in one-third of the patients postoperatively. This may be attributable to the rectal wall above the peritoneal reflection folding inward during the proctography, while the anterior wall of the lower rectum remained fixed and suspended by the mesh. It does seem to make sense anatomically that the greater the anorectal angle (a vertical rectum), the easier the rectal wall folding inward during defecation. Nevertheless, these patients experienced improvement in their presenting symptoms, like the patients who did not have RRI or RAI postoperatively. A previous proctographic study also found that patients with RRI were less likely to experience symptoms of OD and FI than were those with RAI [14, 15].
Two patients had persistent RAI postoperatively. The findings of proctography in both patients showed that the circular RAI before surgery changed to the posterior RAI after surgery. The mechanism of the replacement may be similar to that for new-onset RRI; the posterior wall of the rectum folded inward toward the anal canal, whereas the anterior wall of the rectum remained fixed with the mesh. Both patients experienced improvement of FI and OD symptoms postoperatively. Our previous study showed that the severity of FI was correlated with anterior intussusception descent in patients with RAI [11]. Disappeared anterior intussusception after surgery may have a positive effect on continence in both patients. The reason for postoperative improvement of OD is unclear, but from the anatomical point of view, those with posterior RAI alone may have OD symptoms less seriously than those with circular RAI.
Reason for Improvement of the Symptoms
An improvement in continence was found after LVR in patients with RAI in this study. Our previous study showed that an increase in anal pressure was not seen after LVR in patients with RAI and thus cannot explain the postoperative improvement of FI. Instead, there was an overall increase in both defecatory desire volume and maximum tolerated volume at 12 months postoperatively, and this may have a positive effect on continence [16]. Additionally, the improvement in continence may be explained by elimination of the high-grade intussusception causing inappropriate activation of the anorectal inhibitory reflex [17] or by a decrease in incomplete rectal emptying because of the RAI, which might have caused continuous leakage of stool after evacuation.
A successful symptomatic outcome was not achieved in all patients in this study. Twelve months after the procedure, persistent FI was reported by 6 (14%) patients and OD by 23 (48%). The cause of FI and OD is multifactorial; thus, patients who did not improve after surgery may have other underlying factors causing symptoms of FI and OD, such as anal sphincter failure, a colonic transit disorder or neurogenic factors [18, 19]. In fact, of the six patients with persistent FI, two had a history of obstetric injury or lay open for anal fistula, and one was on hemodialysis for chronic renal failure. Of the 23 patients with persistent OD, 13 were associated with the following conditions: a history of pelvic operation (11), diabetes mellitus (3), endocrine therapy for prostate carcinoma (1), psychiatric disease (1), and hemodialysis for chronic renal failure (1). In this study, one patient appeared to develop new-onset constipation, which has been reported previously, with incidences of 5.5% and 2% [20, 21].
In this study, the proportion of patients who had a significant improvement of OD or FI increased with time. This may be attributable to patients’ adaptation to defecation symptoms or bowel regimens, including stool softeners or bulking agents, which were introduced in most of the patients postoperatively. Another reason may be explained by the findings of our previous study, wherein a significant increase in the rectal volume was not found at 3 or 6 months but was found at 12 months postoperatively [16]. This change with time may be explained by recovery or changed sensation of the anatomic innervation. Our findings are supported by the study by Formijine Jonkers et al., who reported that a better function of the rectum, better sensitivity for feces in the rectum, and less bulging of the rectal wall may improve continence and constipation in patients undergoing LVR [20].
Ris et al. reported that preoperative proctographic findings of the presence of an enterocele and a vertical axis of the rectum at rest were associated with a better resolution of OD symptoms and FI at 3 months postoperatively [22]. However, we did not find any correlation between the proctographic findings and symptomatic relief at each postoperative time until 12 months, probably because of the difference in the follow-up period or the definition of significant improvement of symptoms, for which we used a more rigid standard of improvement.
Limitation
This study was limited by the small sample size, decreasing number of evaluated patients with time, and lack of control group.
Conclusion
LVR for RAI produced adequate (> 50%) improvement of FI in 86% and DO in 52% of patients. The improvements were unrelated to the postoperative occurrence of RRI or posterior RAI, which was found in patients who had a more vertical rectum at rest preoperatively.
References
Orrom WJ, Bartolo DC, Miller R, Mortensen NJ, Roe AM (1991) Rectopexy is an ineffective treatment for obstructed defecation. Dis Colon Rectum 34:41–46
Berman IR, Harris MS, Rabeler MB (1990) Delorme's transrectal excision for internal rectal prolapse. Patient selection, technique, and three-year follow-up. Dis Colon Rectum 33:573–580
Boccasanta P, Venturi M, Stuto A, Bottini C, Caviglia A, Carriero A, Mascagni D, Mauri R, Sofo L, Landolfi V (2004) Stapled transanal rectal resection for outlet obstruction: a prospective, multicenter trial. Dis Colon Rectum 47:1285–1296 discussion 1296-7
D'Hoore A, Cadoni R, Penninckx F (2004) Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg 9:1500–1505
Prasad ML, Pearl RK, Abcarian H, Orsay CP, Nelson RL (1986) Perineal proctectomy, posterior rectopexy, and postanal levator repair for the treatment of rectal prolapse. Dis Colon Rectum 29:547–552
Emile SH, Elfeki HA, Youssef M, Farid M, Wexner SD (2017) Abdominal rectopexy for the treatment of internal rectal prolapse: a systematic review and meta-analysis. Color Dis 19:O13–O24
Tsunoda A, Ohta T, Kiyasu Y, Kusanagi H (2015) Laparoscopic ventral rectopexy for rectoanal intussusception: postoperative evaluation with proctography. Dis Colon Rectum. 58:449–456
Shorvon PJ, McHugh S, Diamant NE, Somers S, Stevenson GW (1989) Defecography in normal volunteers: results and implications. Gut. 30:1737–1749
Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD (1996) A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum 39:681–685
Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, Wexner SD, Bliss D, Lowry AC (1999) Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum 42:1525–1532
Tsunoda A, Takahashi T, Ohta T, Fujii W, Kiyasu Y, Kusanagi H (2016) Anterior intussusception descent during defecation is correlated with the severity of fecal incontinence in patients with rectoanal intussusception. Tech Coloproctol 20:171–176
Faccioli N, Comai A, Mainardi P, Perandini S, Moore F, Pozzi-Mucelli R (2010) Defecography: a practical approach. Diagn Interv Radiol. 16:209–216
Bartram CI, Turnbull GK, Lennard-Jones JE (1988) Evacuation proctography: an investigation of rectal expulsion in 20 subjectswithout defecatory disturbance. Gastrointest Radiol. 13:72–80
Hawkins AT, Olariu AG, Savitt LR, Gingipally S, Wakamatsu MM, Pulliam S, Weinstein MM, Bordeianou L (2016) Impact of rising grades of internal rectal intussusception on fecal continence and symptoms of constipation. Dis Colon Rectum 59:54–61
Collinson R, Cunningham C, D'Costa H, Lindsey I (2009) Rectal intussusception and unexplained faecal incontinence: findings of a proctographic study. Color Dis 11:77–83
Tsunoda A, Takahashi T, Hayashi K, Yagi Y, Kusanagi H (2018) Laparoscopic ventral rectopexy in patients with fecal incontinence associated with rectoanal intussusception: prospective evaluation of clinical, physiological and morphological changes. Tech Coloproctol. 22:425–431
Farouk R, Duthie GS, Bartolo DC, MacGregor AB (1992) Restoration of continence following rectopexy for rectal prolapse and recovery of the internal anal sphincter electromyogram. Br J Surg 79:439–440
Rao SS (2003) Constipation: evaluation and treatment. Gastroenterol Clin N Am 32:659–683
Hayden DM, Weiss EG (2011) Fecal incontinence: etiology, evaluation, and treatment. Clin Colon Rectal Surg 24:64–70
Formijne Jonkers HA, Poierrié N, Draaisma WA, Broeders IA, Consten EC (2013) Laparoscopic ventral rectopexy for rectal prolapse and symptomatic rectocele: an analysis of 245 consecutive patients. Color Dis 15:695–699
Slawik S, Soulsby R, Carter H, Payne H, Dixon AR (2008) Laparoscopic ventral rectopexy, posterior colporrhaphy and vaginal sacrocolpopexy for the treatment of recto-genital prolapse and mechanical outlet obstruction. Color Dis 10:138–143
Ris F, Gorissen KJ, Ragg J, Gosselink MP, Buchs NC, Hompes R, Cunningham C, Jones O, Slater A, Lindsey I (2017) Rectal axis and enterocele on proctogram may predict laparoscopic ventral mesh rectopexy outcomes for rectal intussusception. Tech Coloproctol 21:627–632
Acknowledgments
The authors thank S. Takada for his assistance with statistical analysis.
We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
NO: conception and design of the study, acquisition, analysis and interpretation of data, writing the article. AT: conception and design of the study, acquisition and interpretation of data, writing the article. TT: analysis and interpretation of data, critical revision. SM: acquisition and interpretation of data, critical revision. HK: analysis and interpretation of data, critical revision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study was approved by the Ethical Committee of Kameda Medical Center. (Approval number: 18–158).
Ethical Standards
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Informed Consent
Informed consent was obtained from all patients by authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oka, N., Tsunoda, A., Takahashi, T. et al. Predictive Factors and Effects of Replaced Rectorectal Intussusception on Functional Outcomes in Patients with Rectoanal Intussusception Who Have Undergone Laparoscopic Ventral Rectopexy. Indian J Surg 83, 79–86 (2021). https://doi.org/10.1007/s12262-020-02262-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-020-02262-3