Abstract
Groove pancreatitis (GP) is a rare type of segmental pancreatitis, and it remains largely an unfamiliar entity to most physicians. It is often misdiagnosed as pancreatic cancer and autoimmune pancreatitis. With better understanding of radiological findings, preoperative differentiation is often possible. If there is preoperative diagnosis of GP, one can employ non-surgical treatment. But most of the patients ultimately require surgery. Pancreaticoduodenectomy (PD) is the surgical treatment of choice. We report three cases of GP that were treated by Whipple’s operation at our unit. All the three patients had a history of long-standing alcohol intake. In the first and third patients, we had a preoperative diagnosis of GP. But, in the second patient, our pre-operative and intra-operative diagnosis was a pancreatic head malignancy. Diagnosis of GP was made only after histopathological examination. All the three patients had uneventful postoperative recovery and were well at 55-, 45- and 24-month follow-up respectively. In addition to detail descriptions of our three cases, a detailed review of the current literature surrounding this clinical entity is also provided in this article.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
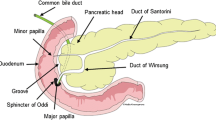
Groove pancreatitis (GP) is a segmental form of chronic pancreatitis characterized by fibrotic scarring of the pancreatoduodenal groove, an anatomic space bordered by the head of the pancreas, duodenum and the common bile duct (CBD). Though the entity was first described by Becker in 1973 in German term as ‘Rinnenpankreatitis’ [1], the term groove pancreatitis was coined by Stolte et al. in 1982 [2]. Because of its rarity, the true incidence of GP is unknown. The reported incidence varies from 2.7 to 24.5% cases of pancreaticoduodenectomies performed for chronic pancreatitis [2,3,4]. Differentiation between GP and pancreatic head cancers may be difficult before operation. However, with increased understanding in the radiological findings, preoperative diagnosis is often possible. In this article, we present three cases of GP treated surgically in our unit over a 7-year period with a brief review of the current medical literature.
Case Reports
Case 1
A 47-year-old male with a history of epigastric pain radiating to the back associated with intermittent non-bilious vomiting and weight loss of about 40 lb in past 1 year was treated at various hospitals as a case of recurrent acute pancreatitis. The patient had a history of alcohol abuse for 8 years. Physical examination revealed mild pallor and features of malnutrition. Laboratory investigations revealed raised serum amylase and lipase with low albumin. His serum CA 19-9 and CEA levels were within normal limits. Computed tomography (CT) scan of the abdomen showed loss of fat planes between head of pancreas and the duodenum with an ill-defined hypodense area in the head of pancreas. Pancreatic duct was not dilated. The second part of the duodenum was thickened. Biliary system was normal. Esophagogastroduodenoscopy revealed an oedematous mucosa with a polypoidal appearance and narrowing of the second part of the duodenum (Fig. 1). Endoscopic biopsy result was benign. Endoscopic ultrasound (EUS) showed multiple hypoechoic areas in the head of the pancreas. Based on imaging and endoscopic findings, we suspected it to be a case of GP. We performed Whipple’s operation. Cut section of the operative specimen showed thickening of the duodenal wall and a thick fibrous band in the pancreato-duodenal groove with few cystic spaces in the head of the pancreas. Histopathological examination was suggestive of GP. Postoperative recovery was uneventful. The patient was well at 55-month follow-up.
Esophagoduodenoscopy showing narrowing of the second part of the duodenum with fine nodular and ulcerated mucosa (case 1)
Case 2
A-45-year old alcoholic male patient presented with 3-month history of recurrent severe postprandial upper abdominal pain with occasional radiation to the back. There was no history of vomiting and jaundice. He was initially treated at a local hospital for about 6 weeks. As there was no improvement of pain, he came to our institution for evaluation. Physical examination was unremarkable except weight loss of 12 lb. Laboratory investigations were within normal limits except raised serum alkaline phosphatase level. Serum CA 19-9 and CEA levels were within normal limits. CT scan (Fig. 2) of the abdomen showed a 4 × 3.5-cm heterogeneously enhancing mass in the head and uncinate process of the pancreas with narrowing of the duodenum. CBD was dilated, and there was periduodenal fat stranding. Esophagogastroduodenoscopy showed narrowing of the second part of the duodenum with fine nodular and ulcerated mucosa. Endoscopic biopsy showed no evidence of dysplasia or malignant changes. We performed Whipple’s operation with a suspicion of pancreatic head malignancy. Cut section of the specimen showed a firm greyish mass at the head of the pancreas. Histopathological examination (Fig. 3a–d) revealed leiomyoma-like mass incorporating the lobules of pancreatic acinar tissue and inflamed disrupted ductal structures mimicking ‘myoadenomatosis’. There were foci of myofibroblastic proliferation. Duodenal wall was thickened with chronic inflammation in the groove area and Brunner’s gland hyperplasia. These features were suggestive of GP. The patient had an uneventful recovery and was well at 45-month follow-up.
CT scan of the abdomen showing heterogeneously enhancing mass in the head and uncinate process of the pancreas with narrowing of the second part of duodenum (case 2)
Histopathological examination showing (case 2). a Myofibrous proliferation with mild chronic inflammation, H&E ×40. b, c Chronic inflammation with glandular proliferation, H&E ×100. d Chronic inflammation in groove area H&E ×400
Case 3
A 40-year-old male with chronic alcohol consumption came to us for recurrent epigastric pain for 3 years, occasional bilious vomiting for 9 months and weight loss for 6 months. Physical examination was unremarkable. Upper GI endoscopy revealed narrowing of the second part of the duodenum with nodular mucosa. Endoscopic biopsy did not reveal any dysplasia or malignancy. CT scan of the abdomen showed typical plate-like mass in the pancreaticoduodenal groove with thickening of adjacent duodenal wall (Fig. 4). Pancreatic duct was dilated (6 mm). As he was not responding to conservative treatments for 6 months, Whipple’s operation was performed. Histopathological examination of the resected specimen confirmed the preoperative diagnosis of GP. The patient had an uneventful recovery and was well at 24-month follow-up.
CT scan of the abdomen showing typical plate-like mass in the pancreaticoduodenal groove with thickening of the duodenal wall (arrow) (case 3)
Discussion
GP is a specific form of chronic pancreatitis characterized by extensive fibrosis and inflammation in the pancreato-duodenal groove. The condition has been reported under several alternative names, including paraduodenal pancreatitis, cystic dystrophy of heterotopic pancreas, paraduodenal wall cyst and myoadenomatosis. In 1991, Becker and Mischke classified GP into pure form and segmental form [3]. The former involves the groove only, with preservation of the pancreatic parenchyma and the main pancreatic duct, while the latter affects both the groove and the head of the pancreas with stenosis of the pancreatic duct causing upstream dilatation.
The pathogenesis of GP remains unclear. Alcohol is the most important predisposing factor. One of the most frequently reported mechanisms is altered pancreatic secretion through Santorini’s duct (SD) related to aggression caused by alcohol [2, 5,6,7,8]. When it is disturbed, the pancreatic secretion via the SD is directed towards the body of the pancreas, to Wirsung’s duct, which forms an acute angle, causing interference with the flow and an accumulation of temporal secretion at the top of the pancreatic head [9]. The increased intraductal pressure in the SD facilitates the formation of pseudocysts and leakage of pancreatic juice into the groove [10, 11]. Other possible reasons for GP are Brunner’s gland hyperplasia, duodenal bud and ectopic pancreas [12]. Moreover, a fibrous scarring secondary to gastrectomy, gastroduodenal ulcer and biliary disease might also be pathogenetic factors [13]. In our patients, we found Brunner’s gland hyperplasia in all the cases.
GP mainly affects middle-aged men with a history of alcohol abuse. Typical symptoms include postprandial abdominal pain, early satiety, nausea, vomiting and weight loss mainly due to duodenal obstruction. These symptoms last from few weeks to more than a year before clinical diagnosis is made. In our cases, the duration between the onset of symptoms and the diagnosis varies from 3 months to 3 years. Jaundice is rare but can occur if there is stenosis of CBD [14]. Jaundice, when present, is usually fluctuating, unlike the progressive jaundice secondary to pancreatic cancer. The course of the disease is often chronic and debilitating. Sometimes, it may be associated with diarrhoea and diabetes mellitus [15]. Laboratory investigations may show a slight elevation of pancreatic enzymes and occasionally hepatic enzymes. Tumour markers are rarely elevated [16].
Preoperative diagnosis of GP is a challenge. It is often misdiagnosed as either pancreatic cancer or autoimmune pancreatitis. Depending upon the clinical features, transabdominal ultrasound and upper GI endoscopy are done as first-line investigations. Upper GI endoscopy often shows an inflamed and polypoid duodenal mucosa with stenosis of the duodenal lumen. Ultrasonography shows increased thickness of the duodenal wall and stenosis of the lumen, with a hypoechoic lesion between the pancreas head and the thickened duodenum. CT scan is an excellent means for diagnosis of GP. In the ‘pure’ form of the disease, a poorly enhancing plate-like hypodense lesion can be identified between the pancreatic head and the duodenum. Multiple cysts located within a thickened duodenal wall exhibiting postcontrast enhancement are also useful indicators of GP [6]. In the ‘segmental’ form of GP, a focal hypodense lesion can be seen in the pancreatic head in close proximity to the duodenal wall. The main pancreatic duct may exhibit mild dilatation in the body and tail region. CBD may be constricted at its distal part leading to upstream dilatation. It should be emphasized that, even in extensive disease, the peripancreatic vessels are preserved, showing no signs of thrombosis or infiltration. MRI shows a sheet-like mass in the groove area, which is hypointense relative to the pancreatic parenchyma on T1-weighted images and iso- to slightly hyperintense on T2-weighted images [13]. Grooves are revealed as hypointense areas on T1-weighted images. T2-weighted imaging is useful to detect intraduodenal wall cysts [6]. Endoscopic ultrasound (EUS) is also effective in diagnosing GP. It reveals smooth stenosis of the CBD; SD is usually undetectable. Duct-penetrating signs are evident in the irregular hypoechoic mass. However, insertion of EUS is often impossible due to duodenal stenosis and the accuracy of EUS is operator dependent [17]. EUS-guided fine-needle aspiration (FNA) may be helpful for preoperative diagnosis of GP. But, the results are variable depending upon the area of sampling. If the area sampled has abundant spindle cells, large numbers of giant cells or hyperplasia of Brunner’s glands, they may mimic neoplasia. This situation is particularly deceptive because the presence of giant cells and hyperplasia of Brunner’s glands are among the characteristic features of GP. Likewise, an area with fibrosis does not rule out neoplasia, since it is common to find a desmoplastic reaction associated with the adenocarcinoma mimicking abnormal inflammatory changes.
Although there are several radiological findings described for GP for preoperative diagnosis, differentiation of GP (principally in the segmental form) from pancreatic head carcinoma preoperatively by these sophisticated investigations is often impossible. CT and MRI are not reliable, especially when the tumour is scirrhous or has a high fibrous component [18]. Some of the pancreatic cancers also originate in the groove, making it difficult to distinguish them from the pure form of GP [19, 20]. In 2010, Ishigami et al. [21] reported the utility of the portal venous phase in CT and MRI to distinguish GP from pancreatic groove carcinoma, finding that GP more frequently presents with irregular focal enhancement in that phase. Recently, Kalb et al. [22] achieved a diagnostic accuracy of 87.2% for GP using three strict MRI criteria: focal thickening of the second duodenal portion, an abnormally increased enhancement of the second duodenal portion and cystic changes in the region of the accessory pancreatic duct. According to the authors, if these three criteria are met, ductal carcinoma of the head of pancreas can be ruled out, with a negative predictive value of 92.9% [22]. Vascular invasion is an important sign for the differentiation of the two entities [6, 19, 23, 24], especially invasion of the gastroduodenal artery which appears infiltrated in the case of tumour and shifted to the left in the case of GP. The presence of cystic lesions in the groove or duodenal wall [6, 18, 19], inflammatory thickening of the duodenal wall [18, 19], and stenosis due to scarring of the duodenal lumen all indicates GP. Endoscopic biopsy can help, although its usefulness is limited if the cancer is small and does not invade the duodenal mucosa (4). The presence of Brunner’s gland hyperplasia is more characteristic of GP and is usually absent in pancreatic cancer. ERCP and EUS may also serve to differentiate these two entities. GP presents a regular smooth bile duct stenosis, while in pancreatic cancer, it is irregular and abrupt. EUS-guided FNA is of great importance in the study of pancreatic lesions because of its high sensitivity and specificity for the diagnosis of pancreatic cancer [25, 26]. However, its value may be limited depending on the site biopsied and its interpretation has to be supported by other complementary tests [25, 26].
There are two therapeutic options for GP: (1) conservative medical and endoscopic measures and (2) surgery. Conservative measures including analgesics, pancreatic rest and abstinence from alcohol are usually successful at treating the initial symptoms but may not be long-lasting. Isayama et al. [27] reported the treatment of GP by endoscopic stenting of the minor papilla, but the long-term clinical course remains obscure. Similarly, Casetti et al. had shown poor results with endoscopic therapy. In their experience, all three endoscopically treated patients eventually needed definitive therapy with pancreaticoduodenectomy (PD) [28]. In a recent study, Arvanitakis et al. [29] showed that a stepwise approach to treatment of GP is feasible and effective and is associated with an acceptable rate of complications. Using both medical and endoscopic treatment, complete clinical response was achieved in almost 80% of cases. Surgery is the treatment of choice when symptoms do not improve or when the condition is too difficult to distinguish from pancreatic carcinoma. In a study by Rahman et al. [30], body weight increased and chronic abdominal pain was relieved in all patients who underwent PD. Complete pain relief was reported in 76% of the patients after PD [28]. The preservation of the pylorus during PD is not always feasible because of extensive fibrosis of the pyloro-duodenal region. We performed Whipple’s procedure in all the three cases. Due to dense fibrosis in the pancreatoduodenal area, the operating time and the blood loss were more in comparison to Whipple’s procedure for periampullary cancer. But, in all the three cases, there were no pancreatic leaks due to firm nature of the pancreas.
Conclusion
Differentiation between GP and pancreatic head cancers may be difficult before operation. However, with increased understanding of the radiological findings, preoperative diagnosis is often possible. In the acute phase, treatment is conservative. GP resistant to medical treatment requires surgery, and Whipple’s operation is the surgery of choice with good long-term results.
References
Becker V (1973) Bauchspeicheldrüse. In: Doerr W, Seifert G, Uhlinger E (eds) Spezielle pathologische Anatomie, vol vol 4. Springer, Berlin, pp 252–445
Stolte M, Weiss W, Volkholz H, Rosch W (1982) A special form of segmental pancreatitis: “groove pancreatitis”. Hepato-Gastroenterology 29:198–208
Becker V, Mischke U (1991) Groove pancreatitis. Int J Pancreatol 10:173–182
Yamaguchi K, Tanaka M (1992) Groove pancreatitis masquerading as pancreatic carcinoma. Am J Surg 163:312–316
Ferreira A, Remalho M, Herédia V et al (2010) Groove pancreatitis: a case report and review of the literature. J Radiol Case Rep 4:9–17
Triantopoulou C, Dervenis C, Giannakou N, Papailiou J, Prassopoulos P (2009) Groove pancreatitis: a diagnostic challenge. Eur Radiol 19(7):1736–1743. doi:10.1007/s00330-009-1332-7
Klöppel G (2007) Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol 20(Supl. 1):S113–S131
Manzelli A, Petrou A, Lazzaro A et al (2011) Groove pancreatitis. A miniseries report and review of literature JOP 12:230–233
Shudo R, Yazaki Y, Sakurai S et al (2002) Groove pancreatitis: report of a case and review of the clinical and radiologic features of groove pancreatitis reported in Japan. Intern Med 41:537–542
Isayama H, Kawabe T, Komatsu Y et al (2005) Successful treatment of groove pancreatitis by endoscopic drainage via the minor papilla. Gastrointest Endosc 61:175–178
Pallisera-Lloveras A, Ramia-Ángel JM, Vicens-Arbona C, Cifuentes-Rodenas A (2015) Groove pancreatitis. Rev Esp Enferm Dig 107(5):280–288
Zamboni G, Capelli P, Scarpa A et al (2009) Nonneoplastic mimickers of pancreatic neoplasms. Arch Pathol Lab Med 133(3):439–453. doi:10.1043/1543-2165-133.3.439
Irie H, Honda H, Kuroiwa T et al (1998) MRI of groove pancreatitis. J Comput Assist Tomogr 22:651–655
Adsay NV, Zamboni G (2004) Paraduodenal pancreatitis: a clinico-pathologically distinct entity unifying “cystic dystrophy of heterotopic pancreas”, “para-duodenal wall cyst”, and “groove pancreatitis”. Semin Diagn Pathol 21:247–254
Rebours V, Lévy P, Vullierme MP et al (2007) Clinical and morphological features of duodenal cystic dystrophy in heterotopic pancreas. Am J Gastroenterol 102:871–879
Levenick JM, Gordon SR, Sutton JE, Suriawinata A, Gardner TB (2009) A comprehensive, case-based review of groove pancreatitis. Pancreas 38(6):e169–e175. doi:10.1097/MPA.0b013e3181ac73f1
Kim JD, Han YS, Choi DL (2011) Characteristic clinical and pathologic features for preoperative diagnosed groove pancreatitis. J Korean Surg Soc 80(5):342–347. doi:10.4174/jkss.2011.80.5.342
Gabata T, Kadoya M, Terayama N et al (2003) Groove pancreatic carcinomas: radiological and pathological findings. Eur Radiol 13:1679–1684
Shanbhogue AK, Fasih N, Surabhi VR et al (2009) A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics 29:1003–1026
Pezzilli R, Santini D, Calculli L et al (2011) Cystic dystrophy of the duodenal wall is not always associated with chronic pancreatitis. World J Gastroenterol 17:4349–4364
Ishigami K, Tajima T, Nishie A et al (2010) Differential diagnosis of groove pancreatic carcinomas vs groove pancreatitis: usefulness of the portal venous phase. Eur J Radiol 74:e95–100
Kalb B, Martin DR, Sarmiento JM et al (2013) Paraduodenal pancreatitis: clinical performance of MR imaging in distinguishing from carcinoma. Radiology 269:475–481
Perez-Johnston R, Sainani NI, Sahani DV (2012) Imaging of chronic pancreatitis (including groove and autoimmune pancreatitis). Radiol Clin N Am 50:447–466
Coakley FV, Hanley-Knutson K, Mongan J et al (2012) Pancreatic imaging mimics: part 1, imaging mimics of pancreatic adenocarcinoma. AJR Am Roentgenol 199:301–308
Chute DJ, Stelow EB (2012) Fine-needle aspiration features of paraduodenal pancreatitis (groove pancreatitis): a report of three cases. Diagn Cytophathol 40:1116–1121
Brosens LAA, Leguit RJ, Vleggaar FP et al (2015) EUS-guided FNA cytology diagnosis of paraduodenal pancreatitis (groove pancreatitis) with numerous giant cells: conservative management by cytological and radiological correlation. Cytopathology 26(2):122–125. doi:10.1111/cyt.12140
Isayama H, Kawabe T, Komatsu Y et al (2005) Successful treatment for groove pancreatitis by endoscopic drainage via the minor papilla. Gastrointest Endosc 61(1):175–178
Casetti L, Bassi C, Salvia R et al (2009) “Paraduodenal” pancreatitis: results of surgery on 58 consecutives patients from a single institution. World J Surg 33(12):2664–2669. doi:10.1007/s00268-009-0238-5
Arvanitakis M, Rigaux J, Toussaint E et al (2014) Endotherapy for paraduodenal pancreatitis: a large retrospective case series. Endoscopy 46:580–587
Rahman SH, Verbeke CS, Gomez D, McMahon MJ, Menon KV (2007) Pancreatico-duodenectomy for complicated groove pancreatitis. HPB (Oxford) 9(3):229–234. doi:10.1080/13651820701216430
Author information
Authors and Affiliations
Contributions
Sukanta Ray wrote and drafted the manuscript; Supriyo Ghatak, Jayanta Dasgupta, Jayanta Biswas, Debottam Bandyopadhyay, Debashis Misra, Ranajoy Ghosh and Sujan Khamrui were involved in literature search and critically revised the manuscript. All authors had read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no competing interest.
Rights and permissions
About this article
Cite this article
Ray, S., Ghatak, S., Misra, D. et al. Groove Pancreatitis: Report of Three Cases with Brief Review of Literature. Indian J Surg 79, 344–348 (2017). https://doi.org/10.1007/s12262-017-1643-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-017-1643-x