Abstract
The objective of this study is to investigate the feasibility and safety of single-port microthoracoscopic thoracic sympathicotomy for the treatment of palmar hyperhidrosis. Between January 2008 and March 2013, 56 patients (36 male, 20 female; mean age 25.6 years, age range 16–39 years) underwent single-port microthoracoscopic thoracic sympathicotomy for palmar hyperhidrosis. Nineteen patients (33.9 %) had moderate palmar hyperhidrosis that could thoroughly wet a handkerchief, and 37 (66.1 %) had severe palmar hyperhidrosis with sweat dripping from the palm. Eight patients (14.3 %) had a positive family history, 34 (60.7 %) had plantar hyperhidrosis, 22 (39.3 %) had axillary hyperhidrosis, and 20 (35.7 %) had both plantar and axillary hyperhidrosis. In addition, 21 patients (37.5 %) had palmar pompholyx, five (8.9 %) had keratolysis exfoliativa, 10 (17.9 %) had chilblains, and nine (16.1 %) had palmar rhagades. A single 10-mm skin incision was made in the third intercostal space at the anterior axillary line, posterior to the pectoralis muscle. A 5-mm microthoracoscope and a 3-mm microelectrocautery hook were inserted through a single port into the thoracic cavity. The third and fourth ribs were identified, and the sympathetic chain was cut using the microelectrocautery hook. The bypassing nerve fibers, such as the Kuntz nerve fiber bundle, were ablated for 2–3 cm along the surface of the rib. The palmar temperature was recorded before and after sympathicotomy. All 56 procedures were completed using single-port microthoracoscopy. No postoperative complications such as hemorrhage, wound infection, hemopneumothorax, bradycardia, or Horner’s syndrome were observed. Bilateral procedures were completed in 20–56 min (mean 30 min). The palmar temperature increased by 2.2 ± 0.3 °C after surgery. The postoperative hospital stay was 1–4 days (mean 2.5 days). Mild compensatory sweating of the back and thigh occurred in five patients (8.9 %) at 2–3 days after surgery and disappeared at 7–15 days. The patients were followed up for 28.5 months (range 1–62 months). Hyperhidrosis resolved in both hands after surgery, and the previously wet, cold hands became dry and warm. The efficacy rate was 100 %. Plantar hyperhidrosis was also significantly reduced in 33 of the 34 patients with this condition (remission rate 97.1 %), and axillary hyperhidrosis was significantly reduced in 19 of 22 patients (remission rate 86.4 %). Eighteen of the 20 patients (90.0 %) with both plantar and axillary hyperhidrosis experienced significant alleviation of their symptoms. Single-port microthoracoscopic thoracic sympathicotomy is a safe, convenient, and effective method of treating palmar hyperhidrosis. This procedure can accurately locate the sympathetic chain with a small incision, minimal invasiveness, and good cosmetic results. The procedure is suitable for extensive clinical use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary palmar hyperhidrosis is caused by autonomic nerve dysfunction and is characterized by abnormally increased sweating of the hands, which may even cause dripping. Most patients also have axillary or plantar hyperhidrosis. This condition affects the social, educational, and professional aspects of patients’ lives, resulting in emotional distress. The cause of palmar hyperhidrosis remains unknown. Patients have excessive secretions from the eccrine sweat glands because of excessive excitability of the sympathetic nerve-dominated target regions. The disorder affects males and females equally starting in childhood or adolescence and has an incidence in the USA of up to 2.8 % [1]. Tu et al. [2] reported an incidence of 4.6 % among university and high school students from Fuzhou, China, with a peak incidence at age 6–16 years, affecting males and females equally. A positive family history is reported in 15.3 % of patients. Conventional therapies include astringents, antiperspirants, botulinum toxin injection, water-absorbing agents, anxiolytic agents, anticholinergic agents, and iontophoresis [3, 4]. However, these therapies have limited usefulness because they are not curative. Thoracoscopic thoracic sympathicotomy is reported to be the most effective method of treating primary palmar hyperhidrosis [3, 4]. In this report, we describe 56 consecutive patients with primary palmar hyperhidrosis who underwent single-port microthoracoscopic thoracic sympathicotomy between January 2008 and March 2013.
Clinical Data and Methods
Data Collection
The patients were 36 males and 20 females with a mean age of 25.6 years (range 16–39 years). The clinical manifestations of palmar hyperhidrosis included abnormally increased sweating of the hands, moist and cold hands, and agitation and anxiety. Moderate palmar hyperhidrosis is characterized by sweaty hands, and severe palmar hyperhidrosis causes sweat to drip from the hands. Forty of the 56 patients (71.4 %) had responded poorly to medication or had relapsed after taking medication. The severity of palmar hyperhidrosis was classified according to the criteria proposed by Lai et al. [5]. Nineteen patients had moderate palmar hyperhidrosis that could thoroughly wet a handkerchief, and 37 patients had severe palmar hyperhidrosis causing sweat to drip from the palm. Eight patients (14.3 %) had a positive family history, 34 (60.7 %) had plantar hyperhidrosis, 22 (39.3 %) had axillary hyperhidrosis, and 20 (35.7 %) had both plantar and axillary hyperhidrosis. In addition, 21 patients (37.5 %) had palmar pompholyx, five (8.9 %) had keratolysis exfoliativa, 10 (17.9 %) had chilblains, and nine (16.1 %) had palmar rhagades. The patients were selected according to the quantified assessment criteria for surgical therapy for palmar hyperhidrosis (Table 1) [6]. Sympathetic chain surgery was recommended for patients with a score of 20–40. Sympathetic chain surgery was not recommended for patients with a score of <20. Patients with a score of >40 may have severe anxiety disorders and should not undergo sympathetic chain surgery. The 56 patients included in this study all had a preoperative score of 20–40, including six (10.7 %) with a score of 20–25, 27 (48.2 %) with a score of 26–30, 20 (35.7 %) with a score of 31–35, and three (5.4 %) with a score of 36–40. All patients underwent chest computed tomography, electrocardiography, blood biochemistry analysis, and thyroid function testing before surgery to exclude hyperhidrosis secondary to hyperthyroidism, diabetes, pulmonary tuberculosis, or anxiety disorders. Severe bradycardia, pleural adhesions, and previous thoracic surgery were considered to be contraindications to surgery. In particular, surgery was avoided in patients with high levels of anxiety. Written informed consent was obtained from all patients prior to surgery. The study protocol was approved by the hospital’s ethics committee.
Surgical Procedures
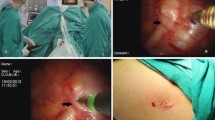
General anesthesia was induced, and the trachea was intubated with a double-lumen tube. The patient was positioned in the semi-Fowler’s position at 45°, with the arms abducted to 90° to expose the axillae. Electrocardiography, blood pressure, blood oxygen saturation, and palmar temperature were monitored throughout the procedure. Surgery was performed on the right side first. After deflation of one lung, a 10-mm incision was made in the third intercostal space at the anterior axillary line and the lateral border of pectoralis major (lateral border of the breast in females). A 5-mm, 30° microthoracoscope and a 3-mm microelectrocautery hook were inserted through the single port to check for severe pleural adhesions. The sympathetic chain was identified in the posterior mediastinum, anterior to the heads of the ribs. The parietal pleura overlying the sympathetic chain at the heads of the third and fourth ribs (R3 and R4) was dissected, and the sympathetic chain was cut by fulguration (Fig. 1). The bypassing nerve fibers, such as the Kuntz nerve fiber bundle, were also cut for 2–3 cm along the surface of the rib (Fig. 2). The palmar temperature was recorded before and after sympathicotomy. An increase in palmar temperature of at least 1–3 °C within 10 min was considered to confirm complete sympathicotomy. The sympathetic chain was first cut at R4, and palmar temperature was immediately recorded. If the increase in palmar temperature was less than 1 °C within 10 min, the sympathetic chain was also cut at R3. The same procedure was then performed on the left side.
Postoperative Follow-Up
The following were checked at postoperative follow-up: (1) postoperative improvement in palmar, plantar, and axillary hyperhidrosis, as well as skin disease; (2) time, degree, cause, site, and factors influencing recurrence of symptoms; (3) comparison of quality of life before and after surgery and the degree of satisfaction with the surgery; (4) time, degree, cause, site, and factors influencing compensatory hyperhidrosis; and (5) complications after surgery.
Criteria for Assessing the Curative Effect
As previously described [7], the surgery was considered effective if the palmar temperature increased by at least 1–3 °C after surgery and the hands became dry and was considered ineffective if the palmar temperature increased by less than 1 °C and the hands remained moist.
Criteria for Assessing Compensatory Sweating
Compensatory sweating was graded as mild, medium, or severe according to the classification by Licht et al. [8]. In the mild grade, sweating was increased after surgery but did not greatly affect the patient’s normal life. In the medium grade, sweating was significantly increased after surgery and affected the patient’s normal life and emotions, causing anxiety or embarrassment. In the severe grade, sweating was significantly increased after surgery, causing distress and even regret about undergoing the surgery. The severity of compensatory sweating was assessed in a quiet environment at a constant room temperature, at a time without physical activity or emotional stress.
Results
In all 56 patients, the procedure was successfully completed using single-port microthoracoscopy. Sympathicotomy was performed at R4 only in 37 patients, R4 and R3 in 15 patients, and R3 only in four patients. The mean operation time was 30 min (range 25–56 min). Intraoperative and postoperative electrocardiography, blood pressure, blood oxygen saturation, and palmar temperature remained stable. The mean postoperative hospital stay was 2.5 days (range 1–4 days). The surgical incision was minimal and well hidden in all patients. Hyperhidrosis resolved in both hands in all patients after surgery, and the previously wet, cold hands became dry and warm. The efficacy rate was 100 %. The palmar temperature increased by 2.2 ± 0.3 °C after surgery. No postoperative complications such as hemorrhage, wound infection, hemopneumothorax, bradycardia, or Horner’s syndrome were observed. Mild compensatory sweating of the back and thigh occurred in five patients (8.9 %) at 2–3 days after surgery and disappeared at 7–15 days. Plantar hyperhidrosis was significantly reduced in 33 of the 34 patients with this condition (remission rate 97.1 %). Axillary hyperhidrosis was significantly reduced in 19 of the 22 patients with this condition (remission rate 86.4 %). Eighteen of the 20 patients (90.0 %) with both plantar and axillary hyperhidrosis experienced significant alleviation of their symptoms. Patients were followed up for 28.5 months (range 1–62 months). All patients could work and study normally, with no recurrence of symptoms, and were satisfied with the outcome of surgery.
Discussion
Video-assisted thoracoscopic thoracic sympathicotomy was developed in the 1990s. This procedure is minimally invasive, can accurately locate the sympathetic chain, has reliable outcomes, and is considered to be the only effective method of treating palmar hyperhidrosis [9]. To achieve the expected clinical outcomes and increase our understanding of the minimal invasiveness, safety, curative effects, and cosmetic outcomes of single-port microthoracoscopic thoracic sympathicotomy, we focused on the following important aspects of the procedure.
Key Points Regarding Surgical Techniques
-
1.
Incision. The site and size of the incision are important. Surgeons often use two or three incisions so that the thoracoscope and other equipment can be introduced through separate ports, to increase the ease of surgery. However, this requires two or three 10–15-mm incisions. In the present series, a single 10-mm incision was made in the third intercostal space at the anterior axillary line and the lateral border of pectoralis major (lateral border of the breast in females) on each side. After dissection of the pleura, the sympathetic chain was dissected, hooked, and cut using a 5-mm, 30° microthoracoscope and a 3-mm microelectrocautery hook. As the procedure was performed through a single port, this required more operator experience and skill than for two- or three-port procedures.
-
2.
Anesthesia and body position. The trachea was intubated with a double-lumen tube to enable single-lung ventilation, which increased the exposure of the surgical field. The patient was placed supine with the head and the side undergoing surgery slightly elevated, to fully expose the posterior superior mediastinum.
-
3.
Identification of the sympathetic chain. The second rib is the highest rib visible during thoracoscopy because the first rib is usually covered by yellow adipose tissue. The first rib can be identified by gently touching the adipose tissue with the electrocautery hook. The sympathetic trunk appears as a white band that travels downward from the cervical pleura over the heads of the ribs. It can be identified overlying the ribs using the electrocautery hook. The sympathetic ganglion should be carefully identified and resected, taking care not to damage the sympathetic chain, and the depth of electrocautery should be carefully controlled. We found that microelectrocautery was effective for this procedure. We routinely started dissection at R4. In four patients, a large tributary of the azygos vein was closely attached to the nerve chain at R4 on the right side. To prevent damage to this vein, we dissected the sympathetic chain at R3 in these patients.
-
4.
The left sympathetic chain has a greater effect on heart function than the right sympathetic chain, and dissection of the left sympathetic chain may influence the heart rate. Surgery was therefore performed on the right side first. In addition, the heart rate, blood pressure, and blood oxygen saturation were monitored throughout surgery.
Immediate Evaluation of Curative Effects
Thoracic sympathetic ganglion excision or thoracic sympathetic chain dissection can treat palmar hyperhidrosis by interrupting the distribution of postganglionic fibers to the sweat glands of the upper limbs. It is important to perform complete sympathicotomy for successful outcomes. In the current series, palmar temperature was monitored to determine the success of sympathicotomy. A rapid increase in palmar temperature of at least 1–3 °C confirmed complete sympathicotomy. In this study, 15 patients who underwent sympathetic ganglion excision at R4 did not have an increase in palmar temperature of at least 1 °C, and these patients underwent further dissection at R3, resulting in a rapid increase in palmar temperature. This method of evaluation ensures good outcomes and prevents the need for repeat surgery. To prevent relapse, we suggest that it is better to dissect the sympathetic chain after hooking it with an electrocautery hook and that the distance between the two ends should be more than 0.5 cm. The dorsal nerve fibers and branches should also be dissected to prevent neural regeneration. We suggest performing cauterization for 2–3 cm lateral to the nerve trunk, to completely dissect the Kuntz nerve fiber bundle and communicating branches. Palmar hyperhidrosis resolved immediately in all patients, and the hands became dry and warm. The palmar temperature increased by a mean of 2.2 °C. All patients experienced complete resolution of their palmar hyperhidrosis and were satisfied with the outcomes of surgery.
Segment Selection in Thoracic Sympathicotomy and Compensatory Sweating After Surgery
Compensatory sweating is a common and uncomfortable complication after thoracic sympathicotomy. The body may sweat excessively in untreated areas, most commonly the lower back and thigh. The exact mechanism of this phenomenon is poorly understood. Hyperhidrosis is alleviated in most patients after surgery, but a gradual increase in excessive sweating can result in marked emotional distress and may cause patients to seek further treatment. The frequency and severity of compensatory sweating are positively correlated with the degree and range of sympathicotomy. Previous studies reported that a wider extent or higher level of dissection of the sympathetic nerves, i.e., a larger area of sympathicotomy, resulted in a higher incidence and increased severity of compensatory sweating [10–13]. Thoracic sympathicotomy may be performed at the second rib (R2), R3, or R4, indicating that the innervation of the palmar sweat glands remains poorly understood. Surgery for primary palmar hyperhidrosis should block the nerve conduction pathway by dissecting the sympathetic chain at a specific level. Yoon et al. [14] compared preliminary results after conventional R2 and R3 thoracoscopic sympathicotomy and found a resolution rate of 100 % in both groups. However, the rate of compensatory sweating was 16.7 % in the R3 sympathicotomy group, which was significantly lower than in the R2 plus R3 sympathicotomy group (45.8 %). Another study [15] reported that R2–R4 sympathicotomy had similar curative effects to R4 clamping, but compensatory sweating and gustatory sweating were less common in the R4 clamping group. These results indicate that R3 or R4 sympathicotomy or clamping can effectively treat palmar hyperhidrosis and that a smaller extent or lower level of dissection can significantly reduce compensatory sweating. Recent evidence [16] indicates that sympathicotomy below R3 can block the thoracic sympathetic nerve of the upper limb while retaining feedback information from the partial thoracic sympathetic nerve to the hypothalamus, effectively reducing the rate of compensatory sweating. R4 sympathicotomy has advantages over R3 sympathicotomy because it reduces the extent of sympathicotomy, thereby reducing the incidence and severity of compensatory sweating. Studies [17, 18] have shown that R3 or R4 sympathicotomy alone can effectively treat primary palmar hyperhidrosis, and R2 sympathicotomy is therefore rarely performed. R4 or R3–R4 sympathicotomy can be used to treat palmar hyperhidrosis combined with plantar hyperhidrosis, and R5 additional sympathicotomy can be used to treat palmar hyperhidrosis combined with axillary hyperhidrosis [3, 4]. In the present study, we performed R4 sympathicotomy to treat palmar hyperhidrosis, because this method can maximize the curative effect and minimize the rate of compensatory sweating. Moreover, ablation of the bypass fibers can prevent relapse. R3 sympathicotomy was additionally performed depending on the increase in palmar temperature during surgery.
In summary, we performed single-port microthoracoscopic thoracic sympathicotomy to treat primary palmar hyperhidrosis. This method can accurately locate the thoracic sympathetic chain and is safe and effective with minimal invasiveness and good cosmetic results. An experienced operator and prevention of complications are important to ensure successful treatment.
References
Strutton DR, Kowalski JW, Glaser DA, Stang PE (2004) US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol 51(2):241–248
Tu YR, Li X, Lin M, Lai FC, Li YP, Chen JF, Ye JG (2007) Epidemiological survey of primary palmar hyperhidrosis in adolescent in Fuzhou of People’s Republic of China. Eur J Cardiothorac Surg 31(4):737–739
Moreno Balsalobre R, Moreno Mata N, Ramos Izquierdo R, Aragón Valverde FJ, Molins López-Rodo L, de Andrés JJ R, García Fernández JL, Cañizares Carretero MÁ, Congregado Loscertales M, Carbajo Carbajo M (2011) SEPAR. Guidelines on surgery of the thoracic sympathetic nervous system. Arch Bronconeumol 47(2):94–102
Cerfolio RJ, De Campos JR, Bryant AS, Connery CP, Miller DL, DeCamp MM, McKenna RJ, Krasna MJ (2011) The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg 91(5):1642–1648
Lai YT, Yang LH, Chio CC, Chen HH (1997) Complications in patients with palmar hyperhidrosis treated with transthoracic endoscopic sympathicotomy. Neurosurgery 41(1):110–113
Lin JZ, Zhou Y (2007) Thoracoscopic sympathicotomy surgery. Zhonghua Wai Ke Za Zhi 45(14):941–944
Li X, Tu YR, Lin M, Lai FC, Chen JF, Miao HW (2009) Minimizing endoscopic thoracic sympathicotomy for primary palmar hyperhidrosis: guided by palmar skin temperature and laser Doppler blood flow. Ann Thorac Surg 87(2):427–431
Licht PB, Pilegaard HK (2004) Severity of compensatory sweating after thoracoscopic sympathicotomy. Ann Thorac Surg 78(2):427–431
Yanagihara TK, Ibrahimiye A, Harris C, Hirsch J, Gorenstein LA (2010) Analysis of clamping versus cutting of T3 sympathetic nerve for severe palmar hyperhidrosis. J Thorac Cardiovasc Surg 140(5):984–989
Reisfeld R (2006) Sympathicotomy for hyperhidrosis: should we place the clamps at T2-T3 or T3-T4? Clin Auton Res 16(6):384–389
Ramsaroop L, Singh B, Moodley J, Partab P, Pather N, Satyapal KS (2003) A thoracoscopic view of the nerve of Kuntz. Surg Endosc 17(9):1498
Licht PB, Pilegaard HK (2006) Gustatory side effects after thoracoscopic sympathicotomy. Ann Thorac Surg 81(3):1043–1047
Yazbek G, Wolosker N, de Campos JR, Kauffman P, Ishy A, Puech-Leão P (2005) Palmar hyperhidrosis—which is the best level of denervation using video-assisted thoracoscopic sympathicotomy: T2 or T3 ganglion? J Vasc Surg 42(2):281–285
Yoon SH, Rim DC (2003) The selective T3 sympathicotomy in patients with essential palmar hyperhidrosis. Acta Neurochir (Wien) 145(6):467–471
Neumayer C, Zacherl J, Holak G, Jakesz R, Bischof G (2003) Experience with limited endoscopic thoracic sympathetic block for hyperhidrosis and facial blushing. Clin Auton Res 13(1):I52–I57
Yang J, Tan JJ, Ye GL, Gu WQ, Wang J, Liu YG (2007) T3/T4 thoracic sympathictomy and compensatory sweating in treatment of palmar hyperhidrosis. Chin Med J (Engl) 120(18):1574–1577
Ahn SS, Wieslander CK, Ro KM (2000) Current developments in thoracoscopic sympathicotomy. Ann Vasc Surg 14(4):415–420
Doolabh N, Horswell S, Williams M, Huber L, Prince S, Meyer DM (2004) Mack MJ thoracoscopic sympathicotomy for hyperhidrosis: indications and results. Ann Thorac Surg 77(2):410–414
Conflict of Interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, H., Shu, Y., Shi, W. et al. Single-Port Microthoracoscopic Sympathicotomy for the Treatment of Primary Palmar Hyperhidrosis: an Analysis of 56 Consecutive Cases. Indian J Surg 77, 270–275 (2015). https://doi.org/10.1007/s12262-015-1288-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-015-1288-6