Summary
Despite the increasing number of patients with metastatic pancreatic adenocarcinoma (mPDAC) receiving second-line chemotherapy, little data have been reported about long-term survivors of mPDAC and prognostic factors. The study analyzes the prognostic variables of mPDAC in patients with a survival of 10 months or longer (long-term survivors). Patients registered in an institutional database receiving chemotherapy for mPDAC were selected, and two models defined the most relevant variables at baseline and after the first cycle of chemotherapy in short- and long-term survivors. A total of 110 patients were included, 69 short- and 41 long-term survivors. At baseline, long-term survivors reported significantly low rates of elevated carbohydrate antigen (CA) 19‑9 (odds ratio [OR] 0.81, confidence interval [CI] 0.67–0.97) and liver involvement (OR 0.35, CI 0.14–0.90). After the first cycle of chemotherapy, polychemotherapy regimens (OR 5.26, CI 1.57–17.67) and neutrophil reduction (OR 0.16, CI 0.03–0.80) were the only independent variables associated with a long-term survivorship. mPDAC long-term survivors rarely present elevated serum CA 19‑9 concentrations or liver metastases at baseline. Although they receive more intensive chemotherapy regimens, they more frequently experienced neutrophil reduction after the first cycle of chemotherapy regardless of the regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal types of cancer, with a 5-year survival of less than 10% [1]. A Surveillance, Epidemiology, and End Results (SEER) analysis on 57,263 patients with PDAC with a diagnosis between 1993 and 2013 documented that in 2013 only 12.7% of cases reported an overall survival (OS) longer than 12 months, while 50.6% died within 2 months from diagnosis [2].
However, median OS of patients with unresectable or metastatic pancreatic adenocarcinoma (mPDAC) has progressively increased since 2011, thanks to the introduction of new chemotherapy regimens [3, 4]. Despite the poor prognosis, the even longer OS and the request of second-line chemotherapy from more and more patients [5], it is important to revisit and standardize the prognostic factors for mPDAC [6] in order to better plan future trials and improve the management of chemotherapy in clinical practice.
To date, the number of reliable prognostic variables of mPDAC is limited, such as performance status, serum carbohydrate antigen (CA) 19‑9, and liver metastases. On the other hand, few observational studies of long-term survivors of mPDAC exist. A retrospective study of two cohorts of 47 mPDAC patients showed that in the cohort of long-term survivors a low neutrophil-to-lymphocyte ratio (NLR) was more prevalent, whereas a lymphopenia was less frequent [7].
The current study aims to retrospectively analyze, at baseline and early during chemotherapy, a set of potential prognostic variables in patients with mPDAC receiving systemic antineoplastic treatment.
Methods
Patients selection
All patients with mPDAC, registered in the database of the division of Medical Oncology of the Ospedale Civile di Sanremo, were evaluated, and data from those who received first-line cytotoxic chemotherapy between May 2010 and May 2018 were collected. Patients were included in the present analysis if they were diagnosed with unresectable locoregional or metastatic disease, signed an informed consent form, and only if the day of death was reported in the medical record.
OS was calculated from the start of first-line chemotherapy until death by any cause. The sample was divided into three tertiles based on OS: the first tertile comprised patients with a median OS of 0–5 months, the second tertile patients with an OS of 5–10 months, and the third tertile comprised patients with a good prognosis exceeding 10 months. All patients with a survival of 10 months or less were classified as short-term survivors, while the others were classified long-term survivors.
Two sets of variables were examined: those at baseline and those calculated after the first cycle of chemotherapy. The first series consisted of 23 baseline variables, which were extracted for each patient from the clinical records, such as Eastern Cooperative Oncology Group performance status (ECOG PS), age, stage, gender, body mass index (BMI), body surface area (BSA), number of comorbid conditions, tumor location, timing of metastasis, previous resection of primary tumor, baseline serum carbohydrate antigen 19‑9 (CA 19-9), baseline serum carcinoembryonic antigen (CEA), presence of liver metastasis, lung metastasis, peritoneal metastasis, neutrophil count, lymphocyte count, platelet count, hemoglobin, derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and trilinear peripheral blood cell score (TRIS). Furthermore, at the beginning of the second cycle the following four variables were measured: first-line chemotherapy regimen (mono- vs. polychemotherapy), chemotherapy-induced neutropenia after 1 month (CIN-1), neutrophil reduction rate (NR), and lymphocyte reduction rate (LR). TRIS was calculated by assigning 1‑point for neutrophil count > 7000/μl, platelet count > 400,000/μl, or hemoglobin decrease (< 11g/dl for women, < 12 for men). Patients who received granulocyte-colony stimulating factors before the second cycle were excluded. The determination of cell blood counts and oncological markers was performed by the Laboratorio Analisi department of the same institution.
Statistical analysis
A correlation analysis was performed using Pearson’s test (rho) to exclude collinearity between similar variables. In the case of a significant Pearson test, the variable with fewer missing cases was retained. Similarly, a distribution analysis of each variable was performed using the Kolmogorov–Smirnov test, and whenever a normal distribution was not confirmed, the scale of the variable was transformed.
Considering OS, patients were classified in short- vs. long-term survivors, and for every variable a bivariate logistic regression was performed to identify which prognostic factors were more frequently expressed among patients with good prognosis.
After selecting the variables that differed significantly on the univariate analysis, the first multivariate analysis of all baseline variables was performed and the second analysis included also those that differed significantly between the two subgroups after the first cycle of chemotherapy. Therefore, two final logistic multivariate regression models defined the most important differences in the distribution of the prognostic variables at baseline and after the first cycle of chemotherapy between short- and long-term survivors.
The odds ratio (OR) and 95% confidence intervals (CIs) were calculated by Cox regression, and a two-tailed P-value < 0.05 was considered statistically significant.
All statistical analyses were conducted with the statistical computing language R (version 3.6.0 for Linux, R Core Team, 2017. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/).
Results
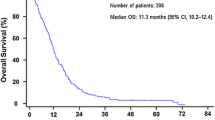
Of 169 patients with mPDAC, 110 were eligible for the present analysis. Median OS was 7.68 months, progression-free survival 3.96 months, whereas disease control rate after first-line chemotherapy was 55.2%. The median duration of chemotherapy was 10.8 weeks, 12.7 weeks for polychemotherapy and 8.7 weeks for monochemotherapy. Patient characteristics are reported in Table 1.
The collinearity of the selected variables was verified, and three variables were excluded: NLR was excluded due to the close relationship with dNLR (rho = 0.908; p-value < 0.001). Likewise, NG (Pearson rho = 0.884; p-value < 0.001) and dNLR R10 (Pearson rho = −0.650; p-value < 0.001) were ruled out because of the close correlation with NR. After the normality test, three variables resulted in a nonnormal distribution, so that a logarithmic transformation was applied (absolute neutrophil count, lymphocyte count, platelet count).
Subsequent analyses were then performed with the 27 remaining variables. The results of the bivariate analyses between the baseline variables and OS as well as the early postchemotherapy variables are compiled in Table 2. After bivariate analysis a significant relationship was reported for the ECOG PS, the baseline CA 19‑9, the occurrence of liver metastases, the baseline hemoglobin, and TRIS. In addition, after the first cycle of chemotherapy, the chemotherapy regimen and the NR appeared to be associated with OS.
Finally, two multivariate regression models were built with baseline variables and with those available after the first cycle of chemotherapy (Table 3). At baseline, long-term survivors had significantly low rates of elevated CA 19‑9 (OR 0.81, CI 0.67–0.97) and liver involvement (OR 0.35, CI 0.14–0.90). After the first cycle, polychemotherapy (OR 5.26, CI 1.57–17.67) and NR (OR 0.16, CI 0.03–0.80) were more frequent among long-term survivors.
Discussion
The current study documents the expression of different prognostic factors in patients with mPDAC surviving more than 10 months, such as a greater likelihood to receive a polychemotherapy regimen, fewer liver metastases, lower serum CA 19‑9 concentrations, and more frequent neutrophil reduction after chemotherapy. Contrary to previous series, the systemic inflammatory response (SIR)-related variables for dNLR, PLR and absolute neutrophil, lymphocyte and platelet counts in the present study were not different between short- and long-term survivors (Table 2). The only exception was TRIS, since the score was significantly lower among long-term survivors (odds ratio [OR] 0.47, confidence interval [CI] 0.24–0.93).
These differences with respect to the few papers published so far could be related to the arbitrary definition of longer survivorship. In fact, the various authors used different OS cut-offs, ranging from 12 months [2] to 18 months [7], up to 24 months [8]. Even the reasons for the long survival are not always clear in the individual case, although OS is likely to be associated with the biology of the disease, as the median OS is longer among patients with mPDAC who are carriers of germline mutations of various predisposing genes such as those of mismatch repair and BRCA. However, given the 10-month cut-off in our series, the different prognosis of long-term survivors may be partly due to the response to chemotherapy even more than in the previous studies [7, 8].
The SIR indicators correlate inversely with the host’s immune system ability to respond to cancer and antineoplastic treatments [9]. A low degree of SIR activation, mostly expressed as low NLR, was correlated with OS independently of other prognostic variables in a large randomized study of two first-line chemotherapy regimens, although this can be explained by the intensity of the chemotherapy regimen [10]. Studies with long-term survivors also documented that NLR was significantly lower than in patients with poor outcome [7, 8]. In our experience, however, the most important SIR-related host variable in prognostic terms is NR, which appears more important than the baseline variables. Unfortunately, to date the role of early neutrophil reduction is little studied [11]; therefore, an evaluation of this variable in prospective studies is strongly recommended. The importance of evaluating this variable as a prognostic factor, but also as an end point of first-line treatment, is also reinforced by the controversial role of the overall response rate according to Response Evaluation Criteria in Solid Tumors (RECIST), given the complexity and low reliability of primary tumor measurements by imaging, due to its inflammatory and desmoplastic component [12].
In our experience, only two baseline variables appear independently unbalanced between short- and long-term survivors: high serum CA 19‑9 concentrations and liver metastases. Both are more frequent among short-term survivors. Baseline CA 19‑9 is a well-known prognostic factor and predictor of response to first-line chemotherapy [13]. As previously reported, it appears to be lower at baseline more often in long-term survivors [8].
In our series, the occurrence of liver metastases is associated with a worse prognosis (Table 3). Few studies have evaluated the impact of metastasis sites on the prognosis of mPDAC [14]. Although various series have reported a good prognosis for isolated lung metastases [15, 16], several trials and a meta-analysis documented that the presence of liver metastases correlates with a poor prognosis, while multiple sites of metastasis were not related with OS different than that of patients with liver-only dissemination [17]. Over 13,000 patients with mPDAC were evaluated in the SEER survey, and patients with isolated hepatic metastases had a poor outcome, in contrast to those with an isolated lung or nodal metastasis, who had longer survival [18]. The study of long-term survivors of mPDAC also suggested a lower frequency of liver metastases in these patients [8] and a negative trend of OS for peritoneal disease [7].
In addition, long-term survivors in our experience more often have a good performance status and higher hemoglobin values, without any difference in age at diagnosis, which is only slightly lower (65 vs 67 years) [8].
Finally, it must be emphasized that there are numerous limits of the study. First, it is retrospective. Moreover, the sample was separated on the basis of OS, with all possible biases related to the role of traditional prognostic variables. This is demonstrated by the performance status and the chemotherapy regimen, which are not balanced in the two subgroups (Table 1). This imbalance may also partly explain the higher prevalence of neutrophil depletion after the first cycle among long-term survivors. However, it should be reiterated that NR remains the only independent prognostic variable together with the chemotherapy intensity (poly- vs. monochemotherapy), a finding that rather suggests an independent role of NR from the therapy regimen. However, it is also necessary to keep in mind that the intent of the present study is purely descriptive of the subgroup of patients with mPDAC and OS longer than 10 months, and that the study sample is homogeneous regarding the fact that all patients had received first-line chemotherapy.
Take home message
mPDAC long-term survivors more often receive intensive chemotherapy regimens and do not present elevated CA 19‑9 or liver metastases, whereas early neutrophil reduction is more frequent.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Golan T, Sella T, Margalit O, Amit U, Halpern N, Shacham-Shmueli E, et al. Short- and long-term survival in metastatic pancreatic adenocarcinoma, 1993–2013. J Natl Compr Cancer Netw. 2017;15(8):1022–7.
Conroy T, Desseigne F, Ychou M, Bouchè O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Wang-Gillam A, Li C‑P, Bodoky G, Dean A, Shan Y‑S, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–57.
ter Veer E, van Rijssen LB, Besselink MG, Mali RMA, Berlin JD, Boeck S, et al. Consensus statement on mandatory measurements in pancreatic cancer trials (COMM-PACT) for systemic treatment of unresectable disease. Lancet Oncol. 2018;9(3):e151–e60.
Rochefort P, Lardy-Cleaud A, Sarabi M, Desseigne F, Cattey-Javouhey A, de la Fouchardiere C. Long-term survivors in metastatic pancreatic ductal adenocarcinoma: a retrospective and matched pair analysis. Oncologist. 2019;4:1543–8.
Kowalczyk M, Mandelson MT, Lin BS‑L, Picozzi VJ. Metastatic pancreatic cancer (MPC): contrast of short-(STS) and long-term(LTS) survivor characteristics. J Clin Oncol. 2018;36(suppl 4S):abstract 415.
Gao Y, Wang WJ, Zhi Q, Shen M, Jiang M, Bian X, et al. Neutrophil/lymphocyte ratio is a more sensitive systemic inflammatory response biomarker than platelet/lymphocyte ratio in the prognosis evaluation of unresectable pancreatic cancer. Oncotarget. 2017;8:88835–44.
Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, et al. nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107(2):dju413.
Colloca GA, Venturino A, Guarneri D. Neutrophil count kinetics during the first cycle of chemotherapy predicts the outcome of patients with locally advanced or metastatic pancreatic cancer. Asia Pac J Clin Oncol. 2020;16(4):247–53.
van der Sijde F, Vietsch EE, Mustafa DAM, Besselinj MG, Koerkamp BG, van Eijck CHJ. Circulating biomarkers for prediction of objective response to chemotherapy in pancreatic cancer patients. Cancers. 2019;11:93.
Yoo T, Lee WJ, Woo SM, Kim TH, Han SS, Park SJ, et al. Pretreatment carbohydrate antigen 19‑9 level indicates tumor response, early distant metastasis, overall survival, and therapeutic selection in localized and unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:e623–e30.
Cannistrà M, Ruggiero M, Zullo A, Serafini S, Grande R, Nardo B. Metastases of pancreatic adenocarcinoma: a systematic review of literature and a new functional concept. Int J Surg. 2015;21(1):S15–S21.
Zheng B, Ohuchida K, Yan Z, Okumura T, Ohtsuka T, Nakamura M. Primary recurrence in the lung is related to favorable prognosis in patients with pancreatic cancer and postoperative recurrence. World J Surg. 2017;41:2858–66.
Wangjam T, Zhang Z, Zhou XC, Lyer L, Faisal F, Soares KC, et al. Resected pancreatic ductal adenocarcinomas with recurrence limited in lung have a significantly better prognosis than those with other recurrence patterns. Oncotarget. 2015;6(34):36903–10.
Jr Uson PLS, D’Avila Sampaio Tolentino F, Montes Santos V, Rother ET, Maluf FC. The impact of metastatic sites in advanced pancreatic adenocarcinoma, systematic review and meta-analysis of prospective randomized studies. PLoS ONE. 2020; https://doi.org/10.1371/journal.pone.0230060.
Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: a Surveillance Epidemiology and End Results database analysis. World J Gastroenterol. 2017;23(10):1872–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G.A. Colloca and A. Venturino declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional research committee of Regione Liguria and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data availability
Data will be shared upon reasonable request to the corresponding author.
Rights and permissions
About this article
Cite this article
Colloca, G.A., Venturino, A. Prognostic factors of long-term survivors with unresectable pancreatic cancer: a retrospective analysis. memo 17, 146–151 (2024). https://doi.org/10.1007/s12254-023-00917-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-023-00917-x