Abstract
We evaluated the clinical and prognostic value of the protein expression of caveolin-1 (CAV1) and p16 at the primary site and metastatic lymph nodes of oral squamous cell carcinoma (OSCC). Primary site specimens from 80 OSCC cases were randomly selected and lymph node specimens from 15 preserved metastatic lymph nodes from among those patients were selected for examination. We evaluated the CAV1 and p16 expression at both the primary site and metastatic lymph nodes, and analyzed the patients’ clinicopathological data in relation to CAV1 and p16 expression. Our analysis revealed significant positive correlations between CAV1 expression at the primary site and pathological metastasis, cell differentiation, and mode of invasion (p = 0.019, p = 0.002, p = 0.015, respectively), but p16 expression was not associated with any clinicopathological factors. Patients with high CAV1 expression at the primary sites showed significantly worse prognoses than those with low or negative CAV1 expression (p = 0.002), and multivariate analysis showed that the T classification and CAV1 expression were independent OSCC prognostic factors. CAV1 expression was also present in the metastatic lymph nodes of the OSCC cases with particularly poor differentiation and high invasive grade, and patients with CAV1-positive metastatic lymph nodes showed significantly worse prognoses than those with CAV1-negative metastatic lymph nodes (p = 0.018). CAV1 may activate metastaticity and the invasive capacity of OSCC cells. CAV1 expression, particularly at metastatic lymph nodes, predicts a worse outcome for OSCC, suggesting that CAV1 could be used as a prognostic marker for OSCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide each year, 270,000 people develop oral cancer and 128,000 of these cases are fatal [1]. Roughly 90% of oral cancer cases are oral squamous cell carcinoma (OSCC). Although the diagnosis of OSCC and the treatments for this cancer have greatly improved, the prognosis has not changed significantly and remains especially poor for advanced-stage OSCC [2]. Improving the prognosis of OSCC is thus the focus of several lines of research.
One of the lines of research concerns the presence of lymph node metastasis, which is an important prognostic factor for OSCC; moreover, in head-and-neck squamous cell carcinoma (HNSCC), lymph node metastasis is the most adverse prognostic factor [3]. The degree of tumor invasiveness is another important prognostic factor for OSCC, as is the tumor’s mode of invasion [4]. The identification of clinicopathological and/or immunohistochemical parameters of lymph node metastasis and tumor invasiveness might thus help determine the malignancy of OSCC and predict its outcomes [5, 6].
Some studies have indicated that the protein caveolin-1 (CAV1) might serve as a prognostic biomarker in OSCC, although the clinical findings regarding CAV1 expression in OSCC are inconsistent. CAV1 is a 22-kDa, 178-amino-acid integral membrane protein that is a major structural protein in caveolae, the 50- to 100-nm protein-coated invaginations of the plasma membrane involved in endocytosis and signal transduction. CAV1, which has been shown to be overexpressed in type I pneumocytes, fibroblasts, endothelial cells, and adipocytes, is one of the adhesion factors that participates in signal transmission through the integrins (which are cell adhesion factors) and various cytokine receptors. Several studies have reported that CAV1 expression is correlated with the prognosis and clinical characteristics of ovarian carcinoma, esophageal squamous cell carcinoma, and prostate cancer [7,8,9]. However, these studies examined only the expression of CAV1 at the primary site; there has been no report investigating the differences in CAV1 expression between the primary site and the metastatic lymph nodes.
Human papillomavirus (HPV) has previously been reported to be involved in oropharyngeal cancer [10,11,12]. p16 is attracting attention as a surrogate marker for HPV infection in oropharyngeal carcinoma, and immunostaining of p16 is used as a diagnostic tool for HPV infection. Oropharyngeal cancer that is positive for p16 is highly radiosensitive and has a good prognosis [13]. However, in OSCC, the relationship between p16 expression and clinicopathological factors and the prognostic significance of p16 expression have not been clearly described.
Here we sought to determine the correlation between the clinicopathological characteristics and CAV1 or p16 expression at both the primary site and metastatic lesions in OSCC, and to clarify the prognostic value of CAV1 or p16 expression for OSCC.
Materials and Methods
Patients and Specimens
We retrospectively analyzed 80 consecutive patients with primary OSCC who had undergone surgical resection at Kanazawa University Hospital’s Department of Oral and Maxillofacial Surgery. The 42 males and 38 females ranged in age from 29 to 91 years (mean 63.4 years). Thirty-one of these cases showed clinical metastasis to the cervical lymph nodes: 18 of the 31 showed pathological metastasis, with 15 having preserved specimens. The Union for International Cancer Control (UICC) system (ver. 7) was used for the TNM classification [14]. The World Health Organization (WHO) criteria were used to determine the grade of tumor differentiation. The Yamamoto et al. [15] classification was used to assess the mode of tumor invasion.
Immunohistochemistry
Eighty primary-site specimens from 80 patients whose primary tumors were resected and 15 preserved lymph node specimens from the subset of these patients with pathologically confirmed metastatic lymph nodes were stained immunohistochemically. The tissue specimens had been fixed in 10% neutral buffered formalin and embedded in paraffin. We examined 4-μm-thick sections from each specimen. We performed the immunohistochemical detection of CAV1 with the use of an anti-caveolin-1 rabbit polyclonal antibody (Cell Signaling Technology, Tokyo) and immunohistochemical detection of p16 with the use of an anti-CDKN2A/p16INK4a antibody (Abcam, Cambridge, MA). We deparaffinized the paraffin-embedded sections, rehydrated them, and subjected them to heat-treatment by microwaving them for 15 min in 10-mM citrate buffer for antigen retrieval. The sections were then left to cool to room temperature.
Endogenous peroxidase was blocked by treatment with 0.3% hydrogen peroxide in methanol for 30 min. Ten-minute blocking with non-specific goat serum was performed next, followed by overnight incubation with primary antibodies at 4 °C. We used the EnVision™ Horseradish Peroxidase (HRP) system (Dako, Kyoto, Japan) to detect the immunoreactive protein. We used diaminobenzidine tetrahydrochloride to visualize the CAV1 expression, and counterstained the specimens with hematoxylin. Non-immune serum instead of the primary antibody was used to treat the negative controls.
Evaluation of Staining
We used light microscopy (100× magnification) to examine the expressions of CAV1 and p16 at the invasive front of each tumor. The expression of CAV1 and p16 were examined for three fields of the invasive front per specimen by microscopy at 100x magnification. The percentage of positive cells was calculated by counting the positive cells in a sampling of 500 cancer cells in each field.
Negative CAV1 expression (CAV1(−)) was defined as CAV1 immunostaining in <5% of cells. The threshold for positive cases was set at 70%, close to the median (70.2%); cases with <70% positive cells were considered to have low CAV1 expression (CAV1(L)), and those with ≥70% positive cells were considered to have high CAV1 expression (CAV1(H)).
In the evaluation of p16 expression, the staining intensity (none 0; weak 1+; moderate 2+; strong 3+) and percentage of stained tumor cells were evaluated. Tumors were considered p16-positive if the nuclear and cytoplasmic staining intensity was moderate (2+) in ≥80% of the tumor cells or the intensity was strong (3+), regardless of the number of stained cells [16].
The expressions of CAV1 and p16 were evaluated and judged by two reviewers (K.K. and H.K.) who were blinded to all details of the tumors. We then analyzed the expression of CAV1 and p16 in each case in relation to the existence and degree of pathological metastasis, the degree of cell differentiation, the mode of tumor invasion, and the following clinical parameters: age, gender, T classification (tumor size), N classification (clinically judged cervical lymph node metastasis) and clinical stage.
Statistical Analysis
For all of the data analyses, we used the JMP 13 software program (SAS, Cary, NC, USA). The Χ2 test was used to determine the relationships between CAV1 or p16 expression and each of the clinicopathological parameters. We used the Kaplan–Meier method to calculate the 5-year survival rates and further evaluated these rates by the log-rank test. When a parameter showed a significant difference, we used Cox’s multivariate proportional hazard model to investigate the parameter’s prognostic values. Probability values <0.05 were accepted as significant.
Results
Staining Patterns of CAV1 or p16 and Correlation with Clinicopathological Parameters
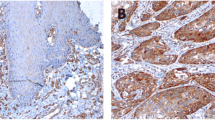
Our immunohistochemical analysis revealed CAV1 expression in the cytoplasm of tumor cells at the primary OSCC sites (Fig. 1). Both adipocytes and stromal cells also showed CAV1 expression. Of the 80 cases, 29 (36.3%) exhibited positive immunostaining for CAV1 in tumor cells, with 11 of these being classified as CAV1(L) and the other 18 as CAV1(H). The correlations between each of the clinicopathological parameters and the expression pattern of CAV1 are provided in Table 1. None of the clinical parameters of patients—i.e., age, gender, T classification, N classification or clinical stage—was correlated with CAV1 expression. However, each of the pathological parameters—i.e., pathological metastasis, cell differentiation and the mode of tumor invasion— was significantly correlated with CAV1 expression (p = 0.019, p = 0.002, p = 0.015, respectively).
Immunohistochemical staining for CAV1 and p16 at the primary site (a: CAV1-high expression (CAV1(H)); b: CAV1-low expression (CAV1(L)); c: CAV1-negative (CAV1(−)); d: p16-negative; e: p16-positive). CAV1 and p16 immunoreactivities are present in the cytoplasm of cancer cells at the invasive front of OSCC (original magnification ×100)
p16 was detected in the nuclei or in both the nuclei and cytoplasm of tumor cells and normal epithelium; none of the cells showed a cytoplasmic signal alone (Fig. 1). Among the 80 cases, 15 cases (18.5%) were p16 positive. The correlations between each of the clinicopathological parameters and the expression pattern of p16 are provided in Table 1. None of the clinicopathological parameters of patients was correlated with p16 expression.
Prognostic Value of CAV1 or p16 Expression at the Primary Site
The median post-surgery follow-up period of the OSCC patients was 37.5 months. The correlation between overall survival and CAV1 or p16 expression at the primary site was tested by the Kaplan–Meier method. As shown in Fig. 2, the 5-year cumulative survival rate of CAV1(−) cases was 72.3% and that of CAV1(L) cases was 72.7%, whereas the 5-year cumulative survival rate of CAV1(H) cases was significantly worse at 37.8% (p = 0.002). In the analysis of the relation between p16 expression and the 5-year cumulative survival rate, no statistically significant difference was observed between the positive cases (46.2%) and the negative cases (67.9%).
To examine the usefulness of the clinicopathological parameters as prognostic factors, we used a Cox proportional hazards model. CAV1 expression, patient age, T classification, N classification and clinical stage were shown by a univariate analysis to be significant prognostic parameters, but only CAV1 expression and T classification were identified by the multivariate analysis as independent prognostic factors (Table 2).
Pathological Characteristics and Prognostic Potential of CAV1 or p16 Expression at the Metastatic Lymph Nodes
The cytoplasm of tumor cells at metastatic lymph nodes also showed CAV1 expression and all CAV1-positive cases had a positive cell rate of 70% or more (Fig. 3a). The expression of p16 was observed in the cytoplasm of tumor cells (Fig. 3b). As shown in Table 3, the results confirmed that CAV1 expression was associated with poor differentiation and high invasive grade. When the relationship between the expression pattern of CAV1 at the primary site and the expression of CAV1 at the metastatic site was examined, a correlation was found, and all cases with CAV1(H) expression at the primary site showed CAV1-positivity at the metastatic lymph nodes (p = 0.002). On the other hand, p16 expression was not associated with either cell differentiation or the mode of invasion (Table 4). All three cases with p16 expression in metastatic lymph nodes also exhibited p16 expression at the primary site. As shown in Fig. 4, the 5-year survival rates were 51.4% for the CAV-1-negative versus 0.0% for the CAV1-positive cases, with the latter having significantly worse prognosis (p = 0.018). However, there was no correlation between p16 expression and life prognosis (positive: 33.3%; negative: 19.4%).
Discussion
Lipid rafts in the plasma membrane play important roles in signaling and transporting substances across the cell membrane. The lipid rafts also carry various protein mediators. Caveolae (Latin for “little caves”) constitute a special kind of lipid raft. The CAV1 protein is a major structural component of the plasma membrane, and an essential structural protein of caveolae. Glenney et al. [17] first identified CAV1 in 1989 as a major v-Src substrate, and Rothberg et al. [18] were the first to clone CAV1 in 1992. Several studies have demonstrated that CAV1 acts as a scaffolding protein by interacting with proteins and kinases such as the HARS protein, Src-family tyrosine kinase, epidermal growth factor (EGF) receptor, and the transforming growth factor beta (TGFβ)/SMAD pathway [19,20,21,22,23,24]. In addition, the phosphorylation of CAV1 on Tyr14 has been suggested to be involved in cell polarization, migration, and focal adhesion [24, 25].
CAV1 appears to function as a tumor suppressor in the early phases of carcinogenesis but as a tumor accelerator in advanced or metastatic tumors [26]. The picture is further complicated by contradictory findings in different cancers. CAV1 has been shown to be downregulated in ovarian cancer [7], whereas CAV1 overexpression was found to be associated with both esophageal squamous cell carcinoma and prostate cancer [8, 9]. CAV1 thus appears to have different functions depending on the types of malignant tumors and/or progression pattern. The precise roles of CAV1 in the development and progression of OSCC and other malignant lesions remain to be identified. Some studies of SCC have reported that there is no significant correlation between CAV1 expression and clinicopathologic parameters [27, 28]; however, we observed that CAV1 expression was significantly correlated with the presence of pathological metastasis, cell differentiation, and mode of invasion at the primary site. In lung SCC, overexpression of CAV1 may be correlated with pathologic T-stage, invasion into surrounding tissues and metastasis [29, 30]. Ando et al. reported that CAV1 overexpression was correlated with tumor progression in patients with esophageal SCC [31]. Our present findings correspond to these studies’ results in suggesting that CAV1 might act as a promoter of tumor progression in OSCC.
So far, CAV1 expression has been studied primarily in relation to invasion. In in vitro research in esophageal SCC cell lines, CAV1 was shown to regulate the migration and invasive and metastatic abilities of cancer cells [32]. A previous study showed that elevated CAV1 enhanced invasion in endometrial cancer [33]. In breast cancer, CAV1 was shown to play an important role in invadopodia formation and extracellular matrix degradation [34, 35]. Taken together, these results suggest that CAV1 affects the invasion of cancer cells. In light of this idea, we used immunohistochemistry to examine the association of CAV1 expression with the invasion of OSCC and confirmed that CAV1 was overexpressed at the invasive front of the primary tumor and that the highly invasive type of OSCC showed higher levels of the CAV1 protein compared to the less invasive type. It can be inferred from the results of this analysis that CAV1 expression promotes invasion in OSCC.
The relationship between prognosis and CAV1 expression was also examined, with the result that the CAV1-positive OSCC patients had significantly worse prognoses than the CAV1-negative patients. Furthermore, in multivariate analysis, CAV1 expression and T classification were revealed to be independent prognostic factors. Auzair et al. [36] reported that high CAV1 expression predicted poor prognosis in their patients with OSCC. In esophageal squamous cell carcinoma, overexpression of CAV1 was correlated with lymph node metastasis, pathological stage, and the patients’ overall survival [8]. Moreover, CAV1 was found to be expressed in cancer stem cells, and high CAV1 expression was found to contribute to therapy resistance, resulting in a poor clinical outcome [37, 38]. Together, these results suggest that CAV1 is a useful prognostic marker in OSCC and that it may play an important role in the progression and treatment-resistance of OSCC.
Regarding metastatic lymph nodes, we observed herein that all of the 8 cases that expressed CAV1 in metastatic lymph nodes expressed CAV1 at the primary site. CAV1 expression in metastatic lymph nodes was also associated with poor differentiation and a high invasive grade. In addition, among the 15 metastatic OSCC cases analyzed here, the patients with CAV1-positive lymph nodes had significantly worse prognoses that those with CAV1-negative lymph nodes. Moreover, the patients with CAV1-positive staining in metastatic lymph nodes had worse prognoses than those with CAV1-positive staining at the primary site. Burgermeister et al. found that gastric cancer cell lines derived from metastatic sites showed higher levels of CAV1 mRNA and protein expression compared to the cell lines derived from primary sites [19]. In gastric cancer, high cytoplasmic CAV-1 expression in metastatic lymph nodes was associated with an unfavorable prognosis [39]. Williams et al. speculated that CAV1 overexpression renders tumor cells biologically aggressive by inducing the expression of proteins related to tumor invasion and metastasis [40]. Taken together, these reports and our present findings suggest that CAV1 expression at metastatic lesions may promote the further aggressiveness and expansion of cancer cells.
The human papillomavirus (HPV) is a high-risk factor for oropharyngeal cancer [41]. It has been reported that the attenuation or deletion of p16 expression is associated with prognosis in head and neck squamous cell carcinomas [42]. Although the relation between p16 expression and OSCC has been examined in several reports, these studies consictently found that p16 expression dose not affect the prognosis of OSCC [43,44,45,46]. In this research, p16 positive patients accounted for as little as 16.3% of the total cases, and there was no association with clinicopathological factors. Moreover, p16 expression in metastatic lymph nodes was not correlated with pathological factors or prognosis. Therefore, it was suggested that p16 expression has little clinical significance in OSCC.
Many viruses enter cells through endocytosis, hijacking the cellular machinery for entry, and invasion of the host cell [47]. The majority of viruses have been demonstrated to use clathrin-mediated endocytosis for entry, whereas a few have been shown to enter through caveolae. A previous study showed that high-risk HPV type 31 (HPV31) enters its natural host cell type via caveola-dependent endocytosis [48]. After initial plasma membrane binding, HPV31 associates with caveolin-1 and transiently localizes to the caveosome before trafficking to the early endosome and proceeding through the endosomal pathway [49]. These results suggested that caveolin-1 plays an important role in HPV infection. Accordingly, we investigated the correlation between the expressions of caveolin-1 and p16, but we found no correlation between the two.
In conclusion, our present study is the first to demonstrate that the overexpression of CAV1 in metastatic lymph nodes is an independent prognostic marker for OSCC. Patients with CAV1 expression in metastatic lymph nodes are unlikely to respond to conventional chemoradiotherapy and are likely to have poor prognosis. In such cases, treatment with molecular-targeted therapy or immunotherapy should be considered as a first-line approach. Further elucidation of the role of CAV1 in OSCC may lead to new therapeutic modalities.
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Vokes EE, Weichselbaum RR, Lippman SM, Hong WK (1993) Head and neck cancer. N Engl J Med 328:184–194
Forastiere A, Koch W, Trotti A, Sidransky D (2001) Head and neck cancer. N Engl J Med 345:1890–1900
Yamamoto E, Miyakawa A, Kohama G (1984) Mode of invasion and lymph node metastasis in squamous cell carcinoma of oral cavity. Head Neck 6:939–947
Järvinen AK, Autio R, Kilpinen S, Saarela M, Leivo I, Grénman R, Mäkitie AA, Monni O (2008) High-resolution copy number and gene expression microarray analyses of head and neck squamous cell carcinoma cell lines of tongue and larynx. Genes Chromosom Cancer 47:500–509
Nakayama S, Sasaki A, Mese H, Alcalde RE, Matsumura T (1998-1999) Establishment of high and low metastasis cell lines derived from a human tongue squamous cell carcinoma. Invasion Metastasis 18:219–228
Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, Arlt K, Zhumabayeva B, Siebert PD, Dietel M, Schäfer R, Sers C (2001) Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol 159:1635–1643
Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H (2002) Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer 94:929–933
Karam JA, Lotan Y, Roehrborn CG, Ashfaq R, Karakiewicz PI, Shariat SF (2007) Caveolin-1 overexpression is associated with aggressive prostate cancer recurrence. Prostate 67:614–622
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35
Allen CT, Lewis JS Jr, El-Mofty SK, Haughey BH, Nussenbaum B (2010) Human papillomavirus and oropharynx cancer: biology, detection and clinical implications. Laryngoscope 120:1756–1772
Marur S, D'Souza G, Westra WH, Forastiere AA (2010) HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11:781–789
Wang MB, Liu IY, Gornbein JA, Nguyen CT (2015) HPV-positive Oropharyngeal carcinoma: a systematic review of treatment and prognosis. Otolaryngol Head Neck Surg 153:758–769
In (2009) International Union against Cancer, LH Sobin, MK Gospodrowicz, CH Wittekind (eds.). TNM Classification of Malignant Tumours. 7th Ed. Wiley-Liss, New York, NY
Yamamoto E, Kohama G, Sunagawa H, Iwai M, Hiratsuka H (1983) Mode of invasion, bleomycin sensitivity, and clinical course in squamous cell carcinoma of the oral cavity. Cancer 51:2175–2180
Heiduschka G, Grah A, Oberndorfer F, Kadletz L, Altorjai G, Kornek G, Wrba F, Thurnher D, Selzer E (2015) Improved survival in HPV/p16-positive oropharyngeal cancer patients treated with postoperative radiotherapy. Strahlenther Onkol 191:209–216
Glenney JR Jr, Zokas L (1989) Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol 108:2401–2408
Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG (1992) Caveolin, a protein component of caveolae membrane coats. Cell 68:673–682
Lu Z, Ghosh S, Wang Z, Hunter T (2003) Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 4:499–515
Burgermeister E, Xing X, Röcken C, Juhasz M, Chen J, Hiber M, Mair K, Shatz M, Liscovitch M, Schmid RM, Ebert MP (2007) Differential expression and function of caveolin-1 in human gastric cancer progression. Cancer Res 67:8519–8526
Li S, Couet J, Lisanti MP (1996) Src tyrosine kinases, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem 271:29182–29190
Couet J, Sargiacomo M, Lisanti MP (1997) Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem 272:30429–30438
Razani B, Zhang XL, Bitzer M, von Gersdorff G, Böttinger EP, Lisanti MP (2001) Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem 276:6727–6738
Torres VA, Tapia JC, Rodríguez DA, Párraga M, Lisboa P, Montoya M, Leyton L, Quest AF (2006) Caveolin-1 controls cell proliferation and cell death by suppressing expression of the inhibitor of apoptosis protein survivin. J Cell Sci 119(Pt 9):1812–1823
Goetz JG, Lajoie P, Wiseman SM, Nabi IR (2008) Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev 27:715–735
Senetta R, Stella G, Pozzi E, Sturli N, Massi D, Cassoni P (2013) Caveolin-1 as a promoter of tumour spreading: when, how, where and why. J Cell Mol Med 17:325–336
Fu P, Chen F, Pan Q, Zhao X, Zhao C, Cho WC, Chen H (2017) The different functions and clinical significances of caveolin-1 in human adenocarcinoma and squamous cell carcinoma. Onco Targets Ther 10:819–835
Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM (2002) Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am J Pathol 161:1647–1656
Chen HL, Fan LF, Gao J, Ouyang JP, Zhang YX (2011) Differential expression and function of the caveolin-1 gene in non-small cell lung carcinoma. Oncol Rep 25:359–366
Cassoni P, Daniele L, Maldi E, Righi L, Tavaglione V, Novello S, Volante M, Scagliotti GV, Papotti M (2009) Caveolin-1 expression in lung carcinoma varies according to tumour histotype and is acquired de novo in brain metastases. Histopathology 55:20–27
Ando T, Ishiguro H, Kimura M, Mitsui A, Mori Y, Sugito N, Tomoda K, Mori R, Harada K, Katada T, Ogawa R, Fujii Y, Kuwabara Y (2007) The overexpression of caveolin-1 and caveolin-2 correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinoma. Oncol Rep 18:601–609
Liu Z, Yu J, Wu R, Tang S, Cai X, Guo G, Chen S (2017) Rho/ROCK pathway regulates migration and invasion of esophageal squamous cell carcinoma by regulating Caveolin-1. Med Sci Monit 23:6174–6185
Diaz-Valdivia N, Bravo D, Huerta H, Henriquez S, Gabler F, Vega M, Romero C, Calderon C, Owen GI, Leyton L, Quest AF (2015) Enhanced caveolin-1 expression increases migration, anchorage-independent growth and invasion of endometrial adenocarcinoma cells. BMC Cancer 15:463
Yamaguchi H, Takeo Y, Yoshida S, Kouchi Z, Nakamura Y, Fukami K (2009) Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res 69:8594–8602
Wang R, Li Z, Guo H, Shi W, Xin Y, Chang W, Huang T (2014) Caveolin 1 knockdown inhibits the proliferation, migration and invasion of human breast cancer BT474 cells. Mol Med Rep 9:1723–1728
Auzair LB, Vincent-Chong VK, Ghani WM, Kallarakkal TG, Ramanathan A, Lee CE, Rahman ZA, Ismail SM, Abraham MT, Zain RB (2016) Caveolin 1 (Cav-1) and actin-related protein 2/3 complex, subunit 1B (ARPC1B) expressions as prognostic indicators for oral squamous cell carcinoma (OSCC). Eur Arch Otorhinolaryngol 273:1885–1893
Ketteler J, Klein D (2018) Caveolin-1, cancer and therapy resistance. Int J Cancer 143:2092–2104
Wang Z, Wang N, Li W, Liu P, Chen Q, Situ H, Zhong S, Guo L, Lin Y, Shen J, Chen J (2014) Caveolin-1 mediates chemoresistance in breast cancer stem cells via β-catenin/ABCG2 signaling pathway. Carcinogenesis 35:2346–2356
Sun S, Hong SA, Won HS, Yoo SH, Lee HH, Kim O, Ko YH (2017) Prognostic value of metastatic Tumoral Caveolin-1 expression in patients with resected gastric Cancer. Gastroenterol Res Pract 2017:5905173
Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP (2004) Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem 279:51630–51646
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35
Namazie A, Alavi S, Olopade OI, Pauletti G, Aghamohammadi N, Aghamohammadi M, Gornbein JA, Calcaterra TC, Slamon DJ, Wang MB, Srivatsan ES (2002) Cyclin D1 amplification and p16(MTS1/CDK4I)deletion correlate with poor prognosis in head and neck tumors. Laryngoscope 112:472–481
Zafereo ME, Xu L, Dahlstrom KR, Viamonte CA, El-Naggar AK, Wei Q, Li G, Sturgis EM (2016) Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol 56:47–53
Kouketsu A, Sato I, Abe S, Oikawa M, Shimizu Y, Takahashi T, Kumamoto H (2016) Detection of human papillomavirus infection in oral squamous cell carcinoma: a cohort study of Japanese patients. J Oral Pathol Med 45:565–572
Götz C, Drecoll E, Straub M, Bissinger O, Wolff KD, Kolk A (2016) Impact of HPV infection on oral squamous cell carcinoma. Oncotarget 7:76704–76712
Nopmaneepaisarn T, Tangjaturonrasme N, Rawangban W, Vinayanuwattikun C, Keelawat S, Bychkov A (2019) Low prevalence of p16-positive HPV-related head-neck cancers in Thailand: tertiary referral center experience. BMC Cancer 19:1050
Sieczkarski SB, Whittaker GR (2005) Viral entry. Curr Top Microbiol Immunol 285:1–23
Smith JL, Campos SK, Ozbun MA (2007) Human papillomavirus type 31 uses a caveolin 1- and dynamin 2-mediated entry pathway for infection of human keratinocytes. J Virol 81:9922–9931
Smith JL, Campos SK, Wandinger-Ness A, Ozbun MA (2008) Caveolin-1-dependent infectious entry of human papillomavirus type 31 in human keratinocytes proceeds to the endosomal pathway for pH-dependent uncoating. J Virol 82:9505–9512
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kato, K., Miyazawa, H., Kobayashi, H. et al. Caveolin-1 Expression at Metastatic Lymph Nodes Predicts Unfavorable Outcome in Patients with Oral Squamous Cell Carcinoma. Pathol. Oncol. Res. 26, 2105–2113 (2020). https://doi.org/10.1007/s12253-019-00791-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00791-1