Abstract
Atorvastatin is the most prescribed cholesterol-lowering statin, while caffeine enhances chemo-sensitivity and induces apoptosis of tumor cells through its DNA repair-inhibiting effect. The present study investigated the effects and mechanisms of atorvastatin and caffeine in combination on human prostate cancer cells cultured in vitro. Cell growth were determined by the trypan blue exclusion assay. The cell apoptosis and colony formation were determined by morphological assessment. The ability of cell migration and invasion were performed using a scratch wound-healing and Transwell assay. Tumorspheres were formed in suspension under the condition of non-adherence and serum-free medium. Finally, the western blot assay was used to determine the levels of proteins. The combination synergistically suppressed proliferation and induced apoptotic death. Meanwhile, the migration, invasion, and the formation of tumorspheres were significantly inhibited by the combination. We found that atorvastatin and caffeine in combination downregulated phospho-Akt, phospho-Erk1/2, anti-apoptotic Bcl-2 and Survivin protein levels. Results of the present study indicate treatment with the combination of caffeine and atorvastatin may be an effective strategy for inhibiting the growth of prostate cancer and should be evaluated clinically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It was estimated that there would be approximately 180,890 diagnosed new cases and 8.3% mortality due to PCa in 2016 in the United States [1, 2]. Androgen deprivation therapy (ADT) is an effective treatment for advanced prostate cancer. Unfortunately, the therapeutic responses to this treatment are temporary, and almost all tumors inevitably progress to castration-resistant prostate cancer (CRPC) [3]. Docetaxel is the standard first-line treatment for CRPC [4]. Although docetaxel chemotherapy has been shown to improve patient survival, resistance and toxicity are still two unsolved and inevitable problems for its utilization as a chemotherapeutic agent [5]. Furthermore, PCa is believed to consist of about 0.1% fraction of cancer stem cells (CSC), which, currently available therapeutic treatment, including hormonal treatment and chemo-radiotherapy, cannot effectively kill [6]. Finally, PCa subsequently develops the ability to grow uncontrollably, relapse and then metastasize. Therefore, the strategy of utilizing a combination of drugs that interact with one another has been demonstrated to solve these problems, leading to increased survival and an improved quality of life [7].
Atorvastatin (Lipitor®) is a statin drug that inhibits the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase. Statins were found to be associated with a decreased risk of recurrence and mortality in PCa [8,9,10,11,12,13]. Recent studies have indicated that statin drugs, including atorvastatin, suppress cell viability and induce cell apoptosis of PCa cells [14, 15]. The results of our previous studies suggest that atorvastatin potentially enhances the antitumor effect of anti-cancer drugs in PCa cells. Atorvastatin in combination with celecoxib or docetaxel have synergistically inhibited cellular proliferation and induced apoptosis. Furthermore, a xenograft PCa model has demonstrated that atorvastatin/celecoxib combination inhibited both the development of androgen-dependent prostate tumors and the growth of androgen-independent prostate tumors more effectively than either agent alone [16,17,18].
Caffeine has been indicated to enhance the antitumor effect of chemotherapy drugs through its DNA repair-inhibiting effect [19, 20]. Additionally, it has been indicated that caffeine directly inhibits proliferation and induces cell apoptosis of tumor cells [21, 22]. Moreover, in our previous studies, we found that oral caffeine and voluntary exercise suppressed progression of androgen-dependent prostate tumors with xenograft cancer models [23]. At present, there are several reports containing promising data utilizing combination anti-cancer drugs with caffeine for certain malignancies, such as pancreatic cancer, stomach cancer, liver cancer and lung cancer. However, there are few reports that show the utility of this combination for PCa. Therefore, to develop combination anti-cancer drugs with caffeine for PCa, we examined whether caffeine potentiates the antitumor effect of atorvastatin in PCa cell lines.
Based on our previous data for caffeine or atorvastatin in PCa, the present study focused on the effects of caffeine/atorvastatin combination treatment on PCa cells. PC-3 cells were exposed to caffeine and atorvastatin alone or in combination, and the cell proliferation, apoptosis, migration, invasion and ability to form tumorspheres were exhibited. Finally, we investigated the underlying mechanisms of the combination.
Materials and Methods
Cells and Reagents
The PC-3 human prostate carcinoma cell line (ATCC, Rockville, MD, USA) were grown in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin in an incubator (37 °C, 5% CO2). Atorvastatin (St. Louis, MO, USA) was dissolved in DMSO and the final concentration of DMSO in all experiments was 0.1%, while caffeine (St. Louis, MO, USA) was dissolved in sterile water.
Experiment Design
Atorvastatin (μM) | Caffeine (mM) | The combination | |
|---|---|---|---|
Trypan blue | 5 | 0.5 | the combination of Atorvastatin (5 μM) and Caffeine (0.5 mM) |
Colony forming assay | 5 | 0.5 | the combination of Atorvastatin (5 μM) and Caffeine (0.5 mM) |
Apoptosis | 5 | 0.5 | the combination of Atorvastatin (5 μM) and Caffeine (0.5 mM) |
Cell migration | 5 | 0.5 | the combination of Atorvastatin (5 μM) and Caffeine (0.5 mM) |
Invasion | 5 | 0.5 | the combination of Atorvastatin (5 μM) and Caffeine (0.5 mM) |
Tumorsphere | 5 | 0.5 | the combination of Atorvastatin (5 μM) and Caffeine (0.5 mM) |
Western blot | 5 | 0.5 | the combination of Atorvastatin (5 μM) and Caffeine (0.5 mM) |
Cell Viability Assay
The cell proliferation activity of PC-3 cells was estimated by Trypan blue exclusion assay as described [17]. Cells (5 × 105 cells/well) were exposed to the test drugs in 6-well plates after 24 h. After 72 h, the number of viable cells was counted via hemocytometer using a light microscope (Nikon Optiphot, Nikon, Tokyo, Japan) with 0.4% trypan blue solution (1:4).
Colony Formation Assay
Cells (100 cells/well) were treated with solvent, 5 μM atorvastatin, 0.5 mM caffeine and a combination of 0.5 mM caffeine and 5 μM atorvastatin in 24-well plates. After 12 days, the colonies (>50 cells) were counted under microscope, which were fixed with methanol and stained with giemsa stain solution as described [24].
Determination of Apoptosis
To evaluate the apoptotic ability of PC-3 cells, morphological assessment was determined with propidium iodide (PI, 1 μg/ml) staining of cells treated with solvent, 5 μM atorvastatin, 0.5 mM caffeine and a combination of 0.5 mM caffeine and 5 μM atorvastatin and analyzed using a fluorescence microscope (Nikon Eclipse TE200, Nikon) as described [26].
Wound-Healing Migration Assay
The ability of cell migration was performed using a scratch wound-healing assay as described previously [25]. Monolayer of PC-3 cells with an artificial wound was treated with 0.5 mM caffeine or 5 μM atorvastatin alone and in combination. Images were obtained at 0, 24 and 48 h through a microscope. Scratch areas were measured by Image J and expressed as a ratio of the original scratch area.
Transwell Migration and Invasion Assay
Millicell chambers (8 mm pore size) in 24-well plate were used for migration assays. After treatment with 0.5 mM caffeine or 5 μM atorvastatin alone and in combination for 48 h, cells underwent a transwell migration assay and were photographed and quantified. Cells were treated with atorvastatin (5 μM) and caffeine (0.5 mM) alone or in combination for 24 h and allowed to invade through Matrigel and invaded cell number was counted. The analyses protocols were described previously [26].
Tumorsphere Culture
Tumorspheres were formed in suspension under the condition of non-adherence and serum-free medium. PC-3 cells (2 × 103 cells/well) were incubated in 24-well plates coated by 1% agarose with keratinocyte serum-free medium. After treatment with atorvastatin (5 μM) and caffeine (0.5 mM) alone or in combination for 14 days, tumorspheres were gathered and imaged to measure the number and size by a BZ-X710 microscope. The analyses protocols were described previously [25].
Western Blotting
Western blot analyses were performed as described previously [25]. Briefly, proteins were extracted from cells, and their concentrations were determined with the Bio-Rad protein assay kit. Samples (30 μg protein) were subjected to SDS-PAGE electrophoresis at 150 V for 60 min on 12% gradient gels. Resolved proteins were then transferred to a PVDF membrane (Bio-Rad), which were incubated with primary antibodies(1:1000), followed by incubated with Donkey anti-rabbit IRDye-R- 680CW (1:1000) for 90 min at room temperature in the dark. Images were acquired using an Odyssey CLx Infrared Imaging System (LI-COR Biosciences) and density of bands were measured by Image Studio Lite software.
Statistical Analyses
Statistical analysis was performed by the software InStat (GraphPad Software, Inc., La Jolla, CA, USA). Data are expressed as the mean ± standard deviation (SD) and were performed, at minimum, in triplicate. Statistical significance was estimated by One-way ANOVA and P-values were labeled as follows: * P < 0.05; ** P < 0.01; *** P < 0.001. IC50 values were evaluated by nonlinear least squares regression. To analyze the potential synergistic effect, the combination index (CI) was determined using the following equation Ac /Ae + Bc/Be = CI. Ac and Bc represent the concentration of drug A and drug B used in combination, while Ae and Be represent the concentration that produced the same magnitude of effect when administered alone. When CI is less than 1, the combined effect of the drugs is considered to be synergistic.
Results
Cytotoxic Effect of Caffeine and Atorvastatin Alone on PC-3 Cells
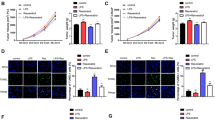
Treatment of PC-3 cells for 72 h with various concentrations of caffeine or atorvastatin inhibited cell growth in a concentration-dependent manner (Fig. 1a). We calculated the mean IC50 values of caffeine and atorvastatin to be 1.71 mM and 18.04 μM, respectively.
Effect of caffeine and atorvastatin on prostate cancer cell viability. (a) Proliferation of PC-3 cells treated with atorvastatin (5–40 μM), caffeine (0.5–5 mM) (B) or caffeine (0.5 mM) + atorvastatin (5 μM) for 72 h. (b) The effects of atorvastatin (5 μM) and caffeine (0.5 mM) alone or in combination on colony formation in PC-3 cells for 12 days. Each value represents mean ± S.E. from three separate experiments. Statistical analysis was performed using one-way ANOVA (* p < 0.05 **p < 0.01, ***p < 0.001)
Effect of Caffeine/Atorvastatin Combination on Anti-Proliferation and Inhibition of Colony Formation in PC-3 Cells
We found that treatment with the drug combination resulted in a stronger growth inhibition when compared to either agent alone (Fig. 1a). In addition, the combination of 0.75 mM caffeine with 5 μM atorvastatin resulted in a 50% decrease in the number of viable cells. Therefore, the combination index (CI) for IC50 was calculated to be 0.72, indicating that the combination of caffeine and atorvastatin synergistically inhibits the growth of PC-3 cells.
To further investigate whether the combination treatment could suppress viability of PC-3 cells, we conducted a clonogenic assay after treatment. As shown in Fig. 1b, the clonogenic assay clearly indicates that clone formation of PC-3 cells was reduced with combinational treatment. These results were consistent with the Trypan blue data. Taken together, those results implied that the combination of caffeine and atorvastatin had a strong cytostatic effect on PC-3 cells.
The Apoptosis Effect was Enhanced by Combining Caffeine with Atorvastatin
Representative micrographs of apoptotic cells are shown in Fig. 2a-d. As shown in Fig. 2e, the combination resulted in 27.2% apoptotic cells, significantly higher than the treatment groups with caffeine or atorvastatin alone (p < 0.001).
Effect of caffeine and atorvastatin on apoptosis induction in prostate cancer cells. Representative micrographs of propidium iodide staining of cells treated with solvent (a), 5 μM atorvastatin (b), 0.5 mM caffeine (c) and a combination of 0.5 mM caffeine and 5 μM atorvastatin (d). (e) Percentage of apoptotic cells (mean ± SD) from three separate experiments. Statistical analysis was performed using one-way ANOVA (***p < 0.001)
The Combination Treatment Impaired Migration and Invasion of PCa Cells
As shown in Fig. 3a, combination treatment for 24 and 48 h resulted in markedly inhibited migration. Additionally, similar results were obtained in the transwell migration assay (Fig. 3b). To investigate the effect of combination treatment on PC-3 cell invasion ability, we conducted a Matrigel invasion assay. Fig. 3c illustrates that the treatment of PC-3 cells with the drug combination impaired the invasive capacity significantly when compared to the drugs alone. These studies demonstrate that caffeine/atorvastatin combination treatment resulted in a strong effect on the migration and invasion abilities of PC-3 cells, implying that the combination treatment might reduce the threat of PCa metastasis, leading to the reduction of mortality rate.
Inhibition of caffeine and atorvastatin on PC-3 cell migration and invasion. (a)The cells were treated with 0.5 mM caffeine or 5 μM atorvastatin alone and in combination. Migration of the cells was determined and expressed as percentages of the original scratch area for 24 and 48 h. (b) After treatment with 0.5 mM caffeine or 5 μM atorvastatin alone and in combination for 48 h, cells underwent atranswell migration assay and were photographed and quantified. (c)Cells were treated with atorvastatin and caffeine alone or in combination for 24 h and allowed to invade through Matrigel. Invaded cell number was counted. Each value represents mean ± S.D. from three separate experiments. Statistical analysis was performed using one-way ANOVA (**p < 0.01;***p < 0.001)
The Capacity of Tumor Sphere Formation in PC-3 Cells Was Diminished by Treatment with both Caffeine and Atorvastatin
After 14 days, spheres were observed under a microscope and photographed (Fig. 4a-d). As Fig. 4e illustrates, the treatment of the combinationmarkedly suppressed the forming efficiency of tumorspheres, while PC-3 sphere formation significantly increased with the exposure of caffeine- treated groups (0.5 mM) when compared to the vehicle control. Fig. 4f indicates that the combination also inhibited the number and size of tumorspheres formed. Taken together, these results imply that PCa tumor sphere formation efficiency and growth were suppressed by combination treatment The combination resulted in the formation of significantly fewer tumorspheres, as well as a dramatic decrease in size.
Effects of caffeine and atorvastatin on the tumor sphere culture in PC-3 cells. The cells were incubated in the presence of caffeine (0.5 mM), atorvastatin (5 μM) or caffeine (0.5 mM) + atorvastatin (5 μM). At the end of the 14-day incubation, images were taken (a-d) and the number (e) and size (f) of the tumorspheres were quantified. Each value represents mean ± S.D. from three separate experiments. Statistical analysis was performed using one-way ANOVA (**p < 0.01, ***p < 0.001)
Caffeine/Atorvastatin Combination Downregulated Anti-Apoptotic Protein Expression and Suppressed Akt and Erk Signaling in PC-3 Cells
Figure 5 shows that both Survivin and Blc-2 expression were dramatically diminished following 24 h exposure to combination treatment when compared to atorvastatin alone (P < 0.001) and were slightly decreased with respect to the caffeine-treated group (Survivin, P < 0.05; Bcl-2, P < 0.01).
Effects of caffeine and atorvastatin on anti-apoptotic protein expression and Akt and Erk Signaling in PC-3 cells. The cells were treated with atorvastatin (5 μM) and caffeine (0.5 mM) alone and in combination for 24 h. Protein levels of Survivin, Bcl-2, phospho-Akt and phospho-Erk1/2 were determined by Western blotting, and the levels of these proteins were analyzed by optical density measurement and normalized toβ-actin. Each value represents mean ± S.D. from three separate experiments. Statistical analysis was performed using one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001)
The serine-threonine kinase, Akt, which is activated downstream of phosphatidylinositol-3-kinase (PI3K), controls cell growth and survival via its key role in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription and cell migration. Hence, it is imperative to evaluate whether the combination could modulate Akt signaling. As Fig. 5 indicates, the levels of phospho-Akt in PC-3 cells treated with the combination were much lower than with alone (P < 0.001), while the total level of the receptor was similar.
Extracellular signal-regulated protein kinases 1 and 2 (Erk 1/2) are members of the mitogen-activated protein kinase super family that mediate cell proliferation and apoptosis. We found that the combination treatment caused a dramatic decrease in the level of both phospho-Erk1 and phospho-Erk2 (P < 0.01, P < 0.001), and had no effect on the level of total Erk1/2 (Fig. 5).
Discussion
Nowadays, ADT is still the primary treatment of this disease. When patients develop PCa with castration resistance, chemotherapy is commonly used as treatment. However, the side effects are awful and most tumors become resistant to the chemotherapy drug. Furthermore, hormonal treatment and chemo-radiotherapy, cannot effectively kill the CSCs of PCa. Therefore, the search of effective clinical approaches for the treatment of this cancer is still a challenge.
In our present study, we demonstrated that the inhibitory effect of caffeine and atorvastatin in combination on the growth of cultured PC-3 cells was much stronger than that of either agent alone. We found that the combination had a synergistic effect on anti-proliferation and induction of apoptosis in PC-3 cells. In addition, the combination dramatically impaired cell migration and invasion. Moreover, the formation of tumorspheres in non-adherent serum-free cultures was significantly inhibited by the combination of caffeine and atorvastatin, suggesting a treatment effect on the cancer stem cells, which was demonstrated for the first time. Statins were shown to inhibit NF-κB, an important transcription factor for regulation of cell growth and death [16, 27,28,29,30]. The target of the statins, HMG-CoA reductase, is involved in the generation of farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP) [31]. FPP and GGPP are required for the process of Ras activation, which is important for regulating cell growth and apoptosis [32]. Our present study showed that atorvastatin inhibited NF-κB activation and reduced the levels of phospho-Akt and phospho-Erk, the downstream targets of the Ras pathway [33]. The oral bioavailability of atorvastatin has been reported 12% due to low aqueous solubility (0.1 mg mL−1), crystalline nature, and hepatic first pass metabolism. Atorvastatin also had side effects including myositis and myasthenia. Using a lower dose of atorvastatin in combination with other drugs my decreased the side effects.
Several mechanisms by which caffeine enhances chemo-sensitivity and inhibits tumor progression have been elucidated; Caffeine is an inhibitor of ataxia telangiectasia mutated (ATM) and ATM-RAD3-related (ATR) kinase activities, which are master regulators of DNA damage-induced cell cycle checkpoints [33]. Caffeine has also been shown to regulate the apoptosis, survival, proliferation and migration of tumor cells, including p53, Bax, Akt/mTOR/S6K, NF-κB pathways, MAPK and Caspase 3 Pathways [34, 35].
Molecular mechanisms for the combined effect of statins and metformin in prostate cancer cells are still largely unknown. We found the drug combination strongly downregulated anti-apoptotic protein expression, such as Survivin and Bcl-2, and suppressed Akt and Erk signaling in PC-3 cells. Our results suggest the combination of atorvastatin and caffeine targets multiple signaling pathways, regulating prostate cancer cell growth and survival. Simultaneous inhibition of these important pathways may result in strong growth inhibition and apoptosis in prostate cancer cells.
Conclusion
In conclusion, our study demonstrates that treatment with the combination of caffeine and atorvastatin had strong inhibitory effects on the growth of human prostate cancer cells, as well as a strong stimulatory effect on apoptosis. The potent combined effects of these two drugs were associated with suppression of migration, invasion and tumor spheres, decreases in the levels of Survivin, Bcl-2, phosphorylated Akt and phosphorylated Erk1/2.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics. CA Cancer J Clin 66(1):7–30
Yap TA, Zivi A, Omlin A, De Bono JS (2011) The changing therapeutic landscape of castration-resistant prostate cancer. Nat Rev Clin Oncol 8(10):597–610
Francini E, Sweeney CJ (2016) Docetaxel activity in the era of life-prolonging hormonal therapies for metastatic castration-resistant prostate Cancer. Eur Urol 70:410–412
Amable L (2016) Cisplatin resistance and opportunities for precision medicine. Pharmacol Res 106:27–36
Zhang L, Li L, Jiao M, Wu D, Wu K, Li X, Zhu G, Yang L, Wang X, Wang X, Hsieh J, He D (2012) Genistein inhibits the stemness properties of prostate cancer cells through targeting hedgehog–Gli1 pathway. Cancer Lett 323(1):48–57
Peng M, Darko KO, Tao T, Huang Y, Su Q, He C, Yin T, Liu Z, Yang X (2017) Combination of metformin with chemotherapeutic drugs via different molecular mechanisms. Cancer Treat Rev 54:24–33. https://doi.org/10.1016/j.ctrv.2017.01.005
Bansal D, Undela K, D'Cruz S, Schifano F (2012) Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS One 7(10):e46691
Flick ED, Habel LA, Chan KA, Van Den Eeden SK, Quinn VP, Haque R, Orav EJ, Seeger JD, Sadler MC, Quesenberry Jr CP, Sternfeld B, Jacobsen SJ, Whitmer RA, Caan BJ (2007) Statin use and risk of prostate cancer in the California Men's health study cohort. Cancer Epidemiol Prev Biomarkers 16(11):2218–2225
Lustman A, Nakar S, Cohen AD, Vinker S (2014) Statin use and incident prostate cancer risk: does the statin brand matter? A population-based cohort study. Prostate Cancer Prostatic Dis 17:6–9
Boudreau DM, Yu O, Buist DS, Miglioretti DL (2008) Statin use and prostate cancer risk in a large population-based setting. Cancer Causes Control 19:767–774
Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, Amling CL, Freedland SJ (2014) Postoperative statin use and risk of biochemical recurrence following radical prostatectomy: results from the shared equal access regional Cancer hospital (SEARCH) database. BJU Int 114:661–666
Yu O, Eberg M, Benayoun S, Aprikian A, Batist G, Suissa S, Azoulay L (2013) Use of statins and the risk of death in patients with prostate cancer. J Clin Oncol 32(1):5–11
Wang C, Tao W, Wang Y, Bikow J, Lu B, Keating A, Verma S, Parker TG, Han R, Wen XY (2010) Rosuvastatin, identified from a zebrafish chemical genetic screen for antiangiogenic compounds, suppresses the growth of prostate cancer. Eur Urol 58(3):418–426
Xie F, Liu J, Li C, Zhao Y (2016) Simvastatin blocks TGF-β1-induced epithelial-mesenchymal transition in human prostate cancer cells. Oncol Lett 11(5):3377–3383
Chen X, Liu Y, Wu J, Huang H, Du Z, Zhang K, Zhou D, Hung K, Goodin S, Zheng X (2016) Mechanistic study of inhibitory effects of atorvastatin and docetaxel in combination on prostate Cancer. Cancer Genomics Proteomics 13:151–160
Zheng X, Cui XX, Gao Z, Zhao Y, Liu Y, Shih WJ, Huang MT, Liu Y, Rabson A, Reddy B, Yang CS, Conney AH (2010) Atorvastatin and celecoxib in combination inhibits the progression of androgen-dependent LNCaP xenograft prostate tumors to androgen independence. Cancer Prev Res 3:114–124
Zheng X, Cui XX, Avila GE, Huang MT, Liu Y, Patel J, Kong AN, Paulino R, Shih WJ, Lin Y, Rabson AB, Reddy BS, Conney AH (2007) Atorvastatin and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice. Clin Cancer Res 13:5480–5487
Sabisz M, Skladanowski A (2008) Modulation of cellular response to anticancer treatment by caffeine: inhibition of cell cycle checkpoints, DNA repair and more. Curr Pharm Biotechnol 9(4):325–336
Tomita K, Tsuchiya H, Sasaki T (1989) DNA repair and drug resistance: enhancement of the effects of anticancer agents by DNA repair inhibitors. Gan To Kagaku Ryoho Cancer Chemother 16(3Pt2):576–584
Li A, Wu N, Zou H, Zhu B, Xiong S, Xiao G (2016) Low concentration of caffeine inhibits cell viability, migration and invasion, and induces cell apoptosis of B16F10 melanoma cells. Int J Clin Exp Pathol 9(11):11206–11213
Okano J, Nagahara T, Matsumoto K, Murawaki Y (2008) Caffeine inhibits the proliferation of liver cancer cells and activates the MEK/ERK/EGFR signalling pathway. Basic Clin Pharmacol Toxicol 102(6):543–551
Zheng X, Cui X, Huang M, Liu Y, Wagner GC, Lin Y, Shih WJ, Lee M, Yang CS, Conney AH (2012) Inhibition of progression of androgen-dependent prostate LNCaP tumors to androgen independence in SCID mice by oral caffeine and voluntary exercise. Nutr Cancer 64(7):1029–1037
Chen Z, Chen S, Lu G, Chen X (2012) Phosphorus limitation for the colony formation, growth and photosynthesis of an edible cyanobacterium, Nostoc sphaeroides. Biotechnol Lett 34(1):137–143
Wang ZS, Huang HR, Zhang LY, Kim S, He Y, Li DL, Farischon C, Zhang K, Zheng X, Du ZY, Goodin S (2017) Mechanistic Study of Inhibitory Effects of Metformin and Atorvastatin in Combination on Prostate Cancer Cells in Vitro and in Vivo. Biol Pharm Bull 40(8):1247
Yu L, Wu X, Chen M, Huang H, He Y, Wang H, Li D, Du Z, Zhang K, Goodin S, Zheng X (2017) The effects and mechanism of YK-4-279 in combination with docetaxel on prostate Cancer. Int J Med Sci 14(4):356–366
Farnier M, Davignon J (1998) Current and future treatment of hyperlipidemia: the role of statins. Am J Cardiol 82:3J–10J
Trialists CT (2005) Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90056 participants in 14 randomised trials of statins. Lancet 366:1267–1278
McFarlane SI, Muniyappa R, Francisco R, Sowers JR (2002) Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab 87:1451–1458
Kim JH, Cox ME, Wasan KM (2014) Effect of simvastatin on castration-resistant prostate cancer cells. Lipids Health Dis 13:56
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343:425–430
Pruitt K, Der CJ (2001) Ras and rho regulation of the cell cycle and oncogenesis. Cancer Lett 171:1–10
Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT (1999) Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res 59(17):4375–4382
Miwa S, Sugimoto N, Yamamoto N, Shirai T, Nishida H, Hayashi K, Kimura H, Takeuchi A, Igarashi K, Yachie A, Tsuchiya H (2012) Caffeine induces apoptosis of osteosarcoma cells by inhibiting AKT/mTOR/S6K, NF-κB and MAPK pathways. Anticancer Res 32(9):3643–3649
He Z, Ma WY, Hashimoto T, Bode AM, Yang CS, Dong Z (2003) Induction of apoptosis by caffeine is mediated by the p53, Bax, and caspase 3 pathways. Cancer Res 63(15):4396–4401
Acknowledgements
Dr. Xi Zheng is the Unilever Chair in Nutrition and Disease Prevention Research. The authors dedicate this paper to Dr. Allan H. Conney, an outstanding and widely recognized cancer researcher who passed away on September 10, 2013.
Funding
The present study was supported by the Guangdong Province Leadership Grant, China National Science Foundation Grants (grant no. 81272452 and 21272043), and the Rutgers Cancer Institute of New Jersey (CCSG P30-CA072720).
Author information
Authors and Affiliations
Contributions
Performed the experiments: ZSW, LYZ, ZW, XFW. Analyzed the data: HPX, YH, HRH. Contributed reagents/materials/analysis tools: KZ, YL.
Wrote the paper: SG, ZYD, XZ.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wang, Z., Zhang, L., Wan, Z. et al. Atorvastatin and Caffeine in Combination Regulates Apoptosis, Migration, Invasion and Tumorspheres of Prostate Cancer Cells. Pathol. Oncol. Res. 26, 209–216 (2020). https://doi.org/10.1007/s12253-018-0415-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-0415-7