Abstract
The percentage of spherical colonies from the trichomes of Nostoc sphaeroides reached 62–73% after 16 days with 50 and 250 μM P, but only10–15% at 0.5 and 5 μM P. During colony formation from microcolonies to macrocolonies, the growth rates were 95, 206 and 244% higher, respectively at 5, 50 and 250 μM P than that at 0.5 μM P. The light-saturated photosynthetic rate, maximum electron transport rate and light-limited photosynthetic efficiency at 0.5 μM P decreased, respectively, by 45, 51 and 32% than those at 250 μM P. These indicated that the colony development, growth and photosynthetic capacities were restricted at low P level, suggesting that P might be an important factor limiting the productivity and distribution of N. sphaeroides in the field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrient limitation to primary productivity is widespread in freshwater and marine ecosystems (Hecky and Kilham 1988). Phosphorus (P) is usually thought to be the major limiting factor for the growth of phytoplankton in aquatic ecosystems and the content of available Pi rarely exceeds 10 μM in natural aquatic environments (Bieleski 1973).
The edible cyanobacterium, Nostoc sphaeroides, which is named as Ge-Xian-Mi in Chinese, has been used as a food delicacy or ingredient in Chinese medicine since the Eastern Jin Dynasty (317–420 a.d.) recorded in The Supplement to Compendium of Materia Medica. It mainly grows in paddy fields around the town of Zouma of Hefeng county in Hubei province, China. During the winter forms microscopically visible spherical and sub-spherical colonies (Qiu et al. 2002). A local survey by Qiu et al. (2002) found that in the paddy fields where N. sphaeroides colonies present, the P content of those irrigated waters was about 3–7 times more than that in the fields with no growth of N. sphaeroides colonies. Another local field survey found that the P content of soil samples (from 3214 ± 535 to 5860 ± 622 μg P g DW−1) from 5 to 15 cm depth in paddy fields with high yield of N. sphaeroides colonies was 2–3 times more than that (less than 1884 ± 75 μg P g DW−1) with no growth of the colonies. In addition, the N. sphaeroides colonies were usually grown around the rice roots where enriched in soil organic matter and microbes, which can greatly enhance the effectiveness of soil P (He et al. 2005). These investigations suggested that there might be an association between the distribution and productivity of N. sphaeroides colonies and the P content in the local paddy fields.
In this study, an extensive investigation about the effects of P concentration on colony development, growth and photosynthesis of N. sphaeroides colonies was carried out in the laboratory. The results demonstrated that the formation of spherical colonies of N. sphaeroides from the trichomes was strictly controlled by P concentrations. During the colony formation from microcolonies to macrocolonies, the growth rates and photosynthetic capacities were limited by low P levels.
Materials and methods
Organism and culture conditions
Nostoc sphaeroides Kützing was obtained from the freshwater Algae culture collection of the Institute of Hydrobiology, the Chinese Academy of Sciences, and it was originally collected from Hefeng County in Hubei Province, China.
Trichomes of N. sphaeroides were obtained from dispersed colonies using a sterilized glass homogenizer according to the method of Deng et al. (2008a). Before preparation of free trichomes, the colonies were firstly incubated in free-P BG110 for 2 days in order to deplete cellular P content of the colonies. Free trichomes were resuspended in 600 ml fresh BG-110 medium containing different P concentrations (0.5, 5, 50 and 250 μM KH2PO4) and incubated at 25°C and 50 μmol photons m−2 s−1 with a 10:14 h light/dark cycle for 16 days. The growth medium was aerated with filtered ambient air at about 300 ml min−1. During 16 days of cultivation, the numbers of trichomes, filaments, asteriated colonies and spherical colonies were respectively counted microscopically by using a haemocytometer every 2 days. The total numbers of N. sphaeroides at four development stages are 350 ± 15. The spherical colony percentage is the ratio of the numbers of spherical colonies to the total numbers of N. sphaeroides.
Microcolonies, diameter of 0.1–0.2 mm, were prepared according to Deng et al. (2008a) and used for the growth and physiology experiments as the following: the microcolonies were also firstly incubated in free-P BG110 for 2 days, and then exposed to various P concentrations under the conditions described as above for 12 days.
Parameters determination
Absolute growth rate (AGR) was calculated according to the following equation: AGR = (Wt2 − Wt1)/T, where Wt1 and Wt2 is the fresh weight at the start and the end of a growth period respectively, and T is the time in days between weighing. Chlorophyll a (chl a) concentration was determined spectrophotometrically in 100% methanol extracts (Porra et al. 1989), and the total P content of the samples was determined according to People’s Republic of China Standard GB12388-12399-90 (1990) in China.
Chlorophyll fluorescence was measured by a modulated chlorophyll fluorometer (FMS2; Hansatech Ltd, King’s Lynn, UK). Parameters for the rapid light response curves of electron transport rate were analyzed according to Platt et al. (1980):
where PAR is the photosynthetically active radiation (μmol m−2 s−1), ETRmax is the asymptotic maximum electron transport rate (μmol electrons m−2 s−1), α1 is the initial slope of light-limited ETR (μmol e− per μmol PAR photons) and is calculated to assess the photosynthetic efficiency.
The photosynthetic O2 evolution of the colonies was measured with a Clark-type oxygen electrode (Chlorolab 2, Hansatech) at 25°C. The samples harvested were resuspended in the fresh BG110 medium with corresponding P concentration buffered with 25 mM bis–tris propane (BTP) at pH 8.0. During the irradiance-dependent photosynthetic measurement, NaHCO3 was set at 2 mM to avoid carbon limitation. Photosynthetic responses to dissolved inorganic carbon (DIC) concentration were measured at 400 μmol m−2 s−1. DIC-free reaction solution (25 mM BTP, pH 8.0) was prepared according to Qiu and Liu (2004).
Parameters for the photosynthetic response to irradiance were analyzed according to Henley (1993):
where I represents irradiance; P, photosynthetic activity at certain irradiance; P max, light-saturated photosynthesis; α, the ascending slope at limiting irradiances, was calculated to assess the photosynthetic efficiency; R d, the dark respiration.
Parameters for the photosynthetic response to DIC were obtained by fitting net photosynthetic rates at various levels of DIC with the Michaelis–Menten formula:
where K 1/2(DIC) is the DIC concentration required to give half maximal photosynthetic rate and Vmax is the DIC-saturated photosynthesis.
Statistical analysis
Statistical calculations are performed using SPSS. One-way analysis of variance (ANOVA) is used to determine the effect of treatments and Tukey honestly significant difference (HSD) test is conducted to test the statistical significance of the differences between means of various treatments.
Results
Figure 1 shows that the trichomes firstly developed a sheath and became a filament after 2 days of cultivation (Fig. 1b). When cell division started within the sheath, aggregated cell mass was formed and expanded into asteriated colonies (Fig. 1c) and finally into spherical colonies (Fig. 1d). The spherical colonies began to occur after 6 days (Fig. 1e). But the spherical colony percentage increased rapidly at high P levels of 50 and 250 μM which reached 62–73% after 16 days, while at low P levels of 0.5 and 5 μM, it increased slowly and only reached to 10–15% (Fig. 1e).
Effects of phosphorus concentrations on the spherical microcolony formation from trichomes of N. sphaeroides. During 16 day of cultivation, the number of trichomes (a), filaments (b), aseriated colonies (c) and spherical colonies (d) were respectively counted microscopically by using a haemocytometer every 2 days. The spherical colony percentage (e) is the ratio of the number of spherical colonies to the total counted number (350 ± 15) of N. sphaeroides. Squares, circles, upward triangles and downward triangles are 0.5, 5, 50 and 250 μM P, respectively
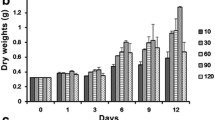
Figure 2 shows that during 12 days’ incubation in the medium with various P concentrations, the colonies displayed a relatively stable growth rate, but the rates were significantly different under different P concentrations (Fig. 2a). The AGR based on fresh weight increased by 95, 206 and 244% respectively at 5, 50 and 250 μM P than that at 0.5 μM P (Turkey HSD, P < 0.05) (Fig. 2b). Figure 3 shows that the Chl a contents of the colonies were significantly higher by 20, 79 and 108%, respectively at 5, 50 and 250 μM P than that at 0.5 μM P (Fig. 3a). There was a concomitant increase in intracellular P content with the rise of P concentration in the external medium, the intracellular P contents of the colonies were significantly higher by 23, 37 and 110% at 5, 50 and 250 μM P than that at 0.5 μM P (Fig. 3b).
Effects of phosphorus concentrations on the growth curves (a) and absolute growth rates (b) of N. sphaeroides colonies after 12 days exposure to different phosphorus concentrations. Squares, circles, upward triangles and downward triangles are 0.5, 5, 50 and 250 μM P, respectively. Data are means ± SD (n = 4)
Figure 4 shows the rapid light response of electron transport rates (ETR) in N. sphaeroides colonies under various P concentrations. The maximum electron transport rate (ETRmax) declined with the decreasing P concentrations, which decreased by 51, 37 and 10% at 0.5, 5 and 50 μM P than that at 250 μM P. Figure 5 shows the photosynthetic responses of various P concentrations to irradiance for N. sphaeroides colonies. When exposed to different P concentrations for 12 days, the light-saturated photosynthetic rates (Pmax) of the colonies decreased by 14, 35 and 45%, and its light-limited photosynthetic efficiencies (α) declined by 32, 20 and 13%, respectively at 0.5, 5 and 50 μM P compared to those at 250 μM P. The photosynthetic responses of various P concentrations to DIC for N. sphaeroides colonies were shown in Fig. 6. The DIC-saturated photosynthetic rates (V max) of the colonies decreased by 48, 31 and 19%, respectively at 0.5, 5 and 50 μM P than those at 250 μM P. With the decrease of P concentration, the K1/2(DIC) tended to decline, and the values decreased by 50 and 17% at 0.5 and 5 μM P than that at 250 μM P.
The rapid light response curves of electron transport rate (a) and their parameters, ETRmax (b), of N. sphaeroides colonies after 12 days exposure to different phosphorus concentrations. Squares, circles, upward triangles and downward triangles are 0.5, 5, 50 and 250 μM P, respectively. Data are means ± SD (n = 4–5)
The photosynthetic response to irradiance (P–I) curves (a) and their parameters, Pmax (b) and α (c), of N. sphaeroides colonies after 12 days exposure to different phosphorus concentrations. Squares, circles, upward triangles and downward triangles are 0.5, 5, 50 and 250 μM P, respectively. Data are means ± SD (n = 4–5)
The photosynthetic response to DIC (P–C) curves (a) and their parameters, Vmax (b) K1/2(DIC) (c), of N. sphaeroides colonies after 12 days exposure to different phosphorus concentrations. Squares, circles, upward triangles and downward triangles are 0.5, 5, 50 and 250 μM P, respectively. Data are means ± SD (n = 3–4)
Discussion
The amount and composition of polysaccharides are considered to play key roles in the formation of microcolonies from trichomes in N. sphaeroides (Huang et al. 1998). Deng et al. (2008a) reported that the formation of N. sphaeroides colonies was strictly controlled by light and temperature and the low light intensity and temperature were more beneficial to the colony information. In this study, we observed the effect of P concentrations on the formation of spherical microcolonies. The results indicated that percentages of spherical colonies from trichomes were significantly lower at 0.5 and 5 μM P than those at 50 and 250 μM P, which suggests that the development of N. sphaeroides colonies was restricted by the low P. Further study is underway to elucidate the physiological and biochemical changes that occur during colony development.
Nostoc sphaeroides has been used as a traditional food for very long time and are also believed to have medicinal qualities. The colonies predominantly live in paddy fields of Hefeng County in China and the yield is low in natural environment. In the past, some attempts were made to culture the colonies on a large scale under controlled indoor culture systems (Deng et al. 2006). However, these systems suffered high production costs. So, an outdoor cultivation was proposed to be an avenue for low production cost, but little study has been conducted to address various issues encountered under outdoor conditions. Deng et al. (2008b) firstly studied the relationship of the biomass productivities and growth kinetics with the differently sized colonies in outdoor raceway ponds. In this experiment, we assessed the effects of P concentrations on the growth and photosynthesis during the growth of N. sphaeroides from microcolonies to macrocolonies. The results indicated that the growth rates and photosynthetic activities were strictly controlled by P concentrations, and high P concentrations were beneficial to the growth and photosynthesis of the N. sphaeroides colonies. In natural aquatic environments, the content of available P rarely exceeds 10 μM (Bieleski 1973). These suggested that to improve the productivity of N. sphaeroides colonies in natural culture outdoors, the P nutrient supply must be necessary.
Phosphorus limitation resulted in a decreased photosynthetic affinity for DIC and down-regulation of the CO2 concentration mechanisms in Chlorella emersonii (Beardall et al. 2005). However, the increased photosynthetic affinities for DIC in this study were observed at low P levels in the N. sphaeroides colonies. Li and Gao (2004) reported that photosynthetic affinity for DIC was dependent on the colony size of N. sphaeroides, and small colonies had higher affinity for DIC. Our results showed that the diameters of the N. sphaeroides colonies became smaller with the decreasing P concentration (Data not shown), which suggested that the increased photosynthetic affinities for DIC at low P levels observed in this study might result from small colonies at low P levels.
The local investigations by Qiu et al. (2002) and He et al. (2005) suggested that P available might play an important role in the growth and distribution of the N. sphaeroides colonies in the area of Zouma. The present study indicated that spherical colonies formation of N. sphaeroides from the trichomes was strictly limited under low P condition, and during the period of formation of macrocolonies from microcolonies the growth rates and photosynthetic capacities were also limited at low P concentrations. Therefore, the results in the laboratory gave a further evidence that P might be an important factor limiting the productivity and distribution of N. sphaeroides resource in natural environment.
References
Beardall J, Roberts S, Raven JA (2005) Regulation of inorganic carbon acquisition by phosphorus limitation in the green alga Chlorella emersonii. Can J Bot 83:859–864
Bieleski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol 24:225–252
Deng Z, Kuang QJ, Hu ZY (2006) Mass culture indoors, the structure of colony and analysis of nutrient composition of Nostoc sphaeroides. J Wuhan Bot Res 24:481–484 (in Chinese)
Deng Z, Hu Q, Lu F, Liu G, Hu Z (2008a) Colony development and physiological characterization of the edible blue-green alga, Nostoc sphaeroides (Nostocaceae, Cyanophyta). Prog Nat Sci 18:1475–1483
Deng Z, Yan C, Lu F, Hu Q, Hu Z (2008b) Growth kinetics of 1–2 mm and 3–4 mm colonies of Nostoc sphaeroides (Cyanophyta) in outdoor culture. Biotechnol Lett 30:1741–1746
He TG, Xiao RL, Shi XH (2005) Analysis on the soil nutrients of citrus orchards in demonstration area of environmental immigrants, Northwest Guangxi. Chin J Eco-Agric 13(1):139–142 (in Chinese)
Hecky RE, Kilham P (1988) Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol Oceanogr 33:796–822
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J Phycol 29:729–739
Huang ZB, Liu YD, Paulsen BS et al (1998) Studies on polysaccharides from three edible species of Nostoc (Cyanobacteria) with different colony morphologies: comparison of monosaccharides from field colonies and suspension culture. J Phycol 34:962–968
Li YG, Gao K (2004) Photosynthetic physiology and growth as a function of colony size in the cyanobacterium Nostoc sphaeroides. Eur J Phycol 39:9–15
People’s Republic of China Standard GB 12388-12399-90 (1990) Methods for Determination of Nutrient Composition in Foods. Chinese Standard Publishing House, Beijing (in Chinese)
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine-phytoplankton. J Mark Res 38:687–701
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Qiu BS, Liu JY (2004) Utilization of inorganic carbon in the edible cyanobacterium Ge-Xian-Mi (Nostoc) and its role in alleviating photo-inhibition. Plant Cell Environ 27:1447–1458
Qiu BS, Liu JY, Liu ZL, Liu SX (2002) Distribution and ecology of the edible cyanobacterium Ge-Xian-Mi (Nostoc) in rice fields of Hefeng County in China. J Appl Phycol 14:423–429
Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant No. 30770172), the Excellent Youth Foundation of Hubei Scientific Committee (No. 2007ABB004), the Science and Technology Plan Projects of Huangshi (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Z., Chen, S., Lu, G. et al. Phosphorus limitation for the colony formation, growth and photosynthesis of an edible cyanobacterium, Nostoc sphaeroides . Biotechnol Lett 34, 137–143 (2012). https://doi.org/10.1007/s10529-011-0747-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0747-9