Abstract
The aim of this study was to investigate the relationship between the H-ras and Cox-2 gene expression in tumors from Iranian Oral Squamous Cell Carcinoma (OSCC) patients. Fresh tumor biopsies removed from oral cavity were collected from 67 new cases. Total RNA was extracted from biopsies and processed for quantification of H-ras and Cox-2 specific RNA expression using real-time PCR (QPCR). In addition, 59 gingival biopsies from apparently normal individuals were processed for QPCR assays. The results showed that Cox-2 expression at mRNA levels was at minimal levels in normal gingival biopsies. However, there was a surge in Cox-2 expression in tumor tissues (11.5 fold, p < 0.0001). Cox-2 expression was elevated depending on the tumor grade and there was a 1.7 fold increase (p = 0.003) in tumors diagnosed as MD/PD compared to that pathologically diagnosed as WD. This inflammatory marker was increased more significantly in smoker patients compared to non-smoker matching group. The H-ras expression at mRNA levels was significantly higher in OSCC samples compared to normal gingival (3 fold; p = 0.044). This expression was significantly higher in tumors diagnosed as MD/PD compared to WD (1.59 fold, p = 0.033). In conclusion, we found a correlation between H-ras expression and Cox-2 induction in OSCC tissue, suggesting that together these genes are contributing to cancer progression. Cox-2 is an early event in cancers of mucosal epithelial cells and a surge in Cox-2 expression in OSCC could be partly due to pro-inflammatory factors such as smoking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral squamous cell carcinoma (OSCC) is the eighth most common cancer worldwide and one of the most common malignant tumors of the oral and maxillofacial, surgery combined with radiotherapy and chemotherapy is still its main treatment [1]. In recent years, increasing incidence of oral cancers, especially in younger age groups, has posed a serious threat to public health [2].
In most countries, oral cancer is more frequent in men than women because of the more prevalent risky habits in men such as alcohol consumption, cigarette smoking, and betel quid chewing [3, 4]. Oral cavity cancers are invasive and often attack and metastasis to lymph nodes and distant organs, thus making them difficult to treat patients [4].
Oral cavity cancers are multi-factorial and include genetic component, environmental factors, viral infections, and social and behavioral factors [5]. Individual variations in susceptibility to tobacco-related oral squamous cell carcinoma have also been attributed to complex interactions between genetic and environmental factors [6].
Inflammation is the immune system’s response to infection and injury and has been implicated in the pathogeneses of arthritis, cancer and stroke, as well as in neurodegenerative and cardiovascular disease [7]. Cyclooxygenase-2 (Cox-2), also named prostaglandin-endoperoxide synthase (PTGS), is a rate-limiting enzyme for transformation of arachidonic acid to inflammatory induced prostaglandins [8].
Overexpression of Cox-2 is observed in many cancers, especially in the upper aerodigestive tract cancers, such as oral cancer [9], gastric cancer [10], colorectal cancer [11] and esophageal cancer [12, 13] and is associated with inflammatory diseases and carcinogenesis, resistance to apoptosis, tumor invasion, and angiogenesis [14].
H-ras oncogene is among several oncogenes which undergoes gene mutation and over expression. It belongs to the ras gene family (H-, K- and N-ras), which appears to be a chief contributor to the development of tumors, since it is associated with about one third of all human cancers [15]. A missense mutation (mainly in codon 12, 13 or 61) reduces the GTPase activity of the protein resulting in a perpetual activated state of H-Ras [16].
There are certain oncogenes that have been identified to be activated in OSCC, such as c-Met, c-SRC and Ras [17, 18]. Ras genes are crucial regulators of several aspects of normal growth and transformation and remarkable variation in the mutation rates of H-ras has been reported ranging from 5 to 40 % in oral carcinomas [19–21]. Over-expression of H-Ras protein determined by IHC in oral cancer tissues suggest that there is a trend towards an increase in later stages of OSCC development [20]. Regardless of the sequential changes in cancer susceptible genes in development of OSCC, mutations in ras gene proved to be a key factor in carcinogenesis pathways in epithelial tissues. Moreover, according to Wong et al., (2013), H-ras signaling pathway is important in regulation of epithelial-mesenchymal transition (EMT) [22]. It has also been demonstrated that H-ras can selectively up-regulate Cox-2 and other inflammatory reactions [23].
The squamous cell lining of the oral cavity epithelia is exposed to various environmental and food-bearing mutagens, most of which are irritants and stimulators of acute inflammatory reactions. However, to our knowledge the interaction of inflammatory reactions with H-ras mutations and downstream activation of this oncogene in OSCC has not been reported. The present study was carried out on human samples from Tehran which were diagnosed as OSCC; the samples collected in this study have unique demographic characteristics to find out if Cox-2 expression at both mRNA and protein levels is associated with H-ras activation and progression of OSCC.
Materials and Methods
Selection of Patients and Sample Collection
In this study, 67 patients diagnosed as OSCC were selected. All the patients were candidates of tumor surgery referred to Cancer Institute, Imam Khomeini Hospital, Tehran, Iran. According to the demographic information shown in Table 1, the mean age of patients was 59.8 ± 13.6 years, consisting of 58 % male and 42 % female patients. All the patients were new case and had no history of radiotherapy or chemotherapy.
The tumor site in the oral cavity of these patients was different, with tongue carcinoma as predominant cancer which comprised about 42 % of the cases. Fresh tissue biopsies from tumors were collected and kept in tubes containing RNAlater buffer and transferred to laboratory. At the same time a portion of the tissue was fixed in formalin solution (10 %) and transferred to the pathology department for examination.
Besides, 59 age and sex-matched apparently healthy candidates were selected from the individuals referred to dentistry for small gingival surgery such as tooth extraction. Gum biopsies were collected by the surgeon and kept in RNAlater for RNA extraction and RT-PCR assay.
This study was approved by the Medical Ethics Committee of the Cancer Institute of Imam Khomeini hospital as well as the Medical Ethics Committee of Tarbiat Modares Univerity. A written informed consent was obtained from each patient before sample collection. The study was conducted in accordance to Helsinki declaration and to Good Clinical Practice guidelines.
The pathological grade and clinical staging of tumors were determined by pathologist according to standard protocols [24].
RNA Extraction from Tissue Samples and cDNA Synthesis
Total RNA was extracted from frozen tumor and matched normal tissue biopsies using the Hybrid-R RNA extraction kit (GeneAll Biotechnology, Republic of Korea), according to manufacturer instructions. The concentration of total RNA in the final eluate was determined using a Nanodrop 2000C Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Two micrograms of total RNA from tissues were reverse-transcribed with oligo (dT) 18 primer using the first strand cDNA synthesis, HyperScript RT master mix (GeneAll Biotechnology, Republic of Korea) in accordance with the manufacturer’s instructions.
Cox-2 and H-ras Genes Expression by Quantitative Real-Time PCR (QPCR)
We quantified the mRNA expression of target genes (Cox-2 and H-ras) by real-time PCR using SYBR green master mix (Ampliqon; Odense, Denmark) on ABI 7500 Realtime PCR system (Applied Biosystems).
The sequences of the primers for Cox-2 gene were: F: 5′- CCCTTCTGCCTGACACCTTT-3′ and R: 5′-TCCTACCACCAGCAACCCT-3′ (product size 150 bp); the sequences of the primers for H-ras gene were; F: 5′-AGCAGGTGGTCATTGATGG-3′ and R: 5′-GTTTGATCTGCTCCCTGTACT-3′ (product size 180 bp).
Simultaneously, hypoxanthine phosphoribosyltransferase 1 (HPRT1) gene was amplified as an internal control using primers designed for HPRT1 gene. The sequence of primers is as follows; F: 5′-CCTGGCGTCGTGATTAGTG-3′ and R: 5′-TCAGTCCTGTCCATAATTAGTCC-3′ (product size 125 bp).
For each qPCR reaction we used 5 μl of diluted cDNA, 5 pmol each of primers, 10 μl of 2× master mix in a total volume of 20 μl. The PCR cycle condition was set as follow: a pre-incubation step for 15 min at 95 °C followed by 40 cycles; each cycle comprised of 15 s at 95 °C, 60 s at 60 °C. A melting curve was generated by linear heating from 60 to 95 °C. The 2-ΔΔCt formula was used to calculate relative quantitative values from data of an individual sample which was normalized with its housekeeping gene. The expression fold for each sample was compared with normal tissues.
Using these quantitative methods requires that the PCR efficiencies of all genes be similar and preferably ≥90 %. Efficiency was measured using a standard curve generated by serial dilutions of the RNA. Consequently, the initial RNA concentration of 100 ng/μl was diluted 5-fold (100 ng, 20 ng, 4 ng and 0.8 ng) for the real-time PCR assay following the standard protocol of Applied Biosystems. The standard curve assays showed an efficient amplification (>90 %) for genes and the specificity was demonstrated by a single peak at the expected temperature on melting curve analyses.
Statistical Analysis
Data analyses performed using SPSS software version 16. The compression between gene expression and clinicopathological factors were analyses by Welch’s t-test and one-way ANOVA. The significant was considered equal or less the 0.05 (p ≤ 0.05).
Results
All the patients were confirmed to have oral cavity SCC based on histological examination by pathologists. From each fresh tissue biopsy, total RNA was isolated and used for RT-PCR using quantitative real-time PCR (QPCR). Using specific primers the Cox-2 and H-ras genes were amplified and the data was compared to HPRT1 gene as internal control and normal gingival tissues. The efficiency of each primer pairs in QPCR was determined to be >90 % as shown by running various cDNA concentrations.
Cox-2 Expression in Samples from Oral Cavity SCC
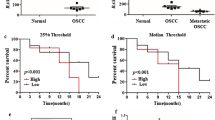
As shown in Fig. 1, the Cox-2 expression at mRNA levels was found to be at minimal levels in gingival tissue biopsies obtained from normal individuals. However, the Cox-2 expression was significantly increased in tumor tissues as compared to normal gingival tissues obtained from healthy individuals (11.5 fold, p < 0.0001).
When the Cox-2 expression was compared in terms of tumor grade/stage, it was shown that Cox-2 expression was elevated depending on the tumor grade. There was a 1.7 fold increase (p = 0.0003) in tumors diagnosed as MD/PD compared to that pathologically diagnosed as WD (Table 2). Changes in Cox-2 expression was also higher in tumors diagnosed as stage III/IV compared to those in stage I/II (p = 0.0012).
The effect of patient’s age on the Cox-2 expression in tumors was also important, since Cox-2 expression was significantly higher (P = 0.0139) in tumors obtained from patients over 50 year of age (50–72 yr) compared to young patients with OSCC (Table 2). However, there were no sex-dependent differences in Cox-2 in these patients.
As shown in Table 2, the smoking habit in patients influences Cox-2 expression in tumors. It was demonstrated that Cox-2 was more significantly expressed in patients with smoking history compared to non-smoker patients (p = 0.0064).
Expression of H-ras in Samples from Oral Cavity SCC
As shown in Fig. 2, the expression of H-ras at mRNA levels was significantly higher in patients with OSCC compared to normal gingival tissues (3 fold; p = 0.044). When compared in terms of tumor grade, the expression of H-ras was found to be significantly higher in tumors diagnosed as MD/PD as compared to WD (p = 0.033). However, there was no significant difference in tumors diagnosed with different pathological stages (Table 2).
Similar to Cox-2, the expression of H-ras in tumors was age-dependent as it was shown that H-ras expression was more in elder patients (50–72 yr). But neither sex nor the smoking habit of patients were considered as important factors in expression of tissue H-ras (Table 2).
Discussion
Chronic inflammation is an important contributor in development of carcinoma of epithelial tissues. Over expression of certain oncogenes during the carcinogenesis can alter the fate of the transformed cells. In case of epithelial tissue particularly oral cancers, the cells are exposed to various environmental carcinogens, leading to gene mutation and activation. The mutations in ras family and changes in ras-related signaling pathways are common to various cancer types particularly carcinomas. Therefore, alterations of ras genes and activity are important in controlling epithelial-mesenchymal transition (EMT) and progression of tumors [22]. Overexpression of H-ras in tissue biopsies of OSCC reported in the present study indicate the contribution of this oncogene in development of cancer in these patients. A 3-fold increase in H-ras expression in OSCC compared to normal tissues indicates the gene modification. According to Wong et al., (2013) H-ras signaling together with inflammation can control EMT during skin tumor progression [22].
Our results show that H-ras is expressed in mucosal OSCC depending on pathological grade of the tumors. This finding is agreement with the report by Vairaktaris et al. (2008), showing that increased H-ras immunoexpression levels from well-differentiated (WD) carcinomas to moderately differentiated (MD) carcinomas suggesting the contribution of H-ras mutations and protein accumulation in later stages of oral oncogenesis [20].
In the present report H-ras and Cox-2 expression in tissue biopsies of OSCC was used as an approach to understand the contribution of inflammatory reactions in oncogene mutation and cancer progression. A highly significant increase in Cox-2 specific RNA in OSCC, determined by QPCR indicates the involvement of Cox-2 related inflammatory reactions in tumors. The correlation of the H-ras and Cox-2 expression at mRNA levels (r = 0.46; p = 0.0005), further suggest the up-regulation of Cox-2 by activation and over-expression of H-ras during carcinogenesis. This finding was in accordance to the report by Lee et al., (2006), suggesting that in invasive rat liver epithelial cells H-ras can selectively regulate MMP-9 and Cox-2 through activation of ERKs and IKK-IkBa-NFkB signaling pathway [23].
The ras family are good targets for various environmental mutagens and factors related to life style such as smoking and alcohol drinking. On the other hands, Cox-2 is an enzyme in prostaglandin pathway readily inducible in response to trauma, smoking and alcohol [25].
In the population selected in this study 53.7 % of cases were smokers, but very rarely the individuals had the drinking habit. Interestingly, the Cox-2 expression was significantly elevated in smoker patients compared to non-smoker patients (p = 0.0064). Whereas, it appears that smoking had little influence on H-ras expression in OSCC patients (Table 2).
A surge in Cox-2 expression in OSCC tissues probably occurs as a result of epithelial changes during cancer progression as well as factors such as smoking, since there was a relatively higher induction of tissue Cox-2 in smoker patients compared to their non-smoker cases.
These data may suggest that Cox-2 is partially influenced by alterations in H-ras activation and part of the Cox-2 related factors could be assigned to external/environments factors. The impact of cigarette smoking on Cox-2 expression has previously been reported. In this connection, a case-control study showed that current tobacco smoking was associated with elevated COX-2 levels compared with never or former smoking (OR 1.68; 95 % CI 1.03–2.75) [25].
The cross-talk between the Cox-2 related factors and oncogenes in development of tumors has been studied. The inter-relationship between these factors may be affected by several factors. Based on the questioners completed in this study, the risk factors in this population are mainly smoking and dietary habits. It is assumed that the population selected in this study has neither tobacco chewing habits nor alcohol drinking.
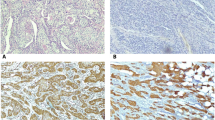
Increased expression of Cox-2 was associated with accumulation of Cox-2 protein in tissues (IHC assay). This finding is in agreement with our previous studies on esophagus SCC samples collected from high-risk region of Iran, showing that 48–74 % of samples had high scores of Cox-2 expression [12, 13].
Among the oral cavity cancers, the prevalence of tongue SCC is higher than other cancer types, comprising of about 42 %. However, the changes in Cox-2 and H-ras presented in this study are not limited to tongue tissue, but all the oral cancers have been included. Studies focused on tongue SCC, show the mRNA expression level of Cox-2 was significantly higher in this tissues compared to the adjacent noncancerous mucosal tissues (median values, 5.865 vs. 3.707, p = 0.018). Also the Cox-2 mRNA expression was found to be significantly (p = 0.037) correlated with lymph node metastasis [26].
The expression of Cox-2 in OSCC patients was associated with the tumor grade and stage. As shown in Table 2, Cox-2 expression was significantly (p = 0.0003) higher in PD/MD tumors compared to the samples diagnosed as WD. Likewise, the Cox-2 expression was found to be significantly greater (p = 0.0012) in pathological stages of III/IV compared to stage I/II. This data clearly show the association of tumor progression and inflammatory reactions in terms of Cox-2 intermediates. Based on our data, Cox-2 expression is also influenced by age and smoking habit. Relatively higher level of Cox-2 expression in smokers is justifiable, because cigarette smoke contains pro-inflammatory agents [27].
Cox-2 induction can be associated with over-expression of different oncogenes, for instance it has been reported that Cox-2 and HER-2 together are important markers for invasion and metastasis of colorectal cancer [11]. Recently, the consequences of Cox-2 induction has been outlined by Wu and Sun (2015) as (1) increase the production of prostaglandins and inhibit the body’s immune response; (2) inhibit tumor cell apoptosis and promote cell proliferation; (3) regulate cell cycle progression; (4) promotion of tumor angiogenesis; (5) increase the expression of matrix metalloproteinases in tumor cells; and (6) induce activation of precursors of carcinogenic substances [11].
In conclusion, we found that although the H-ras and Cox-2 expression were elevated in OSCC tumors, but it seems that over-expression of Cox-2 in the tumors is an early event in OSCC progression which can be readily induced by pro-inflammatory factors such as cigarette smoke. This finding may be implicated in therapeutic interventions using non-steroid anti-inflammatory drugs (NSAID) drugs for cancers vulnerable to chronic mucosal inflammation.
References
Li TJ, Cui J (2013) COX-2, MMP-7 expression in oral lichen planus and oral squamous cell carcinoma. Asian Pac J Trop Med 6(8):640–643. doi:10.1016/S1995-7645(13)60110-8
Li D, Hao SH, Sun Y, Hu CM, Ma ZH, Wang ZM, Liu J, Liu HB, Ye M, Zhang YF, Yang DS, Shi G (2015) Functional polymorphisms in COX-2 gene are correlated with the risk of oral cancer. Biomed Res Int 2015:580652. doi:10.1155/2015/580652
Warnakulasuriya S (2010) Living with oral cancer: epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol 46(6):407–410. doi:10.1016/j.oraloncology.2010.02.015
Yu KT, Ge C, Xu XF, Zou JC, Zou X, Zhen S (2014) CYP1A1 polymorphism interactions with smoking status in oral cancer risk: evidence from epidemiological studies. Tumour Biol 35(11):11183–11191. doi:10.1007/s13277-014-2422-y
Sand L, Wallstrom M, Hirsch JM (2014) Smokeless tobacco, viruses and oral cancer. Oral Health Dent Manag 13(2):372–378
Vlachopoulos C, Aznaouridis K, Bratsas A, Ioakeimidis N, Dima I, Xaplanteris P, Stefanadis C, Tousoulis D (2015) Arterial stiffening and systemic endothelial activation induced by smoking: the role of COX-1 and COX-2. Int J Cardiol 189:293–298. doi:10.1016/j.ijcard.2015.04.029
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31(5):986–1000. doi:10.1161/ATVBAHA.110.207449
Hamzic N, Blomqvist A, Nilsberth C (2013) Immune-induced expression of lipocalin-2 in brain endothelial cells: relationship with interleukin-6, cyclooxygenase-2 and the febrile response. J Neuroendocrinol 25(3):271–280. doi:10.1111/jne.12000
Pandey M, Prakash O, Santhi WS, Soumithran CS, Pillai RM (2008) Overexpression of COX-2 gene in oral cancer is independent of stage of disease and degree of differentiation. Int J Oral Maxillofac Surg 37(4):379–383. doi:10.1016/j.ijom.2008.01.004
He XP, Shao Y, Li XL, Xu W, Chen GS, Sun HH, Xu HC, Xu X, Tang D, Zheng XF, Xue YP, Huang GC, Sun WH (2012) Downregulation of miR-101 in gastric cancer correlates with cyclooxygenase-2 overexpression and tumor growth. FEBS J 279(22):4201–4212. doi:10.1111/febs.12013
Wu QB, Sun GP (2015) Expression of COX-2 and HER-2 in colorectal cancer and their correlation. World J Gastroenterol 21(20):6206–6214. doi:10.3748/wjg.v21.i20.6206
Allameh A, Rasmi Y, Nasseri-Moghaddam S, Tavangar SM, Sharifi R, Sadreddini M (2009) Immunohistochemical analysis of selected molecular markers in esophagus precancerous, adenocarcinoma and squamous cell carcinoma in Iranian subjects. Cancer Epidemiol 33(1):79–84. doi:10.1016/j.canep.2009.05.002
Biramijamal F, Allameh A, Mirbod P, Groene HJ, Koomagi R, Hollstein M (2001) Unusual profile and high prevalence of p53 mutations in esophageal squamous cell carcinomas from northern Iran. Cancer Res 61(7):3119–3123
Liu B, Qu L, Yan S (2015) Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int 15. doi:10.1186/s12935-015-0260-7
Taketomi A, Shirabe K, Muto J, Yoshiya S, Motomura T, Mano Y, Ikegami T, Yoshizumi T, Sugio K, Maehara Y (2013) A rare point mutation in the ras oncogene in hepatocellular carcinoma. Surg Today 43(3):289–292. doi:10.1007/s00595-012-0462-8
Wang Y, Arlt VM, Roufosse CA, McKim KL, Myers MB, Phillips DH, Parsons BL (2012) ACB-PCR measurement of H-ras codon 61 CAA-->CTA mutation provides an early indication of aristolochic acid I carcinogenic effect in tumor target tissues. Environ Mol Mutagen 53(7):495–504. doi:10.1002/em.21710
Sen B, Peng S, Saigal B, Williams MD, Johnson FM (2011) Distinct interactions between c-Src and c-met in mediating resistance to c-Src inhibition in head and neck cancer. Clin Cancer Res 17(3):514–524. doi:10.1158/1078-0432.CCR-10-1617
Murugan AK, Munirajan AK, Tsuchida N (2012) Ras oncogenes in oral cancer: the past 20 years. Oral Oncol 48(5):383–392. doi:10.1016/j.oraloncology.2011.12.006
Chang F, Steelman LS, Shelton JG, Lee JT, Navolanic PM, Blalock WL, Franklin R, McCubrey JA (2003) Regulation of cell cycle progression and apoptosis by the ras/Raf/MEK/ERK pathway (review. Int J Oncol 22(3):469–480
Vairaktaris E, Papakosta V, Derka S, Vassiliou S, Nkenke E, Spyridonidou S, Vylliotis A, Lazaris A, Kokkori A, Moulavassili P, Loukeri S, Perrea D, Donta I, Yapijakis C, Patsouris E (2008) H-ras and c-fos exhibit similar expression patterns during most stages of oral oncogenesis. In Vivo 22(5):621–628
Sathyan KM, Nalinakumari KR, Kannan S (2007) H-ras mutation modulates the expression of major cell cycle regulatory proteins and disease prognosis in oral carcinoma. Mod Pathol 20(11):1141–1148. doi:10.1038/modpathol.3800948
Wong CE, Yu JS, Quigley DA, To MD, Jen KY, Huang PY, Del Rosario R, Balmain A (2013) Inflammation and Hras signaling control epithelial-mesenchymal transition during skin tumor progression. Genes Dev 27(6):670–682. doi:10.1101/gad.210427.112
Lee KW, Kim MS, Kang NJ, Kim DH, Surh YJ, Lee HJ, Moon A (2006) H-ras selectively up-regulates MMP-9 and COX-2 through activation of ERK1/2 and NF-kappaB: an implication for invasive phenotype in rat liver epithelial cells. Int J Cancer 119(8):1767–1775. doi:10.1002/ijc.22056
Lingen MW, Kalmar JR, Karrison T, Speight PM (2008) Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol 44(1):10–22. doi:10.1016/j.oraloncology.2007.06.011
Nguyen T, Tang Z, Younes M, Alsarraj A, Ramsey D, Fitzgerald S, Kramer JR, El-Serag HB (2015) Esophageal COX-2 expression is increased in Barrett’s esophagus, obesity, and smoking. Dig Dis Sci 60(1):65–73. doi:10.1007/s10620-014-3333-x
Fujii R, Imanishi Y, Shibata K, Sakai N, Sakamoto K, Shigetomi S, Habu N, Otsuka K, Sato Y, Watanabe Y, Ozawa H, Tomita T, Kameyama K, Fujii M, Ogawa K (2014) Restoration of E-cadherin expression by selective cox-2 inhibition and the clinical relevance of the epithelial-to-mesenchymal transition in head and neck squamous cell carcinoma. J Exp Clin Cancer Res 33. doi:10.1186/1756-9966-33-40
Liu ES, Shin VY, Ye YN, Luo JC, WK W, Cho CH (2005) Cyclooxygenase-2 in cancer cells and macrophages induces colon cancer cell growth by cigarette smoke extract. Eur J Pharmacol 518(1):47–55. doi:10.1016/j.ejphar.2005.05.018
Acknowledgements
This article is part of the PhD dissertation of A. Moazeni-Roodi. This study has been supported by Tehran University of Medical Sciences and Health Services, Grant #92-02-51-23399.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moazeni-Roodi, A., Allameh, A., Harirchi, I. et al. Studies on the Contribution of Cox-2 Expression in the Progression of Oral Squamous Cell Carcinoma and H-Ras Activation. Pathol. Oncol. Res. 23, 355–360 (2017). https://doi.org/10.1007/s12253-016-0114-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-016-0114-1