Abstract
Tidal marsh plant species commonly zonate along environmental gradients such as elevation, but it is not always clear to what extent plant distribution is driven by abiotic factors vs. biotic interactions. Yet, the distinction has importance for how plant communities will respond to future change such as higher sea level, particularly given the distinct flooding tolerances and contributions to elevation gain of different species. We used observations from a 33-year experiment to determine co-occurrence patterns for the sedge, Schoenoplectus americanus, and two C4 grasses, Spartina patens and Distichlis spicata, to infer functional group interactions. Then, we conducted a functional group removal experiment to directly assess the interaction between sedge and grasses throughout the range in which they cooccur. The observational record suggested negative interactions between sedge and grasses across sedge- and grass-dominated plots, though the relationship weakened in years with greater flooding stress. The removal experiment revealed mutual release effects, indicating competition was the predominant interaction, and here, too, competition tended to weaken, though nonsignificantly, in more flooded, lower elevation zones. Whereas zonation patterns in undisturbed portions of marsh suggest that the sedge will dominate this marsh as flooding stress increases with sea level rise, we propose that grasses may exhibit a competition release effect and contribute to biomass and elevation gain even in sedge-dominated communities as sea level continues to rise. Even as abiotic stresses drive changes in the relative contributions of sedges and grasses, competition among them moderates fluctuations in total plant biomass production through time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal wetlands have relatively low plant richness owing to the combination of flooding, salinity, and other stresses that few species can tolerate. Yet, plant community composition is an especially strong driver of ecosystem processes in coastal wetlands, where plants largely control soil elevation relative to sea level, and thus, the maintenance of the ecosystem as a whole (Cahoon et al. 2020). Changes in plant community composition can have enormous consequences in coastal wetlands because tidal marsh-adapted plant species often have distinct traits that directly control rates of soil elevation gain (White et al. 2012; Mueller et al. 2016; Chen et al. 2018).
To forecast marsh sustainability in a future of accelerating sea level rise, we need to know how plants respond to flooding frequency, but plant interactions can complicate interpretation of flooding tolerances based on distribution patterns alone. Coastal ecologists determine the flooding tolerances of individual species primarily using two methods: vegetation surveys and sea level manipulations, both of which could have limitations in cases where plant interactions are strong. First, surveys provide only a snapshot of plant distribution under current conditions. Marsh plant distributions along gradients such as elevation are often interpreted as reflecting individual species tolerances for abiotic factors such as flooding stress (Marani et al. 2004; Silvestri et al. 2005; Swanson et al. 2015; Langston et al. 2020), but species interactions in mixed communities can engender great deviations between the fundamental niche of a single species and its realized niche in the presence of other species (Pennings and Callaway 1992). For instance, negative interactions, like competition, can narrow the realized elevational range of a plant species while facilitation, a positive interaction among plants commonly observed in marshes (Callaway 1995; McIntire and Fajardo 2014), may expand the realized distribution beyond the fundamental niche. Second, sea level manipulations have mostly used monospecific mesocosms (e.g., Morris et al. 2002; Kirwan and Guntenspergen 2012; Voss et al. 2013; Wigand et al. 2016; Watson et al. 2017) to assess flood tolerances, but the tolerance of intact plant communities may differ from what would be predicted from the sum of individual plant responses. For instance, monospecific mesocosms could underestimate plant elevation ranges by ignoring facilitation, which could cause an underestimation of ecosystem resilience. Failure to account for plant interactions could engender errors in either direction in projections of wetland sustainability.
The direction and strength of plant species interactions also depend on abiotic stresses. For instance, the stress-gradient hypothesis predicts that abiotic stress determines the likelihood that facilitation versus competition will occur (Bertness and Callaway 1994; Brooker and Callaghan 1998; Lortie and Callaway 2006; Maestre et al. 2009). This hypothesis states that under stressful physical conditions, positive interactions are more likely to occur; inversely, under lower levels of stress, competitive interactions will dominate (Bertness and Callaway 1994). Though positive interactions are common in many macrotidal low marshes (Bertness and Hacker 1994; Luo et al. 2010), species interactions have not been frequently examined for the high marsh communities that inhabit vast areas of tidal wetlands from Nova Scotia to Louisiana. In microtidal settings, these so-called “high marsh” communities are commonly the lowest-lying emergent plant communities in the system and will determine the sustainability of the wetlands in the face of rising seas.
Previous studies have indicated the possibility of interactions among common high marsh species, including the C3 sedge, Schoenoplectus americanus, and C4 grasses, Spartina patens and Distichlis spicata (Arp et al. 1993; Cherry et al. 2009; Langley and Megonigal 2010; White et al. 2012; Holmquist et al. 2021), but the extent of interspecific interactions in determining dominance between these species has never been explicitly examined. Sea level manipulations using mesocosms (a.k.a. “marsh organs”) show overlap in the flooding tolerances of S. americanus, S. patens, and D. spicata (Broome et al. 1995; Langley et al. 2013; Kirwan and Guntenspergen 2015; Nicks 2018). Field surveys show that S. americanus tends to dominate the lower, and C4 grasses higher elevations of their common habitat, though there is 92% overlap in their elevation range (Holmquist et al. 2021). To what extent are these plant zones determined by abiotic factors versus interactions with other plants? Plant interactions will partly determine the progression of the plant community compositional change that contribute strongly to elevation maintenance as these marshes experience more frequent flooding with accelerating sea level rise.
To evaluate the direction and strength of plant interactions in a high elevation brackish marsh, we (1) analyzed existing data from a 33-year elevated CO2 experiment and (2) conducted a complementary competition experiment in which we removed sedges or grasses from plots that varied in initial dominance. Based on previous observations from this marsh, we predicted there would be strong competition between the C3 sedge, S. americanus, and C4 grasses, S. patens and D. spicata. Following the stress-gradient hypothesis, we predicted that competition would be most intense at the higher (less flooded) elevations. By testing for the existence of species interactions and resolving their strength, we can better predict marsh plant community response to rising seas.
Methods
Site Description
The Kirkpatrick Marsh is located at the Smithsonian Environmental Research Center (SERC) in Edgewater, MD. The Global Change Research Wetland (GCReW) is a facility that occupies a large portion of the site and is used for long-term experiments and observations. The site is adjacent to the Rhode River, a sub-estuary of Chesapeake Bay. The site in the present study has a 44 cm tidal range (Holmquist et al. 2021), and the marsh plots used are positioned between 17 and 25 cm above NAVD88. Mean growing season sea level has risen 20 cm since the beginning of this study while the marsh surface has risen by roughly 5 cm (unpublished SET data).
We investigated the relationship between competition and flooding tolerance using areas of the marsh either dominated by the sedge Schoenoplectus americanus or dominated by the C4 grass species, Spartina patens and Distichlis spicata. S. americanus is tolerant of 2–17 psu salinity and is relatively flood tolerant (Hess 1975) and accordingly tends to occupy the lower-lying areas of the marsh (Arp et al. 1993). In less flood-stressed conditions at higher elevations, S. americanus appears to be a weaker competitor (Emery et al. 2001). S. patens has a lower flood tolerance than the sedge and is often outcompeted by the sedge in frequently flooded areas (Konisky and Burdick 2004). In response to flooding, this C4 grass develops root aerenchyma, but thrives above the high-water line in most marshes (Callaway and King 1996). D. spicata overlaps greatly with S. patens in physiological tolerance of flooding and salinity. It is considered a poor competitor with other plants (Levine et al. 1998) but may have enhanced ability to colonize gaps and establish under harsh conditions compared to co-occurring plant species (Brewer et al. 1998).
Long-Term Experiment
To explore temporal patterns of plant co-occurrence among plant functional groups, we used data from an ongoing elevated CO2 experiment at the GCReW (Drake et al. 1985, 1989; Drake 2014). Briefly, 10 plots were located in each of three vegetation zones, C3 sedge-dominated (C3), C4 grass-dominated (C4), and mixed-composition (MX) zones (Fig. S5). The MX zones were established with roughly equal initial contributions from sedge and grasses in 1987, though the composition of all plots has shifted considerably over time. All plots were enclosed with 1-m diameter open-top chambers (Drake et al. 1985, 1989). Half of the chambers (Ambient) received ambient air delivered by a blower through a manifold, and the other half (Elevated) received ambient air mixed with CO2 at a rate sufficient to increase the atmospheric [CO2] above ambient by 340 ppm beginning in 1987. Aboveground biomass was estimated in each plot in late July each year using a combination of stem counts and allometry for S. americanus (Lu et al. 2016), and by clipping subplots for D. spicata and S. patens (Drake et al. 1989). In May 1998, wells (sippers) were installed to sample soil porewater at three depths, 20 and 40 cm for analysis of key porewater parameters (Keller et al. 2009). We report salinity, pH, H2S, and NH4+ (analyzed following the methods of Keller et al. 2009) for two depths over the interval of 2002–2013 for sedge- and grass-dominated plots (Table 1).
To assess observational evidence of interactions between the functional groups, we used plot-level plant species biomass from the 33-year experiment. Because we found no strong and consistent treatment effects of elevated CO2 on plant community composition (as opposed to individual or plot-scale plant biomass which does respond to elevated CO2), we lumped data across treatments to yield greater replication within years and zones (n = 10). First, we regressed annual grass biomass by annual sedge biomass across the entire site and all years to determine if the relationship between functional groups was positive, negative, or neutral. Because the sedge and grasses exhibit distinct preferences for environmental conditions such as flooding regime, salinity, and nutrient availability, which vary through space and time, we would expect a negative relationship even in the absence of species interactions. For instance, the sedges dominate the lower elevation plots and the grasses dominate the higher plots regardless of species interactions. Therefore, we partitioned the dataset to examine relationships within vegetation zones and within individual years, then performed the same analysis.
Plant Removal Experiment
To experimentally determine the nature of the interaction between plant functional groups, we performed a removal experiment at the Global Change Research Wetland (GCReW) beginning in May 2013 (Reid 2013). The study site was located within 300 m (Fig. S5) of the study described above along a similar elevation profile. The plots contained a mixture of a sedge, S. americanus, and two grasses, S. patens and D. spicata, spanning a slight elevation gradient ranging from 0.14 to 0.23 m NAVD88. Based on hourly tidal data from the Annapolis gauge (gauge 8575512 https://tidesandcurrents.noaa.gov), which is 12 km from the site, for the 2013 growing season (May 1–Sept 30), these elevations were flooded 52% and 32% of the time. Because hydroperiod and other abiotic factors play a role in which species should be favored, a block design was used to account for confounding environmental influences. Six blocks were established: two blocks dominated by grass based on percent cover, two dominated by sedge and two intermediates. The grass-dominated zones tend to be at higher elevation than the sedge-dominated zones, but there is considerable overlap in elevation among each zone (Holmquist et al. 2021).
Each block contained four plots (0.5 × 0.5 m), linearly arranged and separated by at least 1.2 m. Each plot was randomly assigned to one of four treatments: no removal (NR) control plots that were marked and measured like the others, but the plant community was not manipulated; sedge removal (SR) plots, from which all green sedges were clipped at soil level; grass removal (GR) plots, from which all green grass (S. patens and D. spicata) was cut at ground level and removed; and all removal (AR) plots, from which all vegetation was cut at ground level at the beginning of the growing season in May 2013 to assess impacts of the clipping disturbance.
The sedge removal and grass removal treatments were administered three times throughout the growing season: May, June, and August 2013. Those plants removed were dried at 60 °C for 72 h and weighed. The AR treatment was administered only once in May to examine which species would recover and re-sprout faster following an aboveground disturbance. During measurements, a 25 cm square was placed in the center of each plot to allow a 25 cm buffer zone around the measurement area. Counts of the number of green stems of each functional group were taken. For sedge, counts consisted of the number of green stems found within the 25 cm square. For grass, a smaller 10 cm square was placed in the corner of the 25 cm square. The number of small squares needed to reach a statistically robust number of 100 grass stems was then recorded in addition to the count. Sedge and grass were measured in the NR and AR plots, while only sedge was measured in the GR plot and only grass was measured in the SR plots.
These measurements were recorded in May, June, and August 2013. In September, which marked the end of the growing season, and a time when the sedge was approximately halfway senesced, the plots were clipped at the soil surface and bagged. Plant mass from the 25 cm center square was kept separate from the remaining buffer portion. Clippings were sorted to remove brown stems that were found in the plots from previous seasons in the NR treatment. Clipped grasses were sorted by species. Any stems that had viable green tissue were considered to have grown in the current growing season. Plant mass was dried and weighed as described above. Stem densities are shown in Fig. S3, but biomasses were analyzed for testing hypotheses.
Data Analysis
Long-Term Experiment
To investigate whether sedge and grass biomass were negatively related over the course of the long-term experiment, we used a linear mixed-effects model that accounted for repeated measurements over time. More specifically, we fit the linear model of grass biomass versus sedge biomass when adjusting for the effects of year, plant community, and CO2 treatment, with a random intercept across chambers to allow chamber-specific variability and to account for possible correlation among repeated measurements within the same plot over time (model 1).
To display these data, we show two summary metrics from these relationships, the slope in a simple linear regression model of annual grass biomass versus sedge biomass in each plant community and the corresponding correlation coefficient, r. A strong negative relationship between the abundance of the two species should yield a negative slope, and a negative r with large magnitude. A positive interaction would tend to yield a positive slope and a positive r with relatively large magnitude. Weak interactions yield r near zero.
Plant Removal Experiment
Treatments were blocked to account for abiotic conditions that co-vary with relative elevation, so we used the absolute release effect, the difference in biomass between removal treatments (SR or GR) and the control plots (NR) in each block to evaluate the effect of removing competition, otherwise known as the intensity of competition (Grace 1993). For sedge, the release effect was estimated as (Sedge biomass)GR—(Sedge biomass)NR. For grass, the release effect was estimated as (Grass biomass)SR—(Grass biomass)NR.
We focused our analyses on absolute competition intensity because low biomasses in some control plots (the denominator) lead to highly variable estimates for relative competition intensity. We used one-sample t test to determine if the mean index of competition differed from zero (n = 6). If the mean release effect was greater than zero, it suggested that competition occurred. If the mean release effect was lower than zero, it suggested that removing other species from the plot was detrimental to the target species (i.e., facilitation). If the mean release effect did not differ from zero it indicated no interactions. We used one-way ANOVA to test whether on average the release effect differed among plant communities. The Shapiro–Wilk test and the Bartlett’s test were used to check normality and homogeneity of variance, respectively.
Results
Long-Term Experiment
We found sedge biomass and grass biomass were negatively correlated (Fig. 1), i.e., the slope estimate was −0.45 g m−2 when time, plant community, and CO2 treatment were held fixed (Table 2, t958 = − 11.49, p < 0.01), which indicated competition between these species. On average, elevated CO2 reduced the grass biomass by 57.60 g m−2 compared with the ambient treatment (t26 = −3.69, p < 0.01) adjusting for other variables in the model (Table 2). We fit another similar linear mixed-effects model of sedge biomass versus grass biomass, time, plant community, and CO2 treatment to test on the effect of elevated CO2 on sedge growth, but we did not find this effect statistically significant (results not shown).
The magnitude of the slope in the simple linear regression of grass biomass versus sedge biomass varied through time in all plant communities (Fig. 2), which indicates a potential interaction between the linear relationship of grass biomass versus sedge biomass and time. To further investigate whether the linear relationship between sedge and grass biomass changed over time and whether this interactive effect differed among plant communities, we fit a linear mixed-effects model with random intercept across chambers of grass biomass against sedge biomass, time, the interaction between sedge biomass and time, and the CO2 treatment in each of the three communities (models 2–4). We found that this linear relationship weakened over time in all communities (Table 3). That is, for every 1 g m−2 increase in the sedge biomass, the reduction in the mean grass biomass decreased by 0.0056 g m−2, 0.059 g m−2, and 0.021 g m−2 every year in the sedge-dominated, grass-dominated, and the mixture community, respectively. This interactive effect was statistically significant in the grass-dominated (t317 = 2.02, p = 0.044) and the mixed community (t317 = 3.21, p < 0.01), but not in the sedge-dominated community (t317 = 1.31, p = 0.19).
The slope (top panels) and correlation coefficient, r, (bottom panels) of the linear relationship between sedge and grass biomass through time in each of the three elevation zones in the long-term experiment. Negative slopes indicate negative relationships (i.e., competition) between sedge and grass abundance. Each point represents the relationship for 10 plots within a community for a year. In cases where either sedge or grasses were present in < 2 plots, no data are shown
Plant Removal Experiment
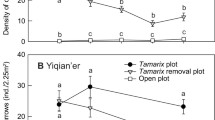
In the control (no removal) group, grass-dominated plots were composed of 80% grass and 20% sedge; mixed plots were 63% sedge, 47% grass, and sedge-dominated plots were 83% sedge and 17% grass by mass (Fig. 3). Regarding the two grasses, the mean biomass of S. patens was greater in the grass-dominated community than in the other two communities (Fig. 3, F2,3 = 10.21, p = 0.046), while no difference in mean biomass of D. spicata was observed (F2,3 = 0.069, p = 0.94).
On average, removing a functional group (either sedge or grass) resulted in enhanced growth of the other functional group (a positive release effect) across different communities, i.e., the mean sedge release effect (107.3 g m−2with standard error 21.5 g m−2) and the mean grass release effect (97.8 g m−2 with standard error 16.8 g m−2) were both greater than 0 (sedge release effect: t5 = 4.99, p < 0.01; grass release effect: t5 = 5.84, p < 0.01). The mean sedge release effect and the mean grass release effect seemed to differ across plant communities (Fig. 4), but a one-way ANOVA showed that these differences were not statistically significant (sedge release effect: F2,3 = 2.84, p = 0.20; grass release effect: F2,3 = 2.72, p = 0.21). Of the two grass species, D. spicata responded to sedge removal more strongly than S. patens in sedge-dominated community (t2 = 4.15, p = 0.053), but not in other communities (Fig. 3). Results from AR plots and total biomass across species, which were not used in competition calculations, are shown in Fig. S6.
Competition intensity in the plant-removal experiment estimated as the release effect, the magnitude of the difference in end-of-season biomass of the focal functional group between unmanipulated control plots and plots from which the competitors have been removed in each block. Greater positive values indicate stronger competition. For both species, release effects were greater than zero (p < 0.01), but did not differ among communities. Bars represent means ± standard error (n = 2 in each community)
Discussion
Our findings indicate interspecific competition shapes the dominance patterns among the sedge, S. americanus, and C4 grasses, S. patens and D. spicata, throughout a large portion of their ranges at this site. The long-term experiment yielded an inverse relationship between sedge and grass biomass (Fig. 1), indicative of a negative interaction among the species. Accounting for other variables, a 1 g m−2 increase in sedge biomass related to a decrease in the mean grass biomass of 0.45 g m−2 (Table 2). To aid visualization of the negative relationship between the abundance of different species adjusting for the influence of abiotic conditions such as flooding regime or soil conditions, which vary over space and through time, we examined the strength of the relationship within the same elevation zone in individual years. Still, negative relationships between grass and sedge biomass were prevalent, which was consistent with the results of model 1 (Fig. 2, Table 3). To unequivocally determine the nature of plant interactions, we conducted a plant removal experiment along an elevational gradient in adjacent plots. This experiment yielded a significant increase in biomass for both the sedge and grass species when the other functional group was removed (Figs. 3 and 4), corroborating the evidence that competition drives plant community composition observed in the long-term experiment.
Our finding that competition structures this marsh plant community agrees with some previous work in other marshes (Valiela et al. 1978; Bertness and Ellison 1987; Brose and Tielbörger 2005; Pennings et al. 2005), but the existence of mutual competitive effects, i.e., that each species inhibited the other, differs from other findings. In previous studies, the zonation patterns of Spartina alterniflora, a C4 grass, and Juncus roemerianus, a C3 rush, were attributed to a trade-off between stress tolerance and competitive ability. The range of J. roemerianus had limitations set by abiotic factors while the range of S. alterniflora was determined by competition (Pennings et al. 2005). Other studies only removed one competitor and did not examine mutual competition (e.g., Brose and Tielbörger 2005). In our experiment, both functional groups exhibited a release from competition, suggesting that the realized distributions of both grasses and sedge were determined by mutual competitive interactions.
Marshes are considered stressful habitats owing to high porewater salinity and soil anoxia, but also commonly exhibit facilitation among plants (Bertness and Hacker 1994; Cui et al. 2011). Past findings of facilitation contrast with results from our experiments, where negative relationships in abundance dominated and release effects indicated competition across a range of elevations. That we found no evidence for facilitation could be explained by differences in the traits of the plant species that comprise the community. The most severe agent of stress in this brackish marsh where salinity typically peaks below 12 ppt is anoxia (Erickson et al. 2007). Facilitation would be expected at low elevations where flooding is more frequent provided that the plant species present release O2 to the rhizosphere and thereby reduce stress for surrounding plants (Callaway and King 1996). Much of facilitation literature has included plants such Typha latifolia and Spartina alterniflora, which can strongly oxidize the rhizosphere. Neither of those strongly aerating species were present in our plots. Though S. americanus can be highly aerenchymous, perhaps none of the focal species in the present study release sufficient oxygen to facilitate other plant species.
Mechanisms of Competition
This release effect, an increase in target species growth when competitors are removed, likely derives from increased availability of resources, most probably nutrients and light. Previous work at this site has shown a strong positive association between grass biomass and porewater [N] (Keller et al. 2009, Table 1) which we interpret as the manifestation of grasses having a lower affinity for N than the sedge (Cott et al. 2018). Moreover, fertilization with N greatly increases grass biomass (Langley and Hungate 2014). Both patterns suggest that N scarcity plays a role in mediating competition particularly where the sedge dominates over the grasses. Where nutrients are more available, grasses, such as S. patens and D. spicata, may be superior competitors for light over Juncus gerardi (Levine et al. 1998). These grasses commonly grow horizontally in thick mats that greatly diminish light penetration to the soil surface. While live S. americanus stems grow vertically and linearly, the resulting litter can remain suspended in the canopy for several years, accumulating in thick layers that also intercept a large portion light. So, competition for light could be mutual in the present marsh.
Flooding stress has increased with relative sea level rise and the plant communities have generally shifted towards increasing dominance by the sedge (Figs. S1 and S2). Increasing flooding frequency over the past decades also appears to diminish the strength of competition in the long-term experiment (Fig. 2, Tables 2 and 3). Similarly, release effects tended to have smaller magnitude in sedge-dominated (lower elevation) plots (Fig. 4), though there were no statistically significant differences. Both patterns agree with the stress gradient hypothesis. Moreover, the observation that relative abundance of these species varies along elevation gradients (i.e., grasses tend to dominate higher and sedge dominates lower), even though grasses and sedges are capable of growing throughout most of the common elevation range (Holmquist et al. 2021) in this marsh, indicates that flooding regime has a strong influence on the outcome of competition.
The evidence found herein for the prevalence of competition helps explain patterns in the long-term experiment. For instance, whereas the sedge has followed our prediction of increasing in abundance through time in the higher-elevation C4 plots as sea level has risen, D. spicata drove an unexpected resurgence of grass biomass in the low-elevation plots (Supplemental Fig. S1, right panel) as sea level rose rapidly (Fig. S2). In fact, many areas of the marsh that are disturbed by human or animal activity become vigorously colonized by D. spicata (Brewer et al. 1998), even at elevations below where it occurs in undisturbed marsh (Fig. S4). Moreover, other perturbations such as adding nitrogen can reverse the outcome of competition (Levine et al. 1998) even in low-elevation plots in this marsh (Langley and Megonigal 2010). The distribution of plant species that comprise plant community zonation patterns must be understood to be limited to the ecological context in which they occur, because patterns of dominance can change as species respond to physical disturbance, altered nutrient regime, or climate change according to species-specific and genotype-specific traits.
Implications for Marsh Sustainability
Plant species interactions can drive changes in plant community composition with consequences for the soil-building processes that sustain coastal wetlands. The prevalence of competition in this marsh, even under stressful conditions, appears to have the effect of stabilizing the production of plant biomass over time. Despite substantial shifts in plant dominance as sea level rise has accelerated above historical rates and an unexplained surge of grasses in sedge-dominated plots around 1995 (Fig. S1, top panels), total biomass has remained strikingly consistent (Fig. S1, bottom panels). The outcome for plant productivity may be quite different in marshes where positive interactions, such as facilitation, are common. In those cases, positive interactions among plant species may yield less stable total biomass production over time, which could result in a more sudden decline in plant biomass, and therefore, marsh elevation gain, in response to sea level rise. We posit that competitive plant communities may exhibit distinct patterns of resilience from facilitative communities.
Our results illustrate the point that researchers may need to consider more than the most flood-tolerant species for forecasting marsh sustainability. In this marsh, one may presume to base projections of plant productivity, and therefore elevation gain, on the production patterns of the sedge because it tends to dominate lower portions of the marsh in these plots. However, projecting the marsh response to sea level rise assuming a monospecific stand of S. americanus would ignore the contributions of grasses when and where sedges decline and would result in an underestimation of marsh resiliency in this ecosystem. Marshes have optimum elevations relative to sea level at which plant production peaks. With smoothly accelerating relative sea level rise, a marsh that falls below the optimum elevation will not recover. Even though some plants may be able to grow below this elevation, they will not produce enough biomass to sustain elevation gain at the rate required to counteract accelerating sea level rise (Morris 2006). Therefore, it is the whole community that occurs above the elevation of total peak biomass, not the individual species with the lowest range, which is most important for determining the fate of the ecosystem. Perturbation of the flooding regime or other global changes may release previously subdominant species from competition, allowing them to contribute meaningfully to elevation gain.
Plants play a critical role in determining future marsh elevation gain as they protect against soil loss to erosion (Cahoon et al. 2020), and individual species differ in their contributions to elevation gain (Krauss et al. 2003; Chen et al. 2018). We recommend future sea level manipulations allow for a realistic assemblage of plant species (e.g., Peng et al. 2018) and or competition treatments (e.g., Schile et al. 2017) to allow assessment of how interactions may alter flooding tolerance of individual plant species. Observational surveys that are used to infer elevational ranges should be paired with manipulative studies to separate the effects of ecological interactions from physical stressors.
References
Arp, W.J., B.G. Drake, W.T. Pockman, P.S. Curtis, and D.F. Whigham. 1993. Interactions between C3 and C4 salt marsh plant species during four years of exposure to elevated atmospheric CO2. Vegetation 104: 133–143.
Bertness, M.D., and R. Callaway. 1994. Positive interactions in communities. Trends in Ecology & Evolution 9: 191–193.
Bertness, M.D., and A.M. Ellison. 1987. Determinants of pattern in a New England salt marsh plant community. Ecological Monographs 57: 129–147.
Bertness, M.D., and S.D. Hacker. 1994. Physical stress and positive associations among marsh plants. The American Naturalist 144: 363–372.
Brewer, J.S., T. Rand, J.M. Levine, and M.D. Bertness. 1998. Biomass allocation, clonal dispersal, and competitive success in three salt marsh plants. Oikos 82: 347–353.
Brooker, R.W., and T.V. Callaghan. 1998. The balance between positive and negative plant interactions and its relationship to environmental gradients: A model. Oikos 81: 196–207.
Broome, S.W., I.A. Mendelssohn, and K.L. McKee. 1995. Relative growth of Spartina patens (Ait.) Muhl. and Scirpus olneyi gray occurring in a mixed stand as affected by salinity and flooding depth. Wetlands 15: 20–30.
Brose, U., and K. Tielbörger. 2005. Subtle differences in environmental stress along a flooding gradient affect the importance of inter-specific competition in an annual plant community. Plant Ecology 178: 51–59.
Cahoon, D.R., K.L. McKee, and J.T. Morris. 2020. How plants influence resilience of salt marsh and mangrove wetlands to sea-level rise. Estuaries and Coasts 44: 883–898.
Callaway, R.M. 1995. Positive interactions among plants. The Botanical Review 61: 306–349.
Callaway, R.M., and L. King. 1996. Temperature-driven variation in substrate oxygenation and the balance of competition and facilitation. Ecology 77: 1189–1195.
Chen, Y., Y. Li, C. Thompson, X. Wang, T. Cai, and Y. Chang. 2018. Differential sediment trapping abilities of mangrove and saltmarsh vegetation in a subtropical estuary. Geomorphology 318: 270–282.
Cherry, J.A., K.L. McKee, and J.B. Grace. 2009. Elevated CO2 enhances biological contributions to elevation change in coastal wetlands by offsetting stressors associated with sea-level rise. Journal of Ecology 97: 67–77.
Cott, G.M., J.S. Caplan, and T.J. Mozdzer. 2018. Nitrogen uptake kinetics and saltmarsh plant responses to global change. Scientific Reports 8: 1–10.
Cui, B.-S., Q. He, and Y. An. 2011. Community structure and abiotic determinants of salt marsh plant zonation vary across topographic gradients. Estuaries and Coasts 34: 459–469.
Drake, B.G. 2014. Rising sea level, temperature, and precipitation impact plant and ecosystem responses to elevated CO2 on a Chesapeake Bay wetland: Review of a 28-year study. Global Change Biology 20: 3329–3343.
Drake, B.G., P.W. Leadley, W.J. Arp, D. Nassiry, and P.S. Curtis. 1989. An open top chamber for field studies of elevated atmospheric CO2 concentration on saltmarsh vegetation. Functional Ecology 3: 363–371.
Drake, B.G., H.H. Rogers, and L.H. Allen. 1985. Methods of exposing plants to elevated carbon dioxide. In B. R. Strain and J. D. Cure (Eds.), Direct effects of increasing carbon dioxide on vegetation (pp. 11–31). DOE/ER-0238. U.S. Department of Energy, Washington, DC.
Emery, N.C., P.J. Ewanchuk, and M.D. Bertness. 2001. Competition and salt-marsh plant zonation: Stress tolerators may be dominant competitors. Ecology 82: 2471–2485.
Erickson, J.E., J.P. Megonigal, G. Peresta, and B.G. Drake. 2007. Salinity and sea level mediate elevated CO2 effects on C3–C4 plant interactions and tissue nitrogen in a Chesapeake Bay tidal wetland. Global Change Biology 13: 202–215.
Grace, J.B. 1993. The effects of habitat productivity on competition intensity. Trends in Ecology & Evolution 8: 229–230.
Hess, T.J. 1975. An evaluation of methods for managing stands of Scirpus olneyi. MS thesis. Louisiana State University, Baton Rouge.
Holmquist, J.R., L. Schile-Beers, K. Buffington, M. Lu, T.J. Mozdzer, J. Riera, D.E. Weller, M. Williams, and J.P. Megonigal. 2021. Scalability and performance tradeoffs in quantifying relationships between elevation and tidal wetland plant communities. Marine Ecology Progress Series 666: 57–72.
Keller, J.K., A.A. Wolf, P.B. Weisenhorn, B.G. Drake, and J.P. Megonigal. 2009. Elevated CO2 affects porewater chemistry in a brackish marsh. Biogeochemistry 96: 101–117.
Kirwan, M.L., and G.R. Guntenspergen. 2012. Feedbacks between inundation, root production, and shoot growth in a rapidly submerging brackish marsh. Journal of Ecology 100: 764–770.
Kirwan, M.L., and G.R. Guntenspergen. 2015. Response of plant productivity to experimental flooding in a stable and a submerging marsh. Ecosystems 18: 903–913.
Konisky, R.A., and D.M. Burdick. 2004. Effects of stressors on invasive and halophytic plants of New England salt marshes: A framework for predicting response to tidal restoration. Wetlands 24: 434–447.
Krauss, K.W., J.A. Allen, and D.R. Cahoon. 2003. Differential rates of vertical accretion and elevation change among aerial root types in Micronesian mangrove forests. Estuarine, Coastal and Shelf Science 56: 251–259.
Langley, J.A., and B.A. Hungate. 2014. Plant community feedbacks and long-term ecosystem responses to multi-factored global change. AoB Plants 6.
Langley, J.A., and J.P. Megonigal. 2010. Ecosystem response to elevated CO2 levels limited by nitrogen-induced plant species shift. Nature 466: 96–99.
Langley, J.A., T.J. Mozdzer, K.A. Shepard, S.B. Hagerty, and P.J. Megonigal. 2013. Tidal marsh plant responses to elevated CO2, nitrogen fertilization, and sea level rise. Global Change Biology 19: 1495–1503.
Langston, A.K., O. Durán Vinent, E.R. Herbert, and M.L. Kirwan. 2020. Modeling long-term salt marsh response to sea level rise in the sediment-deficient Plum Island Estuary, MA. Limnology and Oceanography 65: 2142–2157.
Levine, J.M., J.S. Brewer, and M.D. Bertness. 1998. Nutrients, competition and plant zonation in a New England salt marsh. Journal of Ecology 86: 285–292.
Lortie, C.J., and R.M. Callaway. 2006. Re-analysis of meta-analysis: Support for the stress-gradient hypothesis. Journal of Ecology 94: 7–16.
Lu, M., J.S. Caplan, J.D. Bakker, T.J. Mozdzer, B.G. Drake, J.P. Megonigal, and J.A. Langley. 2016. Allometry data and equations for coastal marsh plants. Ecology 97: 3554–3554.
Luo, W., Y. Xie, X. Chen, F. Li, and X. Qin. 2010. Competition and facilitation in three marsh plants in response to a water-level gradient. Wetlands 30: 525–530.
Maestre, F.T., R.M. Callaway, F. Valladares, and C.J. Lortie. 2009. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. Journal of Ecology 97: 199–205.
Marani, M., S. Lanzoni, S. Silvestri, and A. Rinaldo. 2004. Tidal landforms, patterns of halophytic vegetation and the fate of the lagoon of Venice. Journal of Marine Systems 51: 191–210.
McIntire, E.J., and A. Fajardo. 2014. Facilitation as a ubiquitous driver of biodiversity. New Phytologist 201: 403–416.
Morris, J.T. 2006. Competition among marsh macrophytes by means of geomorphological displacement in the intertidal zone. Estuarine, Coastal and Shelf Science 69: 395–402.
Morris, J.T., P.V. Sundareshwar, C.T. Nietch, B. Kjerfve, and D.R. Cahoon. 2002. Responses of coastal wetlands to rising sea level. Ecology 83: 2869–2877.
Mueller, P., K. Jensen, and J.P. Megonigal. 2016. Plants mediate soil organic matter decomposition in response to sea level rise. Global Change Biology 22: 404–414.
Nicks, D. 2018. Response of S. patens and D. spicata productivity to experimental sea-level rise. MS Thesis, The College of William & Mary, Williamsburg, Virginia.
Peng, D., L. Chen, S.C. Pennings, and Y. Zhang. 2018. Using a marsh organ to predict future plant communities in a Chinese estuary invaded by an exotic grass and mangrove. Limnology and Oceanography 63: 2595–2605.
Pennings, S.C., and R.M. Callaway. 1992. Salt marsh plant zonation: The relative importance of competition and physical factors. Ecology 73: 681–690.
Pennings, S.C., M.-B. Grant, and M.D. Bertness. 2005. Plant zonation in low-latitude salt marshes: Disentangling the roles of flooding, salinity and competition. Journal of Ecology 93: 159–167.
Reid, J.N. 2013. C3 and C4 plant competition in a mid-Atlantic tidal marsh under current and increased nitrogen concentrations. M.S. Thesis, Villanova University, Villanova, PA.
Schile, L.M., J.C. Callaway, K.N. Suding, and N.M. Kelly. 2017. Can community structure track sea-level rise? Stress and competitive controls in tidal wetlands. Ecology and Evolution 7: 1276–1285.
Silvestri, S., A. Defina, and M. Marani. 2005. Tidal regime, salinity and salt marsh plant zonation. Estuarine, Coastal and Shelf Science 62: 119–130.
Swanson, K.M., J.Z. Drexler, C.C. Fuller, and D.H. Schoellhamer. 2015. Modeling tidal freshwater marsh sustainability in the Sacramento–San Joaquin Delta under a broad suite of potential future scenarios. San Francisco Estuary and Watershed Science 13.
Valiela, I., J.M. Teal, and W.G. Deuser. 1978. The nature of growth forms in the salt marsh grass Spartina alterniflora. The American Naturalist 112: 461–470.
Voss, C.M., R.R. Christian, and J.T. Morris. 2013. Marsh macrophyte responses to inundation anticipate impacts of sea-level rise and indicate ongoing drowning of North Carolina marshes. Marine Biology 160: 181–194.
Watson, E.B., C. Wigand, E.W. Davey, H.M. Andrews, J. Bishop, and K.B. Raposa. 2017. wetland loss patterns and inundation-productivity relationships prognosticate widespread salt marsh loss for southern New England. Estuaries and Coasts 40: 662–681.
White, K.P., J.A. Langley, D.R. Cahoon, and J.P. Megonigal. 2012. C3 and C4 biomass allocation responses to elevated CO2 and nitrogen: Contrasting resource capture strategies. Estuaries and Coasts 35: 1028–1035.
Wigand, C., K. Sundberg, A. Hanson, E. Davey, R. Johnson, E. Watson, and J. Morris. 2016. Varying inundation regimes differentially affect natural and sand-amended marsh sediments. PLoS One 11.
Acknowledgements
This work was supported the NSF LTREB Program (DEB-0950080, DEB-1457100, DEB-1557009, and DEB-2051343) and the Smithsonian Environmental Research Center. J. Gabriel was supported by the Villanova University Research Fellows Program. J. Reid was supported by a Villanova Graduate fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Scott Warren
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gabriel, J.R., Reid, J., Wang, L. et al. Interspecific Competition is Prevalent and Stabilizes Plant Production in a Brackish Marsh Facing Sea Level Rise. Estuaries and Coasts 45, 1646–1655 (2022). https://doi.org/10.1007/s12237-021-01043-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-021-01043-9