Abstract
The occurrence of low dissolved oxygen (hypoxia) in coastal waters may alter trophic interactions within the water column. This study identified a threshold at which hypoxia in the northern Gulf of Mexico (NGOMEX) alters composition of fish catch and diet composition (stomach contents) of fishes using fish trawl data from summers 2006–2008. Hypoxia in the NGOMEX impacted fish catch per unit effort (CPUE) and diet below dissolved oxygen thresholds of 1.15 mg L−1 (for fish CPUE) and 1.71 mg L−1 (for diet). CPUE of many fish species was lower at hypoxic sites (≤ 1.15 mg L −1) as compared to normoxic regions (> 1.15 mg L −1), including the key recreational or commercial fish species Atlantic croaker Micropogonias undulatus and red snapper Lutjanus campechanus. Overall, fish diets from hypoxic sites (≤ 1.71 mg L−1) and normoxic sites (> 1.71 mg L−1) differed. Fish caught in normoxic regions consumed a greater mass of benthic prey (ex. gastropods, polychaetes) than fish caught in hypoxic regions. Hypoxia may increase predation risk of small zooplankton, with observations of increased mass of small zooplankton in fish stomachs when bottom hypoxia was present. Changes in contributions of small zooplankton and benthic prey to fish diet in hypoxic areas may alter energy flow in the NGOMEX pelagic food web and should be considered in fishery management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seasonal bottom hypoxia has emerged as one of the major global problems in freshwater, estuarine, and coastal marine ecosystems (Diaz and Rosenberg 2008). One of the most widely known reoccurring summer hypoxic zones exists in the northern Gulf of Mexico (NGOMEX). The NGOMEX hypoxic zone is one of the largest areas of coastal hypoxia identified worldwide, with an area exceeding 20,000 km2 in some years (Turner et al. 2008; Bianchi et al. 2010). The occurrence and size of the zone is a result of high nutrient loading from the heavily agricultural Mississippi River watershed (Turner et al. 2008; Bianchi et al. 2010). The effects of hypoxia on NGOMEX living resources are of particular interest, not only because of the extent of hypoxia, but also given the economic importance of this region for commercial and recreational fishing, and the efforts and costs to control the size of the hypoxic zone through landscape/watershed management and nutrient reduction (Rabotyagov et al. 2014).
Effects of hypoxia on fish may occur through direct and indirect processes including changes in spatial distributions (Ludsin et al. 2009), reproduction and recruitment (Shang and Wu 2004; Thomas and Rahman 2012), vital rates (e.g., growth and mortality), and increased susceptibility to other stressors (Breitburg et al. 2009). Examples of direct effects include reduced fish catch per unit effort (CPUE) in the Chesapeake Bay (Buchheister et al. 2013) and in the northeast Pacific (Hughes et al. 2015) and increased CPUE of menhaden in the NGOMEX (Langseth et al. 2014). Reductions in abundance of sensitive fish species occur due to fish kills (Thronson and Quigg 2008) or changes in the spatial distribution, with mobile species avoiding low oxygen waters, but occurring above or congregating at the horizontal edges of hypoxic regions (Craig and Crowder 2005; Hazen et al. 2009; Ludsin et al. 2009; Zhang et al. 2009). Concentration of fish in small pockets of suitable habitat, or changes in fish behavior due to hypoxia, may increase fish CPUE by rendering fish more susceptible to fishing gear (Breitburg et al. 2009; Langseth et al. 2014).
Hypoxia-induced changes in food webs result from shifts in the abundance and spatial distribution of lower trophic levels (Breitburg et al. 1997; Ekau et al. 2010; Roman et al. 2019). Hypoxia is associated with high zooplankton mortality and low zooplankton biomass (Kimmel et al. 2009). Sensitive species include some commonly found in the NGOMEX such as the copepods Acartia tonsa (Elliott et al. 2013), Centropages hamatus (Stalder and Marcus 1997), Paracalanus sp., and Oithona sp. (Zhang and Wong 2011). Small zooplankton in particular may be more susceptible to low oxygen; in the Chesapeake Bay, hypoxia has been associated with zooplankton communities composed of large individuals (Kimmel et al. 2009).
In addition to the direct effects of hypoxia on specific taxa, hypoxia can alter trophic interactions by affecting predator or prey escape/capture responses. For example, hypoxia decreases prey escape response and increases efficiency of capture by predators (Breitburg et al. 1997; Decker et al. 2004; Domenici et al. 2007); hypoxia can also reduce (Keister et al. 2000; Taylor and Rand 2003; Ludsin et al. 2009) or increase (Prince and Goodyear 2006; Costantini et al. 2008) the spatial overlap between predators and prey. Observations of hypoxia-related changes in the spatial distribution of fish, the vertical distribution of zooplankton, and the size structure of the zooplankton community (Zhang et al. 2009; Kimmel et al. 2010; Roman et al. 2012) all suggest that altered trophic interactions are an important ecological consequence of hypoxia for zooplankton and their predators in the NGOMEX.
The relationship between hypoxia and trophic dynamics (e.g., zooplanktivory, benthivory, and piscivory) remains largely hypothetical (Costantini et al. 2008; Arend et al. 2011; Brandt et al. 2011; Zhang et al. 2014). Many studies have documented diets of NGOMEX species (Sutton and Hopkins 1996; Bethea et al. 2007; Wells et al. 2008), but few estimate how fish diet may be altered in hypoxic areas (though see Aku and Tonn 1999; Pothoven et al. 2009). Increasingly, simulations and modeling studies that incorporate several components of the marine food web have been used to examine increases or decreases in fisheries production under various scenarios of hypoxia severity (de Mutsert et al. 2016; Rose et al. 2018). However, these studies are limited by the available knowledge of fish diet, especially for some common forage species such as Atlantic bumper Chloroscombrus chrysurus (Glaspie et al. 2018).
Assessing thresholds of dissolved oxygen at which sublethal or lethal effects occur for a particular species or community of organisms is essential to manage marine systems experiencing hypoxia. This information can be used to predict when fisheries will fail (Renaud 1986) or to set targets to avoid mortality of fish and invertebrates (Vaquer-Sunyer and Duarte 2008). In the literature, hypoxia thresholds typically refer to bottom dissolved oxygen levels ≤ 2 mg L−1 (Renaud 1986). However, in laboratory studies, the median lethal oxygen concentration (LC50) for major groups of marine organisms varies from 0.89 (gastropods) to 2.45 mg L−1 (crustaceans) (Vaquer-Sunyer and Duarte 2008). This indicates that thresholds other than 2 mg L−1 may be more meaningful for fish and invertebrate communities. Few studies have examined hypoxia thresholds in situ (through see Eby and Crowder 2002). Alternative hypoxia thresholds for fish species in the NGOMEX have not been assessed, nor have thresholds incorporating hypoxia-related changes in diet composition. An improved ability to understand how hypoxia influences foraging interactions between fish and zooplankton in the NGOMEX should generally benefit our ability to model and forecast the long-term consequences of hypoxia on pelagic fish populations and fisheries productivity, which has thus far remained elusive in nearly all ecosystems (Rose et al. 2004; Breitburg et al. 2009; Hazen et al. 2009). Here, we describe changes in the diets of fish and the structure of the pelagic food web relative to the occurrence of hypoxia in the NGOMEX.
Water column dissolved oxygen, fish CPUE and spatial distribution, and fish diet composition data collected in the NGOMEX during 2006–2008 were used to identify thresholds of bottom DO below which fish CPUE and diet (stomach contents) were altered. Hypoxia thresholds were then used to (1) examine the effects of hypoxia on fish CPUE and (2) determine if diet composition differs for fish caught in normoxic and hypoxic areas for zooplanktivorous, benthivorous, and piscivorous fish.
Methods
Sample Collection
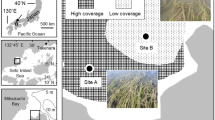
Samples were collected from the NGOMEX aboard the R/V Pelican (Louisiana Universities Marine Consortium, LUMCOM), with cruises on August 4–13, 2006, July 30–October 14, 2007, and August 1–August 11, 2008 (Fig. 1). Physical properties of the water column, including temperature and dissolved oxygen, were measured with a CTD (Seabird SBE 9 with a SBE 43 dissolved oxygen probe).
Fish were collected using a bottom trawl (7.62 m head-rope, 3.66 m mouth depth; 38 mm stretch mesh; 12 mm cod-end liner) or a mid-water trawl (9.14 m wide, 6.10 m tall, 12 mm cod-end liner). Trawling occurred day and night, and trawl duration varied between 10 and 60 min to ensure adequate collection of fish. After capture, fish were identified, counted, and frozen at − 20 °C. Because the bottom trawl net was not opening and closing, resulting samples may have included fish from higher in the water column that would have been captured during net deployment and retrieval. Trawl times used to calculate an index of fish abundance, catch per unit effort (CPUE, number of fish min−1), reflected only the amount of time the bottom trawl was on the shelf bottom or at mid-water targeted depth.
To determine fish diet composition, a minimum of 15 non-empty fish stomachs per species were analyzed from each trawl station whenever possible. Fish were thawed and total length (TL) measured to the nearest 1 mm. Stomachs were removed and dissected under a microscope. Fish were dried in a drying oven and weighed to the nearest 0.0001 g dry mass. All zooplankton in stomachs were identified to the lowest possible taxon and counted using a dissecting microscope. A minimum of 50 individuals in each taxon were digitized and measured to the nearest 0.01 mm with ImagePro Plus (Media Cybernetics, Inc. Silver Spring, MD). Partial animals were counted as individuals, but not measured for length. Lengths of zooplankton were converted to dry mass using relationships reported in the literature (Fontaine and Neal 1971; Uye 1982; Cadman and Weinstein 1985; Chisholm and Roff 1990; Webber and Roff 1995; Hopcroft et al. 1998; Tita et al. 1999; Ara 2001; Remsen et al. 2004; Rose et al. 2004). Mean dry mass of individuals in each category was multiplied by the total number to calculate the total dry mass for each prey category. Dry mass of stomach contents was divided by fish total dry mass (g) to account for differences in fish size, and thus stomach capacity.
Due to the wide range of prey species examined, fish species were classified as zooplanktivores, benthivores, or piscivores for analysis. We used k-means clustering with the Hartigan–Wong algorithm (Hartigan and Wong 1979) to partition the species into three groups based on an 8 column matrix summarizing their stomach contents. Each prey item in stomach contents was categorized as either small zooplankton (≤ 5 μg mean dry mass), large zooplankton (> 5 μg mean dry mass), benthic organisms, or mobile prey (fish, shrimp, and squid). The first four columns of the stomach content matrix were the proportion of prey in each category (mean mass from each category divided by mean total mass). The next four columns were the frequency of occurrence of each category. The bootstrapped Jaccard coefficient was calculated to assess fit of the k-means groupings (Hennig 2007). K-means clustering was only completed on taxa for which at least 10 fish were processed in both normoxic and hypoxic areas, which included 14 fish species (Table 1). Clustering identified one grouping of six species that consumed small and large zooplankton more frequently than the other fish species analyzed, and this group was considered zooplanktivorous (Jaccard coefficient 0.89); one grouping of five species that frequently consumed benthic prey, but did not often consume zooplankton or fish, and this group was considered benthivorous (Jaccard coefficient 0.81); and one grouping of three species that frequently consumed fish or highly mobile prey which was considered piscivorous (Jaccard coefficient 0.77) (Table 1). These clusters were considered stable (Jaccard coefficient 0.75–0.84) or highly stable (0.85–1.00) (Hennig 2008).
Threshold Analysis
Hypoxic sampling stations were identified using Threshold Indicator Taxa Analysis (TITAN) of stomach contents and bottom DO (Baker and King 2010). TITAN identified thresholds in community data by combining change-point analysis (nCPA) with indicator species analysis. For each taxon in a community, the analysis produced a score (IndVal) estimating the association of the taxon to two groups separated at candidate change points (xi) along a gradient of a univariate indicator variable, x (bottom DO). The IndVal for each taxon was standardized as a z-score and the sum of z-scores for all taxa, sum(z), was calculated. The value of x that maximized sum(z) was identified as a community-level change point, xcp. Bootstrapped 95% confidence intervals for xcp are calculated by resampling the observations (sampled with replacement, to create a bootstrap sample the same size as the original dataset) 500 times.
We conducted TITAN analysis to identify thresholds (change points) in (1) fish CPUE and (2) fish stomach contents. CPUE was calculated for each fish species caught in each trawl. Fish stomach contents were calculated as the mean mass of each taxon found in stomach contents, divided by fish total dry mass (g), and averaged across all fish caught in each trawl. Only fish taxa or stomach content taxa that appeared in ≥ 10 trawls were used to complete this analysis.
Diet Composition Analysis
To test if diet composition differed between hypoxic and normoxic areas, stomach content composition data were analyzed using PERMANOVA (Anderson 2008). The community-level change point xcp (threshold) for fish stomach contents was used to assign the category “hypoxic” or “normoxic” to each trawl, depending on the bottom DO at each trawl location, as determined from CTD data. PERMANOVA analysis included only taxa for which at least 10 fish were processed in both normoxic and hypoxic areas (Table 1). The PERMANOVA was completed using Bray Curtis dissimilarity matrices calculated from fourth-root transformed biomass (Anderson 2014), and models had the following factors: dissolved oxygen (two levels: normoxic and hypoxic), time of day the sample was collected (two levels: day and night), diet classification (three levels: zooplanktivore, benthivore, and piscivore), species (14 levels), and year (3 levels). Bottom temperature was included as a covariable. If multiple individuals of the same species were captured in a single trawl, the mean prey biomass for all fish of that species in the trawl was used to avoid pseudo-replication.
Effects of spatial variability on the PERMANOVA results were examined by running two additional PERMANOVA models: the first included the sampling site latitude and longitude (normalized using z-score transformation; Anderson 2005) as covariables, and the second included latitude/longitude and sampling day nested within year (assuming trawls taken on the same day were more closely related, both in space and time). Repeating the analysis with covariables generated very similar results to those obtained from the original PERMANOVA model, and only results from the original model are shown here (Benedetti-Cecchi and Osio 2007).
For all two-group comparisons, 95% confidence intervals were calculated using non-parametric bootstrap hypothesis testing with 10,000 simulations (DiCiccio and Efron 1996). All analyses were completed in R (R Core Team 2019). All data and code for this study have been archived and can be found at https://doi.org/10.5063/F10Z71M7.
Results
Threshold Analysis
A threshold in fish community composition was identified at bottom DO 1.15 mg L−1 (95% CI [0.99, 3.24]). A threshold in diet composition was identified at bottom DO 1.71 mg L−1 (95% CI [0.98, 3.33]). A threshold of 1.15 mg L−1 was used to categorize bottom DO as “hypoxic” or “normoxic” for analysis of fish CPUE, and a threshold of 1.71 mg L−1 was used to categorize bottom DO as “hypoxic” or “normoxic” for analysis of fish diet.
Hypoxia (≤ 1.71 mg L −1) was extensive throughout the study period; across all 3 years, 29% of sites were hypoxic, and bottom water dissolved oxygen ranged from 0.0 to 5.7 mg L−1 (Fig. 1). Mean bottom dissolved oxygen was 2.6 mg L−1 (S.D. 1.2) in 2006, 2.8 mg L−1 (S.D. 0.9) in 2007, and 1.8 mg L−1 (S.D. 1.5) in 2008. Bottom temperature ranged from 20.6 to 31.4 °C.
Fish CPUE
The final dataset consisted of fish collected from n = 91 trawls over 1707 min in regions identified as normoxic (> 1.15 mg L −1), and fish collected from n = 46 trawls over 943 min in regions identified as hypoxic (≤ 1.15 mg L−1) (Fig. 1; Table 1). We found differences in fish species composition and catch statistics between normoxic and hypoxic areas of the NGOMEX (Fig. 2). The most abundant fish (in terms of CPUE) in both normoxic and hypoxic regions were striped anchovy Anchoa hepsetus, Atlantic bumper C. chrysurus, sand seatrout Cynoscion arenarius, Atlantic croaker Micropogonias undulatus, and Atlantic cutlassfish Trichiurus leptutus (Fig. 2). The CPUE of many species was lower in hypoxic than in normoxic regions, including red snapper Lutjanus campechanus, Gulf butterfish Peprilus burti, M. undulatus, longspine porgy Stenotomus caprinus, bay anchovy Anchoa mitchelli, gray triggerfish Balistes capriscus, dwarf sand perch Diplectrum bivittatum, pinfish Lagodon rhomboides, lane snapper Lutjanus synagris, Atlantic thread herring Opisthonema oglinum, Spanish sardine Sardinella aurita, and least puffer Sphoeroides parvus (Fig. 2). The CPUE of all species listed in Table 1 can be found in Supplementary Fig. 1.
Diet Composition Analysis
Zooplankton in stomach contents included large zooplankton > 5 μg mean dry mass, such as Acartia sp., Centropages sp.; Eucalanus sp.; Temora sp.; cladocerans such as Evadne sp., Penilia sp., and Podon sp.; other calanoids such as Clausocalanus sp., Labidocera sp., Pseudodiaptomus sp., Undinula sp., Euchaeta sp., and Pontella sp.; barnacle larvae; crab larvae; fish larvae; shrimp larvae; and urochordates. Small zooplankton < 5 μg mean dry mass in fish diets included Corycaeus sp., Oithona sp., Oncaea sp., Paracalanus sp., Saphirella sp., copepod nauplii, and harpacticoid copepods. Benthic organisms found in fish diets included amphipods, bivalves, crabs, cumaceans, echinoderms, gastropods, isopods, mantis shrimp, nematodes, oligochaetes, ostracods, polychaetes, and tanaids. Large, mobile prey, such as fish and squid, was also found in fish diets.
Large zooplankton made up a major portion of the diet for most species (Fig. 3). The most commonly found large zooplankton species in fish diets were shrimp larvae (found in 24% of fish stomachs, mean 1001 μg g−1 fish dry weight), Temora sp. (12% of stomachs, mean 5 μg g−1 fish dry weight), other calanoids (10% of stomachs, mean 20 μg g−1 fish dry weight), and Centropages sp. (10% of stomachs, mean 6 μg g−1 fish dry weight). Other common prey items were benthic organisms (Fig. 3). The most commonly found benthic species were nematodes (21% of fish stomachs, mean 24 μg g−1 fish dry weight), polychaetes (15% of stomachs, mean 203 μg g−1 fish dry weight), and gastropods (14% of stomachs, mean 31 μg g−1 fish dry weight). Large, mobile prey made up a substantial component of the diet for a few species, including C. arenarius, L. campechanus, and T. lepturus (Fig. 3). The most commonly found large, mobile prey groups were fish (7% of fish stomachs, mean 872 μg g−1 fish dry weight) and squid (2% of stomachs, mean 263 μg g−1 fish dry weight). Small zooplankton made up a smaller component of the diets of most fish species, although several zooplankton taxa were commonly found in fish diets, including Corycaeus sp. (17% of stomachs, mean 12 μg g−1 fish dry weight), Paracalanus sp. (12% of stomachs, mean 11 μg g−1 fish dry weight), harpacticoid copepods (12% of stomachs, mean 2 μg g−1 fish dry weight), and Oncaea sp. (10% of stomachs, mean 5 μg g−1 fish dry weight). Means reported are for all fish, not just those that had prey in stomachs. The diet of all species listed in Table 1 can be found in Supplementary Fig. 2.
Diet composition for six zooplanktivorous fish species (a–f), five benthivorous fish species (g–k), and three piscivorous fish species (l–n) from samples taken in the northern Gulf of Mexico during summer of 2006–2008. Numbers above bars represent the total fish stomachs processed for that species, in either normoxic (Norm.) or hypoxic (Hyp.) regions. Full species names can be found in Table 1
An interaction between dissolved oxygen and diet class was identified with PERMANOVA (Table 2). A post hoc test was completed to interpret the main effect of dissolved oxygen separately for each diet class. We conducted separate PERMANOVA analyses for zooplanktivores, benthivores, and piscivores. Dissolved oxygen was a significant variable for zooplanktivores (F1,138 = 8.75, p = 0.001; Supplementary Table 1), and benthivores (F1,90 = 2.36, p = 0.03; Supplementary Table 2), but not for piscivores (F1,71 = 1.53, p = 0.20; Supplementary Table 3). For zooplanktivores, greater mass of some small zooplankton taxa (Oithona sp., Paracalanus sp.) was found in the stomachs of fish caught in hypoxic areas, as compared to normoxic areas (Fig. 4). The mass of many other prey taxa was greater in the stomachs of zooplanktivorous fish caught in normoxic areas as compared to those caught in hypoxic areas, including crab larvae, urochordates, amphipods, mantis shrimp, and ostracods (Fig. 4). Several prey taxa had greater mass in the stomachs of benthivores caught in normoxic areas, as compared to hypoxic areas, including Paracalanus sp., Eucalanus sp., amphipods, gastropods, and polychaetes (Fig. 4). There were few oxygen-related differences in the mass of prey found in stomachs of piscivores, though a greater mass of polychaetes was found in the stomachs of piscivorous fish from normoxic regions, as compared to hypoxic regions (Fig. 4). Compared to fish diets in hypoxic areas, there was a tendency for fish from all diet classes to consume greater mass of squid in normoxic areas, and for benthivores to consume greater mass of fish in normoxic areas (Fig. 4).
Fourth-root transformed mean mass of prey in stomachs of fish from hypoxic (closed circles) and normoxic (open triangles) regions in the northern Gulf of Mexico. Means are calculated for zooplanktivores (top), benthivores (middle), and piscivores (bottom) for all fish species included in PERMANOVA analysis (taxa for which at least 10 fish were processed in both normoxic and hypoxic areas). If multiple individuals of the same species were captured in a single trawl, the mean prey biomass for all fish of that species in the trawl was used and the means presented were not weighted by the number of fish caught in each trawl
Discussion
Community Thresholds
This study examined changes in fish diet composition and fish community composition relative to the occurrence of hypoxia in the NGOMEX. Hypoxia in the NGOMEX was associated with changes in fish catch per unit effort (CPUE) and diet below dissolved oxygen thresholds of 1.15 mg L−1 (for fish CPUE) and 1.71 mg L−1 (for fish diet). The dissolved oxygen threshold for fish diet composition was higher than the threshold for fish catch. As dissolved oxygen levels decline in the NGOMEX (especially off the coast of Texas, Karnauskas et al. 2017), changes in the fish diet can be expected to occur before changes in the fish community occur. Thus, changes in trophic transfer are a likely consequence of NGOMEX hypoxia.
The thresholds for fish CPUE and fish diet in the NGOMEX were below the traditional threshold used to identify hypoxia (2 mg L−1). Studies using a threshold of 2 mg L−1 may be missing the potential impacts of hypoxia on the fish community and food web in this system. Many species may forage near their metabolic limits at the hypoxic boundary, creating a hypoxic “edge effect” (Zhang et al. 2009). It is important to consider the possibility that community-level hypoxia thresholds in the NGOMEX may be lower than in other systems.
Fish CPUE
The relationship between hypoxia and pelagic fish catch is not well-understood, and few studies have related fish catch to hypoxia in other systems (Buchheister et al. 2013; Hughes et al. 2015). To our knowledge, this study is the first to relate catch of a suite of both demersal and pelagic fish species to dissolved oxygen in the NGOMEX. The second objective of this study was to examine the effects of hypoxia on fish CPUE in the NGOMEX. In agreement with published literature (Buchheister et al. 2013; Hughes et al. 2015), our results indicate that local fish catch is reduced when hypoxia is present in the NGOMEX.
Hypoxia-related decreases in catch may have implications for fisheries management and conservation. Key commercial or recreational species were caught less often in hypoxic areas, including Atlantic croaker and red snapper. Over 1 million Atlantic croaker are harvested annually in the recreational fishery (NMFS 2017a). The red snapper fishery was worth nearly $28 million in 2017 (NMFS 2018). Some of the species relatively absent from hypoxic areas, contributing to the lower catch in those areas, are also species of concern. For example, both red snapper and gray triggerfish have been identified as overfished species (NMFS 2017b). Gray triggerfish were rarely caught in trawls in hypoxic areas. Several species that had lower CPUE in hypoxic regions, including Gulf butterfish, bay anchovy, Atlantic thread herring, and Spanish sardine, are also key prey species for large predators in the NGOMEX (Manooch and Hogarth 1983; Meyer and Franks 1996; Hoffmayer and Parsons 2003). Given the importance of species such as red snapper to the economy of the NGOMEX, future research on the relationship between hypoxia, local displacements of fish, and population-level fishery trends or catches is needed.
CPUE is often used as an index of abundance for fish populations, but CPUE is not necessarily proportional to local abundance. CPUE depends upon catchability, which can increase when fish are aggregated along the edge of the hypoxic zone (Breitburg et al. 2009; Craig 2012; Langseth et al. 2014), increasing CPUE without an increase in local abundance. Finally, even if CPUE and local abundance are proportional, we are unable to determine a mechanism for any changes in fish abundance. Possible mechanisms for any decline in CPUE may include mortality (direct or due to predation; Thronson and Quigg 2008), vertical or horizontal migration (Craig and Crowder 2005; Hazen et al. 2009; Ludsin et al. 2009; Zhang et al. 2009), or changes in reproduction or recruitment (Shang and Wu 2004; Thomas and Rahman 2012).
Diet Composition
The third objective of this study was to determine if diet composition differs for fish caught in normoxic and hypoxic areas for zooplanktivores, benthivores, and piscivores. There was a significant impact of hypoxia on fish diet composition for both zooplanktivores and benthivores. Fish caught in hypoxic areas consumed less mass of large, mobile prey such as fish (for benthivores) and squid (a tendency for all diet classes). This result can be explained by a distribution shift in mobile prey when hypoxia is present. Squid in particular are known to be sensitive to hypoxia (Zielinski et al. 2000) and likely avoid hypoxic conditions. Small forage fishes such as juvenile anchovies also avoid hypoxic waters (Taylor et al. 2007).
Hypoxia may result in increased predation risk for small zooplankton, since zooplanktivores in hypoxic areas consumed greater mass of small zooplankton than those in normoxic areas. In the Chesapeake Bay, there was lower biomass of small zooplankton in hypoxic bottom water than in normoxic surface water, indicating a possible vertical distribution shift, and most (> 60%) of the zooplankton that were in hypoxic waters were dead (Kimmel et al. 2009). Zooplankton moving out of the hypoxic waters may aggregate on the edges of the hypoxic zone (Craig and Crowder 2005; Hazen et al. 2009; Zhang et al. 2009), bringing dense concentrations of zooplankton in contact with their predators. Several studies have suggested that hypoxia (and subsequent habitat compression) of zooplankton may lead to increased predation by fish (Vanderploeg et al. 2009a; Vanderploeg et al. 2009b; H. Zhang et al. 2009; Brandt et al. 2011; Roman et al. 2012). A zooplankton distribution shift likely brings predators in closer contact with zooplankton prey, and this effect may increase habitat quality for fish foraging in the surface waters of the hypoxic zone.
Fish in hypoxic areas consumed less benthic prey such as gastropods and polychaetes. Previous studies in the Chesapeake Bay suggest that when hypoxia is intermittent or moderate, some fish species consume more benthic prey because benthic organisms such as clams and polychaetes have been shown to reduce burial depth under hypoxia, making them more susceptible to predation (Pihl et al. 1992; Long et al. 2008; Long and Seitz 2008). The low mass of benthic prey consumed by fish in hypoxic areas of the NGOMEX may be a result of fish avoiding hypoxic bottom waters, as fish are rarely seen at oxygen concentrations below 2 mg L−1 (Rabalais and Turner 2001). However, fish are known to conduct foraging forays into hypoxic bottom waters in many systems (Pihl et al. 1992; Rahel and Nutzman 1994; Roberts et al. 2009). Future research should focus on the availability of benthic prey resources in the NGOMEX hypoxic zone, and the likelihood that some of the common fish species are able to forage on benthic organisms for short periods under hypoxic conditions.
Understanding how hypoxia might affect NGOMEX food web dynamics can help inform ecosystem models and help agencies understand and predict how this ecologically and economically important region might change with various hypoxia management scenarios. The results of this study suggest hypoxia may alter food web dynamics and trophic transfer in the NGOMEX by decreasing fish CPUE and modifying fish diets. Increased consumption of zooplankton by fish may increase flow of energy to upper trophic levels in hypoxic regions, fueling increased growth and reproduction. Habitat compression of prey has been credited for increasing body size of marlin and sailfish (Prince and Goodyear 2006) and providing an opportunity for population growth in Chesapeake Bay striped bass Morone saxatilis (Costantini et al. 2008). In contrast, lower CPUE of many small forage fish, along with decreased consumption of fish, squid, and benthic organisms, may prevent nutrients from making their way to higher consumers. This may alter food web structure in the NGOMEX, for example through increased dominance by gelatinous zooplankton (Breitburg et al. 2003). The consequences of hypoxia and altered food web interactions remain speculative and have only been explored in modeling studies and small-scale laboratory studies (Breitburg et al. 1997; Brandt and Mason 2003; Breitburg et al. 2003; Brandt et al. 2011; Zhang et al. 2014; de Mutsert et al. 2016); thus, further investigation into how localized shifts in distribution, growth, and diets affect short-term or long-term growth in fish is warranted.
Management of NGOMEX fisheries in an ecosystem context requires ecosystem models that incorporate the impacts of hypoxia on interactions between fish and their prey. This study is an important step to understanding local changes in fish food web dynamics in areas experiencing hypoxia, but more research is needed to scale up to the entire NGOMEX, and ultimately forecast the long-term effects of hypoxia on pelagic fish populations. Such forecasts will be valuable to understand changes in fisheries productivity, and to prevent catch limits from slipping into the realm of overfishing if hypoxia reduces fish survival, recruitment, or growth. Incorporating hypoxia into ecosystem models and ultimately estimates of catch will improve fisheries management and ensure these resources are available for future generations.
References
Aku, Peter M.K., and William M. Tonn. 1999. Effects of hypolimnetic oxygenation on the food resources and feeding ecology of cisco in Amisk Lake, Alberta. Transactions of the American Fisheries Society 128 (1): 17–30.
Anderson, M.J. 2005. PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance. https://doi.org/10.1002/9781118445112.stat07841.
Anderson, Marti J. 2008. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26 (1): 32–46.
Anderson, M. J. 2014. Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef: Statistics Reference Online. https://doi.org/10.1002/9781118445112.stat07841, .
Ara, Koichi. 2001. Length-weight relationships and chemical content of the planktonic copepods in the Cananeia Lagoon estuarine system, Sao Paulo, Brazil. Plankton Biology and Ecology 48: 121–127.
Arend, Kristin K., Dmitry Beletsky, Joseph DePinto, Stuart A. Ludsin, James J. Roberts, Daniel K. Rucinski, Donald Scavia, David J. Schwab, and Thomas O. Höök. 2011. Seasonal and interannual effects of hypoxia on fish habitat quality in Central Lake Erie. Freshwater Biology 56 (2): 366–383. https://doi.org/10.1111/j.1365-2427.2010.02504.x.
Baker, Matthew E., and Ryan S. King. 2010. A new method for detecting and interpreting biodiversity and ecological community thresholds. Methods in Ecology and Evolution 1 (1): 25–37. https://doi.org/10.1111/j.2041-210X.2009.00007.x.
Benedetti-Cecchi, Lisandro, and Giacomo Chato Osio. 2007. Replication and mitigation of effects of confounding variables in environmental impact assessment: Effect of marinas on rocky-shore assemblages. Marine Ecology Progress Series 334: 21–35. https://doi.org/10.3354/meps334021.
Bethea, Dana M., Loraine Hale, John K. Carlson, Enric Cortés, Charles A. Manire, and James Gelsleichter. 2007. Geographic and ontogenetic variation in the diet and daily ration of the bonnethead shark, Sphyrna tiburo, from the eastern Gulf of Mexico. Marine Biology 152 (5): 1009–1020. https://doi.org/10.1007/s00227-007-0728-7.
Bianchi, Thomas S., Steven F. DiMarco, James H. Cowan, Robert D. Hetland, Piers Chapman, John W. Day, and Mead A. Allison. 2010. The science of hypoxia in the northern Gulf of Mexico: A review. Science of the Total Environment 408 (7): 1471–1484. https://doi.org/10.1016/j.scitotenv.2009.11.047.
Brandt, Stephen B., and Doran M. Mason. 2003. Effect of nutrient loading on Atlantic menhaden (Brevoortia tyrannus) growth rate potential in the Patuxent River. Estuaries 26 (2): 298–309. https://doi.org/10.1007/BF02695968.
Brandt, Stephen B., Marco Costantini, Sarah Kolesar, Stuart A. Ludsin, Doran M. Mason, Christopher M. Rae, and Hongyan Zhang. 2011. Does hypoxia reduce habitat quality for Lake Erie walleye (Sander vitreus)? A bioenergetics perspective. Canadian Journal of Fisheries and Aquatic Sciences 68 (5): 857–879. https://doi.org/10.1139/f2011-018.
Breitburg, Denise L., Timothy Loher, Carol A. Pacey, and Adam Gerstein. 1997. Varying effects of low dissolved oxygen on trophic interactions in an estuarine food web. Ecological Monographs 67 (4): 489–507.
Breitburg, Denise L., Aaron T. Adamack, Kenneth A. Rose, Sarah E. Kolesar, Beth Decker, Jennifer E. Purcell, Julie E. Keister, and James H. Cowan. 2003. The pattern and influence of low dissolved oxygen in the Patuxent River, a seasonally hypoxic estuary. Estuaries 26 (2): 280–297.
Breitburg, Denise L., J. Kevin Craig, Richard S. Fulford, Kenneth A. Rose, Walter R. Boynton, Damian C. Brady, Benjamin J. Ciotti, et al. 2009. Nutrient enrichment and fisheries exploitation: Interactive effects on estuarine living resources and their management. Hydrobiologia 629 (1): 31–47. https://doi.org/10.1007/s10750-009-9762-4.
Buchheister, Andre, Christopher F. Bonzek, James Gartland, and Robert J. Latour. 2013. Patterns and drivers of the demersal fish community of chesapeake bay. Marine Ecology Progress Series 481: 161–180. https://doi.org/10.3354/meps10253.
Cadman, Linda R., and Michael P. Weinstein. 1985. Size-weight relationships of postecdysial juvenile blue crabs (Callinectes sapidus Rathbun) from the Lower Chesapeake Bay. Journal of Crustacean Biology 5 (2): 306–310.
Chisholm, Laurie A., and John C. Roff. 1990. Size-weight relationships and biomass of tropical neritic copepods off Kingston, Jamaica. Marine Biology 106 (1): 71–77.
Costantini, Marco, Stuart A. Ludsin, Doran M. Mason, Xinsheng Zhang, William C. Boicourt, and Stephen B. Brandt. 2008. Effect of hypoxia on habitat quality of striped bass (Morone saxatilis) in Chesapeake Bay. Canadian Journal of Fisheries and Aquatic Sciences 65 (5): 989–1002. https://doi.org/10.1139/f08-021.
Craig, J. Kevin. 2012. Aggregation on the edge: Effects of hypoxia avoidance on the spatial distribution of brown shrimp and demersal fishes in the Northern Gulf of Mexico. Marine Ecology Progress Series 445: 75–95. https://doi.org/10.3354/meps09437.
Craig, J. Kevin, and Larry B. Crowder. 2005. Hypoxia-induced habitat shifts and energetic consequences in Atlantic croaker and brown shrimp on the Gulf of Mexico shelf. Marine Ecology Progress Series 294: 79–94. https://doi.org/10.3354/meps294079.
de Mutsert, Kim, Jeroen Steenbeek, Kristy Lewis, Joe Buszowski, James H. Cowan, and Villy Christensen. 2016. Exploring effects of hypoxia on fish and fisheries in the northern Gulf of Mexico using a dynamic spatially explicit ecosystem model. Ecological Modelling 331: 142–150. https://doi.org/10.1016/j.ecolmodel.2015.10.013.
Decker, Mary Beth, Denise L. Breitburg, and Jennifer E. Purcell. 2004. Effects of low dissolved oxygen on zooplankton predation by the ctenophore Mnemiopsis leidyi. Marine Ecology Progress Series 280: 163–172. https://doi.org/10.3354/meps280163.
Diaz, Robert J., and Rutger Rosenberg. 2008. Spreading dead zones and consequences for marine ecosystems. Science 321 (5891): 926–929. https://doi.org/10.1126/science.1156401.
DiCiccio, Thomas J., and Bradley Efron. 1996. Bootstrap cinfidence intervals. Statistical Science 11: 189–212. https://doi.org/10.1007/sl0869-007-9037-x.
Domenici, Paolo, Cristel Lefrançois, and A. Shingles. 2007. Hypoxia and the antipredator behaviours of fishes. Philosophical Transactions of the Royal Society B: Biological Sciences 362 (1487): 2105–2121. https://doi.org/10.1098/rstb.2007.2103.
Eby, Lisa A., and Larry B. Crowder. 2002. Hypoxia-based habitat compression in the Neuse River Estuary: context-dependent shifts in behavioral avoidance thresholds. Canadian Journal of Fisheries and Aquatic Sciences 59 (6): 952–965. https://doi.org/10.1139/f02-067.
Ekau, Werner, Holger Auel, Hans O. Portner, and Denis Gilbert. 2010. Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences 7 (5): 1669–1699. https://doi.org/10.5194/bg-7-1669-2010.
Elliott, David T., James J. Pierson, and Michael R. Roman. 2013. Predicting the effects of coastal hypoxia on vital rates of the planktonic copepod Acartia tonsa Dana. PLoS One 8 (5): e63987. https://doi.org/10.1371/journal.pone.0063987.
Fontaine, Clark T., and Richard A. Neal. 1971. Length-weight relations for three commercially important penaeid shrimp of the Gulf of Mexico. Transactions of the American Fisheries Society 100 (3): 584–586.
Glaspie, Cassandra N., Melissa A. Clouse, Stuart A. Ludsin, Doran M. Mason, Michael R. Roman, Craig A. Stow, and Stephen B. Brandt. 2018. Effect of hypoxia on diet of Atlantic Bumpers in the Northern Gulf of Mexico. Transactions of the American Fisheries Society 147 (4): 740–748. https://doi.org/10.1002/tafs.10063.
Hartigan, John A., and Melissa A. Wong. 1979. A k-means clustering algorithm. Applied Statistics 28: 100–108.
Hazen, Elliott L., J. Kevin Craig, Caroline P. Good, and Larry B. Crowder. 2009. Vertical distribution of fish biomass in hypoxic waters on the Gulf of Mexico shelf. Marine Ecology Progress Series 375: 195–207. https://doi.org/10.3354/meps07791.
Hennig, Christian. 2007. Cluster-wise assessment of cluster stability. Computational Statistics and Data Analysis 52 (1): 258–271. https://doi.org/10.1016/j.csda.2006.11.025.
Hennig, Christian. 2008. Dissolution point and isolation robustness: Robustness criteria for general cluster analysis methods. Journal of Multivariate Analysis 99 (6): 1154–1176. https://doi.org/10.1016/j.jmva.2007.07.002.
Hoffmayer, Eric R., and Glenn R. Parsons. 2003. Food habits of three shark species from the Mississippi Sound in the Northern Gulf of Mexico. Southeastern Naturalist 2 (2): 271–280.
Hopcroft, Russell R., John C. Roff, and Heather A. Bouman. 1998. Zooplankton growth rate: the larvaceans Appendicularia, Fritillaria and Oikopleura in tropical waters. Journal of Plankton Research 20 (3): 539–555.
Hughes, Brent B., Matthew D. Levey, Monique C. Fountain, Aaron B. Carlisle, Francisco P. Chavez, and Mary G. Gleason. 2015. Climate mediates hypoxic stress on fish diversity and nursery function at the land–sea interface. Proceedings of the National Academy of Sciences 112 (26): 8025–8030. https://doi.org/10.1073/pnas.1505815112.
Karnauskas, Mandy, Christopher R. Kelble, Seann Regan, Charline Quenée, Rebecca Allee, Michael Jepson, Amy Freitag, et al. 2017. 2017 Ecosystem Status Report Update for the Gulf of Mexico.
Keister, Julie E., Edward D. Houde, and Denise L. Breitburg. 2000. Effects of bottom-layer hypoxia on abundances and depth distributions of organisms in Patuxent River, Chesapeake Bay. Marine Ecology Progress Series 205: 43–59. https://doi.org/10.3354/meps205043.
Kimmel, David G., William C. Boicourt, James J. Pierson, Michael R. Roman, and Xinsheng Zhang. 2009. A comparison of the mesozooplankton response to hypoxia in Chesapeake Bay and the northern Gulf of Mexico using the biomass size spectrum. Journal of Experimental Marine Biology and Ecology 381. Elsevier B.V.: S65–S73. https://doi.org/10.1016/j.jembe.2009.07.012.
Kimmel, David G., William C. Boicourt, James J. Pierson, Michael R. Roman, and Xinsheng Zhang. 2010. The vertical distribution and diel variability of mesozooplankton biomass, abundance and size in response to hypoxia in the northern Gulf of Mexico USA. Journal of Plankton Research 32 (8): 1185–1202.
Langseth, Brian J., Kevin M. Purcell, J. Kevin Craig, Amy M. Schueller, Joseph W. Smith, Kyle W. Shertzer, Sean Creekmore, Kenneth A. Rose, and Katja Fennel. 2014. Effect of changes in dissolved oxygen concentrations on the spatial dynamics of the Gulf menhaden fishery in the Northern Gulf of Mexico. Marine and Coastal Fisheries 6 (1): 223–234. https://doi.org/10.1080/19425120.2014.949017.
Long, W. Christopher, and Rochelle D. Seitz. 2008. Trophic interactions under stress: Hypoxia enhances foraging in an estuarine food web. Marine Ecology Progress Series 362: 59–68. https://doi.org/10.3354/meps07395.
Long, W. Christopher, Bryce J. Brylawski, and Rochelle D. Seitz. 2008. Behavioral effects of low dissolved oxygen on the bivalve Macoma balthica. Journal of Experimental Marine Biology and Ecology 359 (1): 34–39. https://doi.org/10.1016/j.jembe.2008.02.013.
Ludsin, Stuart A., Xinsheng Zhang, Stephen B. Brandt, Michael R. Roman, William C. Boicourt, Doran M. Mason, and Marco Costantini. 2009. Hypoxia-avoidance by planktivorous fish in Chesapeake Bay: Implications for food web interactions and fish recruitment. Journal of Experimental Marine Biology and Ecology 381: 121–131. https://doi.org/10.1016/j.jembe.2009.07.016.
Manooch, C.S., and W.T. Hogarth. 1983. Stomach contents and giant trematodes from wahoo, Acanthocybium solanderi, collected along the South Atlantic and Gulf coasts of the United States. Bulletin of Marine Science 33: 227–238.
Meyer, Gabriele H., and James S. Franks. 1996. Food of cobia, Rachycentron canadum, from the Northcentral Gulf of Mexico. Gulf Research Reports 9: 161–167. https://doi.org/10.18785/grr.0903.02.
NMFS. 2017a. Fisheries Economics of the United States 2015. NOAA Technical Memorandum: 247. https://doi.org/10.1017/CBO9781107415324.004.
NMFS. 2017b. Fisheries Economics of the United States 2015. Washington, D.C.: NOAA Technical Memorandum. https://doi.org/10.1017/CBO9781107415324.004.
NMFS. 2018. Fisheries of the United States. https://doi.org/10.2307/j.ctv157bc5.9.
Pihl, Leif, Susanne P. Baden, Robert J. Diaz, and Linda C. Schaffner. 1992. Hypoxia-induced structural changes in the diet of bottom-feeding fish and Crustacea. Marine Biology 112 (3): 349–361. https://doi.org/10.1007/BF00356279.
Pothoven, Steven A., Henry A. Vanderploeg, Thomas O. Höök, and Stephen B. Brandt. 2009. Feeding ecology of emerald shiners and rainbow smelt in central Lake Erie. Journal of Great Lakes Resources 35 (2): 190–198.
Prince, Eric D., and C. Phillip Goodyear. 2006. Hypoxia-based habitat compression of tropical pelagic fishes. Fisheries Oceanography 15 (6): 451–464. https://doi.org/10.1111/j.1365-2419.2005.00393.x.
R Core Team. 2019. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Rabalais, Nancy N., and R. Eugene Turner. 2001. Hypoxia in the northern Gulf of Mexico: Description, causes and change. In Coastal Hypoxia: Consequences for Living Resources and Ecosystems, ed. Nancy N. Rabalais and R. Eugene Turner. Washington D. C.: American Geophysical Union.
Rabotyagov, Sergey S., Todd D. Campbell, Michael White, Jeffrey G. Arnold, Atwood Jay, M. Lee Norfleet, Catherine L. Kling, et al. 2014. Cost-effective targeting of conservation investments to reduce the northern Gulf of Mexico hypoxic zone. Proceedings of the National Academy of Sciences 111 (52): 18530–18535. https://doi.org/10.1073/pnas.1405837111.
Rahel, Frank J., and Julie W. Nutzman. 1994. Foraging in a lethal environment: Fish predation in hypoxic waters of a stratified lake. Ecology 75 (5): 1246–1253.
Remsen, Andrew, Thomas L. Hopkins, and Scott Samson. 2004. What you see is not what you catch: A comparison of concurrently collected net, Optical Plankton Counter, and Shadowed Image Particle Profiling Evaluation Recorder data from the northeast Gulf of Mexico. Deep Sea Research Part I: Oceanographic Research Papers 51 (1): 129–151.
Renaud, Maurice L. 1986. Hypoxia in Louisiana coastal waters during 1983: Implications for fisheries. Fishery Bulletin 84 (10): 19–26. https://doi.org/10.1063/1.1453944.
Roberts, James J., Tomas O. Höök, Stuart A. Ludsin, Steven A. Pothoven, Henry A. Vanderploeg, and Stephen B. Brandt. 2009. Effects of hypolimnetic hypoxia on foraging and distributions of Lake Erie yellow perch. Journal of Experimental Marine Biology and Ecology 381: S132–S142. https://doi.org/10.1016/j.jembe.2009.07.017.
Roman, Michael R., James J. Pierson, David G. Kimmel, William C. Boicourt, and Xinsheng Zhang. 2012. Impacts of hypoxia on zooplankton spatial distributions in the Northern Gulf of Mexico. Estuaries and Coasts 35 (5): 1261–1269. https://doi.org/10.1007/s12237-012-9531-x.
Roman, Michael R., Stephen B. Brandt, Edward D. Houde, and James J. Pierson. 2019. Interactive effects of hypoxia and temperature on coastal pelagic zooplankton and fish. Frontiers in Marine Science 6: 139. https://doi.org/10.3389/fmars.2019.00139.
Rose, Kenneth A., John C. Roff, and Russell R. Hopcroft. 2004. Production of Penilia avirostris in Kingston Harbour, Jamaica. Journal of Plankton Research 26 (6): 605–615.
Rose, Kenneth A., Sean B. Creekmore, Dubravko Justić, Thomas Peter, J. Kevin Craig, Rachel Miller Neilan, Lixia Wang, Md S. Rahman, and David Kidwell. 2018. Modeling the population effects of hypoxia on Atlantic croaker (Micropogonias undulatus) in the Northwestern Gulf of Mexico. Estuaries and Coasts 41 (1): 233–254. https://doi.org/10.1007/s12237-017-0266-6.
Shang, Eva H.H., and Rudolf S.S. Wu. 2004. Aquatic hypoxia is a teratogen and affects fish embryonic development. Environmental Science and Technology 38 (18): 4763–4767. https://doi.org/10.1021/es0496423.
Stalder, L.C., and Nancy H. Marcus. 1997. Zooplankton responses to hypoxia: Behavioral patterns and survival of three species of calanoid copepods. Marine Biology 127 (4): 599–607.
Sutton, Tracy T., and Thomas L. Hopkins. 1996. Trophic ecology of the stomiid (Pisces: Stomiidae) fish assemblage of the eastern Gulf of Mexico: Strategies, selectivity and impact of a top mesopelagic predator group. Marine Biology 127 (2): 179–192. https://doi.org/10.1007/BF00942102.
Taylor, J. Christopher, and Peter S. Rand. 2003. Spatial overlap and distribution of anchovies (Anchoa spp.) and copepods in a shallow stratified estuary. Aquatic Living Resources 16 (3): 191–196. https://doi.org/10.1016/S0990-7440(03)00012-3.
Taylor, J. Christopher, Peter S. Rand, and Jacqueline Jenkins. 2007. Swimming behavior of juvenile anchovies (Anchoa spp.) in an episodically hypoxic estuary: Implications for individual energetics and trophic dynamics. Marine Biology 152 (4): 939–957.
Thomas, Peter, and Md. Saydur Rahman. 2012. Extensive reproductive disruption, ovarian masculinization and aromatase suppression in Atlantic croaker in the northern Gulf of Mexico hypoxic zone. Proceedings of the Royal Society B: Biological Sciences 279 (1726): 28–38. https://doi.org/10.1098/rspb.2011.0529.
Thronson, Amanda, and Antonietta Quigg. 2008. Fifty-five years of fish kills in Coastal Texas. Estuaries and Coasts 31 (4): 802–813. https://doi.org/10.1007/sl2237-008-9056-5.
Tita, Guglielmo, Magda Vincx, and Gaston Desrosiers. 1999. Size spectra, body width and morphotypes of intertidal nematodes: An ecological interpretation. Journal of the Marine Biological Association of the United Kingdom 79 (6): 1007–1015.
Turner, R. Eugene, Nancy N. Rabalais, and Dubravko Justic. 2008. Gulf of Mexico hypoxia: Alternate states and a legacy. Environmental Science and Technology 42 (7): 2323–2327.
Uye, Shin-Ichi. 1982. Length-weight relationships of important zooplankton from the Inland Sea of Japan. Journal of the Oceanographical Society of Japan 38 (3): 149–158.
Vanderploeg, Henry A., Stuart A. Ludsin, Joann F. Cavaletto, Tomas O. Höök, Steven A. Pothoven, Stephen B. Brandt, James R. Liebig, and Gregory A. Lang. 2009a. Hypoxic zones as habitat for zooplankton in Lake Erie: Refuges from predation or exclusion zones? Journal of Experimental Marine Biology and Ecology 381: S108–S120. https://doi.org/10.1016/j.jembe.2009.07.015.
Vanderploeg, Henry A., Stuart A. Ludsin, Steven A. Ruberg, Tomas O. Höök, Steven A. Pothoven, Stephen B. Brandt, Gregory A. Lang, James R. Liebig, and Joann F. Cavaletto. 2009b. Hypoxia affects spatial distributions and overlap of pelagic fish, zooplankton, and phytoplankton in Lake Erie. Journal of Experimental Marine Biology and Ecology 381: S92–S107. https://doi.org/10.1016/j.jembe.2009.07.027.
Vaquer-Sunyer, Raquel, and Carlos M. Duarte. 2008. Thresholds of hypoxia for marine biodiversity. Proceedings of the National Academy of Sciences 105 (1): 15452–15457. https://doi.org/10.4319/lo.2007.52.1.0338.
Webber, Mona K., and John C. Roff. 1995. Annual biomass and production of the oceanic copepod community off Discovery Bay, Jamaica. Marine Biology 123 (3): 481–495.
Wells, R., J. David, James H. Cowan, and Brian Fry. 2008. Feeding ecology of red snapper Lutjanus campechanus in the northern Gulf of Mexico. Marine Ecology Progress Series 361: 213–225. https://doi.org/10.3354/meps07425.
Zhang, G.T., and C.K. Wong. 2011. Changes in the planktonic copepod community in a landlocked bay in the subtropical coastal waters of Hong Kong during recovery from eutrophication. Hydrobiologia 666 (1): 277–288. https://doi.org/10.1007/s10750-010-0422-5.
Zhang, Hongyan, Stuart A. Ludsin, Doran M. Mason, Aaron T. Adamack, Stephen B. Brandt, Xinsheng Zhang, David G. Kimmel, Michael R. Roman, and William C. Boicourt. 2009. Hypoxia-driven changes in the behavior and spatial distribution of pelagic fish and mesozooplankton in the northern Gulf of Mexico. Journal of Experimental Marine Biology and Ecology 381: S80–S91. https://doi.org/10.1016/j.jembe.2009.07.014.
Zhang, Hongyan, Doran M. Mason, Craig A. Stow, Aaron T. Adamack, Stephen B. Brandt, Xinsheng Zhang, David G. Kimmel, Michael R. Roman, William C. Boicourt, and Stuart A. Ludsin. 2014. Effects of hypoxia on habitat quality of pelagic planktivorous fishes in the northern gulf of Mexico. Marine Ecology Progress Series 505: 209–226. https://doi.org/10.3354/meps10768.
Zielinski, S., P.G. Lee, and H.O. Portner. 2000. Metabolic performance of the squid Lolliguncula brevis during hypoxia: An analysis of the critical PO2. Journal of Experimental Marine Biology and Ecology 243 (2): 241–259.
Acknowledgements
We thank James Roberts, Craig Stow, Stephen Lozano, Jennifer Metes, Aly Peacy, and Katharine Bush for help with collecting the field data. We also thank the captains and crew of the RV Pelican for their help with field sampling.
Funding
This research was supported by NOAA-CSCOR Award NA06NOS4780148 and NA09NOS4780198 and National Academies award NAS-GRP-2000006418. This is NOAA GLERL contribution No. 1931 and UMCES contribution No. 5713.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Wim J. Kimmerer
Rights and permissions
About this article
Cite this article
Glaspie, C.N., Clouse, M., Huebert, K. et al. Fish Diet Shifts Associated with the Northern Gulf of Mexico Hypoxic Zone. Estuaries and Coasts 42, 2170–2183 (2019). https://doi.org/10.1007/s12237-019-00626-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-019-00626-x