Abstract
Fish communities in tidal tributaries have received considerable attention, but the relative value of nontidal tributaries (having a tidal amplitude of < 5 cm) may represent an under-valued habitat. A multi-gear sampling approach was used to collect fish and macroinvertebrates from one tidal and two nontidal tributaries to describe and compare the respective nekton communities and habitat use patterns. Nekton communities in tidal and nontidal tributaries were markedly different even though habitats were similar (e.g., temperature, DO, depths, shoreline vegetation). While catch-per-unit-effort (CPUE) of estuarine-dependent species (e.g., red drum, spot, common snook) was lower in nontidal tributaries, the overall nekton CPUE was twice that of the tidal tributary, and the community was comprised mostly of freshwater marsh species (e.g., eastern mosquitofish, sailfin molly, bluefin killifish). Based on the life histories of the fishes that differed between tributary types, the proximity of coastal inlets and availability of effective larval transport mechanisms for estuarine-dependent species may be greater determinants of community differences than factors related to tributary size or shoreline habitat type. These results recognize smaller nontidal tributaries as undervalued nursery habitats and suggest the function as secondary nursery habitats is a critical service to the overall estuarine community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tributaries that flow into estuaries are an important habitat in the life history of many estuarine and estuarine-dependent fish and invertebrate species (Chittenden 1971; Love et al. 2009). Estuarine-dependent species may use low-salinity areas of tributaries as a refuge from predation early in their life histories (Gibson 2003; Love et al. 2006; Stewart and Scharf 2008), while mature individuals of some species migrate into upstream habitats to spawn (Harris et al. 2007). Local hydrodynamic characteristics, including tidal regimes, estuarine circulation patterns, and tributary flow rates, can affect access to freshwater tributaries by estuarine-dependent species, particularly during early life history stages (Peterson 2003; Nicolas et al. 2010). Throughout the world, tributaries are subject to increasing stress from encroaching development, pollutant loading, habitat destruction, channel dredging, hydrologic alterations, and the introduction of exotic species that may affect available habitats and their suitability for local fauna (Kirby-Smith et al. 2001; Freeman et al. 2003; Daniels et al. 2005).
Much of our understanding of community composition, life histories, and habitat-use patterns in estuarine tributaries comes from investigations in tidally influenced systems in which larval dynamics and fish movements are facilitated by strong tidal transport mechanisms (Peterson and Ross 1991; Ayvazian et al. 1992; Able et al. 2001; Schultz et al. 2003; Paperno et al. 2006), but tributaries associated with microtidal estuaries (such as Florida’s Indian River Lagoon [IRL] and Texas’ Laguna Madre), particularly those far from ocean passes, can be nontidal (nontidal for this study is defined as having a tidal amplitude of < 5 cm, Smith 1983). In systems with varying degrees of hydrodynamic and habitat characteristics, studies of what factors are most important in structuring fish communities have provided conflicting results (Malavasi et al. 2004; Franco et al. 2006), and, as a result, the role of nontidal tributaries as nurseries or critical habitat for estuarine-dependent fish is not well understood. For example, in nontidal areas of estuaries far from passes or inlets, flushing times are greater than those areas close to the inlets (Smith 1993), and the degree to which weaker transport systems affect larval movement into the tributaries within the nontidal area is unknown. Comparisons among tributaries may clarify the importance of physical and biotic traits influencing nekton community distribution and abundance.
The IRL is a barrier-island estuary along the east coast of Florida, situated between the Carolinian and Caribbean zoogeographic provinces, and contains one of the most diverse ichthyofaunas in North America (Gilmore 1995, 2001; Engle and Summers 1999). That diversity makes the IRL a unique location in which to investigate nekton communities in estuarine and adjacent tributary habitats. In addition, the northern portion of the IRL system has limited exchange with the open ocean through inlets, which drastically reduce tidal influence there (Smith 1993). These unique morphological characteristics in the northern IRL provide an opportunity to compare communities from tributaries that feed into tidal and nontidal environments. A substantial body of research exists on the estuarine fish communities in the lagoon (Gilmore et al. 1981, 1983a, b; Snelson 1983; Tremain and Adams 1995; Kupschus and Tremain 2001). To a lesser extent, the fish communities within larger tidal-freshwater tributaries have also been described (Springer 1960; Gunter and Hall 1963; Christensen 1965; Paperno and Brodie 2004; Paperno et al. 2006), but no quantitative information is available about the communities in the tributaries of the nontidal portion of the northern IRL, and the relative importance of these habitats to estuarine-dependent species is unknown. Therefore, the objective of this 2-year study was to compare the seasonal composition of the nekton community in tidal and nontidal tributaries of the IRL. We hypothesized that the tidal tributaries, which experience greater variability in the degree of seawater influence, would support more species and display more seasonal variability in community structure than would the nontidal tributaries.
Material and Methods
Study Area
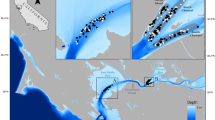
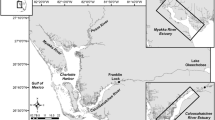
The IRL is a shallow estuary extending 253 km between Jupiter Inlet and Ponce Inlet on the east coast of Florida (Fig. 1). Most of the freshwater enters the lagoon via three navigable tidal rivers (the St. Sebastian, St. Lucie, and Loxahatchee rivers) in the southern half of the lagoon and numerous non-navigable tidal and nontidal tributaries (Smith 1987). This study focused on three tributaries situated in the central portion of the lagoon, which included one tidal system (St. Sebastian River [SSR]) and two nontidal systems (Turkey Creek [TC] and Crane Creek [CC]. The SSR was selected because it is the northernmost tidal tributary in the IRL and closest in proximity to the nontidal portion of the lagoon. The TC and CC tributaries were selected because they are the closest navigable nontidal tributaries to the SSR.
The SSR has a total drainage area of approximately 40,000 ha (Bergman and Donnangelo 1998), is located directly opposite Sebastian Inlet (27° 51′ N, 80° 26′ W), and provides more than 10% of the freshwater to the lagoon (Smith 1993). The SSR is composed of a northern and southern prong that provide natural drainage and two man-made canals (the C 54 and Fellsmere canals) that receive and convey excess floodwater from adjacent agricultural land and the St. Johns River marshes (Steward and Van Arman 1987). Water control structures regulate inflow from both canals into the north prong (Steward and Van Arman 1987; Bergman and Donnangelo 1998), and a distinct salt wedge is present in both prongs year-round.
The majority of the SSR shoreline is covered by vegetation that we classified as either overhanging or emergent. The adjacent upland is characterized as a hydric pine (Pinus sp.) flatwood while the overhanging shoreline vegetation in this area is largely composed of mangroves (Rhizophora mangle, Avicennia germinans, Laguncularia racemosa), oaks (Quercus spp.), wax myrtle (Morella (= Myrica) cerifera), leather fern (Acrostichum danaeifolium), primrose willow (Ludwigia octovalvis), and the nonnative Brazilian Pepper (Schinus terebinthifolius) (USFWS 1999). Typical emergent vegetation types include cattails (Typha spp.), swamp lily (Crinum americanum), panic grasses (Panicum spp.), common reed (Phragmites australis), and mild smartweed (Persicaria hydropiperoides). The unvegetated portions of the SSR shoreline have been denuded through specific clearing practices (e.g., removal of Brazilian pepper) or the construction of docks and seawalls.
The nontidal TC and CC tributaries are located approximately 22 and 27 km north of Sebastian Inlet, respectively, along the western shore of the IRL (Fig. 1). Each creek receives freshwater from surrounding municipal watersheds but provides less net freshwater input (< 5% and ~ 1%, respectively) to the IRL than does the SSR (Smith 1993). The shorelines of these tributaries are characterized by hardened seawalls or various types of native vegetation (marsh, oak and pine forest and bald cypress [Taxodium distichum]) and nonnative Brazilian Pepper similar to that on the tidal SSR, although the presence of mangrove communities in these nontidal tributaries is less prominent (Suphunvorranop and Clapp 1984).
Sampling Methods
From July 2007 to June 2009, we used three sampling gears to collect nekton in the tributaries. Fish and macroinvertebrates from all three tributaries were collected with a stratified-random sampling design by using center-bag seine and electrofishing methods. We conducted monthly sampling for smaller individuals along shorelines where the water was < 1.8 m deep by using a 21.3-m center-bag seine (3.1 mm mesh) in the tidal SSR (N = 14 hauls) and two nontidal TC (N = 4 hauls) and CC (N = 4 hauls) tributaries. Large, mobile species were sampled along shorelines where the water was < 2.5-m deep by using a 61-m seine (25.4 mm mesh) in the SSR (N = 3 hauls) and TC (N = 2 hauls) tributaries (the habitat in the CC tributary was not suitable for this seine). Both seines were deployed from the stern of a boat, and were set in a semi-circle along the shoreline by operating the boat parallel to shore into the prevailing current.
Electrofishing was conducted along the shorelines in tidal and non-tidal systems to complement the net collections by including samples collected from a larger variety of freshwater habitats. Each quarter during a 2-day period, a maximum of two random 100-m transects were sampled in waters with salinities typically less than 5 in each tributary. The available sampling area within both tributary types was likely reduced during the dry season. All electrofishing was conducted with a double-boom electrofishing boat equipped with a 7.5 Generator Powered Pulsator (GPP) Smith-Root electrofisher (170, 340, 500 or 1000 V; 6–38 A and 60 pulses/s DC). Amperage was set at the minimum necessary to incapacitate fish and macroinvertebrates under the ambient water conditions. At each sampling site, the boundaries of the 100-m transect were determined by GPS and marked with buoys. Next, the booms were slowly and repeatedly eased up to the shoreline along the transect length, and current was applied at each approach for at least 15 s and until no new incapacitated individuals were observed in the electric field. The temporarily incapacitated specimens were removed from the water with a dip net (3.1-mm mesh) and transferred into a live well until sampling was completed at each site.
At each sampling site, recorded water quality data consisted of water temperature (°C), salinity, pH, dissolved oxygen (ppm), and water clarity (Secchi depth in m). Detailed habitat characteristics (water depth, shoreline vegetation, substrate composition) and prevailing environmental conditions (weather, wind speed, cloud cover, rainfall) were also recorded at each site. For sites in water deeper than 0.4 m, surface and bottom water quality measurements were taken and presented as means of the two measurements. From each sample, all individuals were counted, identified to the lowest practical taxonomic level, measured to nearest mm (standard length for fish, carapace width for crabs, post-orbital head length for shrimp), and released except any fish or invertebrate not identified to species, which was returned to the laboratory for further identification. Nekton abundance was reported as catch-per-unit effort (CPUE), which was defined as animals haul−1 (seines) or animals transect−1 (electrofishing).
Statistical Analysis
Because our sampling design lacked true replication among tributary types (Hurlbert 1984), we used a nonparametric analysis approach with the understanding that while permutational methods can provide reasonable estimates of p values (Anderson et al. 2008), the ability to ascribe causal differences to specific factors may be limited. In this study, each gear type characterized a different segment of the nekton community within the tributaries and was, therefore, analyzed separately. To examine physical and community differences between tidal and nontidal tributaries, we conducted multivariate analyses on sample data, pooled across month within each season. While pooling data in this manner can reduce the resolution of within-season differences, this study focused on the large-scale seasonal changes in communities related to dry (December–May) and wet (June–November) periods defined in Arnold et al. (1998). Mean water quality data were plotted by gear type to examine seasonal changes in the physical environment of the tributaries. The physical parameters, which are measured on difference scales, were transformed (square-root) and normalized by taking the value of each measurement, subtracting the mean, and then dividing the result by the standard deviation. All community multivariate analyses and ordinations were conducted using nonparametric analytical methods and PRIMER-E with PERMANOVA+ software (Anderson et al. 2008; Clarke et al. 2014; Clarke and Gorley 2015). Differences in nekton assemblage structure between the tidal and nontidal tributaries were first tested using a two-way, permutational analysis of variance (PERMANOVA) that included tributary type and season (along with all possible interactions). The analysis was applied to the Bray-Curtis similarity matrix (Bray and Curtis 1957) that used square-root-transformed CPUE data to reduce the influence of overly abundant species. In addition, all species (n = 35) encountered during just one sampling event were removed from the data set because, while rare species may be useful in examining local disturbances, they usually have little influence on community matrices and complicate statistical computations and interpretations (Lyons 1996). Where significant differences were detected in model effects (p < 0.05), pairwise post hoc comparisons were conducted between tributaries within seasons and between seasons within tributaries. Differences in assemblage structure, based upon the similarity matrix, were explored visually by constructing nonmetric multidimensional scaling plots using year, season, and tributary type as pooling factors. Taxonomic groups contributing to any observed differences in assemblage structure were identified using the similarity percentage analysis (SIMPER, cutoff at 90% contribution) routine. In addition, preliminary analyses with analysis of similarity (ANOSIM) within each tributary indicated that there were no significant physical (R = − 0.13, p > 0.05) or community (R = 0.042, p > 0.05) differences between years; therefore, year was not treated as a factor in subsequent analyses.
Bay anchovies (Anchoa mitchilli) were excluded from all multivariate analyses because of concerns that their high abundance during the entire study period might reduce our ability to resolve differences in community structure. Species complexes that hybridize in the IRL region (e.g., weakfish-sand seatrout [Cynoscion complex], Tringali et al. 2004; silversides [Menidia sp.], Chernoff et al. 1981; menhaden [Brevoortia sp.], Dahlberg 1970; and cichlids [e.g., Oreochromis, Sarotherodon, and Tilapia], Gestring, FWC-Non-Native Fish and Wildlife Program, personal communication) or were not distinguishable macroscopically at small juvenile sizes (e.g., mojarras [Eucinostomus sp.], gobies [Gobiosoma sp.], and centrarchids [Lepomis sp.]), were classified and treated as single species for all analyses and discussion. Species richness values were calculated for each gear type by standardizing differences in sampling effort using the rarefaction methods of Colwell et al. (2012). Rarefaction values were calculated using the software EstimateS (Colwell 2013), which estimates the expected number of species (ES) as a function of the number of accumulated samples.
The data from all three sampling methods were pooled to compare size frequencies (Kolmogorov-Smirnoff test (KS), α = 0.05) of selected species between the two tributary types. Common snook (Centropomus undecimalis), red drum (Sciaenops ocellatus), and spot (Leiostomus xanthurus) were selected to represent species that spawn in the ocean or near ocean inlets and use the low-salinity tributaries as nursery areas. Bluegill (Lepomis macrochirus), largemouth bass (Micropterus salmoides), and brook silverside (Labidesthes sicculus) were selected to represent freshwater species in the lower reaches of the tributaries.
Results
Each tributary type displayed seasonal variation in salinity, temperature, and dissolved oxygen. The summer and fall (wet season) were characterized by warmer temperatures and lower dissolved oxygen, whereas the winter and spring (dry season) were characterized by cooler temperatures with higher dissolved oxygen levels (Fig. 2). Seasonal physical conditions varied between tributary type, particularly for mean salinity. The difference in salinities between nontidal and tidal tributaries during the dry season (difference in seasonal means = 6.1) were more pronounced than during the wet season (difference in seasonal means = 4.6). Multivariate analyses of the physical data indicated significant differences between tributary type at the 21.3-m seine (PERMANOVA, p < 0.02) and 61-m seine sites (PERMANOVA, p < 0.03), but not at the electrofishing sites (PERMANOVA, p = 0.829).
During the study, more than 470,000 animals (representing 131 fish species and 8 macroinvertebrate species) were collected in the three tributaries (Appendix Table 1). The nontidal tributaries had higher CPUE than did the tidal tributary regardless of sampling gear (21.3-m seines: 1554.1 versus 394.0 animals haul−1; 61-m seines: 62.6 versus 33.3 animals haul−1: electrofishing: 42.2 versus 26.5 animals transect−1). This relationship was also consistent between nontidal and tidal tributaries during each season. In contrast, no apparent patterns were observed in comparisons of species richness between tributary types, sampling gear, or seasons (Fig. 3).
Multivariate analyses of the 21.3-m seine data indicated community differences (PERMANOVA, p < 0.01) between the tidal and nontidal tributaries (Fig. 4a, Appendix Table 2). Differences in community composition were observed between the main effects (tributary type, p < 0.001; season, p < 0.01), while the interaction term (tributary type*season) was not significant (p = 0.22; Appendix Table 2). Pairwise comparisons of all tributary and season combinations were significantly different (p < 0.05). Thirty-three species contributed to the observed differences in community structure between the tributary types (SIMPER, 53.6% dissimilarity). The nontidal community was best characterized by freshwater species such as juvenile sunfishes (Lepomis sp.), bluefin killifish (Lucania goodei), and brook silverside along with marsh species such as eastern mosquitofish (Gambusia holbrooki) and sailfin molly (Poecilia latipinna). The tidal community was characterized by seasonal estuarine recruits such as white mullet (Mugil curema), striped mullet (Mugil cephalus), Atlantic croaker (Micropogonias undulatus), pinfish (Lagodon rhomboides) and invertivores consisting primarily of mojarras (Eucinostomus harengulus and juvenile Eucinostomus spp.).
Multivariate analyses of the 61-m seine data also indicated the nekton communities differed (PERMANOVA, p < 0.01) between the tidal and nontidal tributaries (Fig. 4b, Appendix Table 3). Differences in community composition were observed between the main effects (tributary type, p < 0.01; season, p < 0.01), while the interaction term (tributary type*season) was not significant (p = 0.15; Appendix Table 3). Pairwise comparisons of all tributary and season combinations were significantly different (p < 0.05). Thirty-one species contributed to the observed differences in community structure between the tributary types (SIMPER, 53.3%). Many of the species did not differ markedly in CPUE between the tributary types. The nontidal community was differentiated by the CPUE of hogchoker (Trinectes maculatus), Irish pompano (Diapterus auratus), and striped mojarra (Eugerres plumieri), while the tidal community was differentiated by the CPUE of mojarras, white mullet, and blue crabs (Callinectes sapidus) (Appendix Table 1).
Multivariate analyses of the electrofishing data indicated that the nekton communities differed between the main effect of tributary type (PERMANOVA, p < 0.05), while season (p = 0.13) and the interaction term (tributary type*season) was not significant (p = 0.26; Fig. 4c, Appendix Table 4). Pairwise comparisons of the nekton community differed only between seasons in the tidal tributary (p < 0.05), while all other pairwise comparisons did not reveal significant differences (p > 0.05; Appendix Table 4). In the freshwater reaches where electrofishing methods are most effective, 20 species were identified as contributing to observed differences in community structure between the tributary types (SIMPER, 50.6%). The CPUE of many of these species differed markedly between tributary types. These differences were attributed to the CPUE of freshwater fishes (e.g., centrarchids) and two cichlids (Tilapia mariae and Cichlasoma urophthalmus) in the nontidal tributaries and a greater CPUE of estuarine species such as gray snapper (Lutjanus griseus) and common snook in the tidal tributary (Appendix Table 1).
Size distributions of the six species selected for comparison differed between the tributary types. The length-frequency distributions of estuarine-dependent species (common snook, red drum, spot) that spawn in the ocean or near ocean inlets differed significantly between tidal and nontidal tributaries (KS test, all D > 0.274, p < 0.05). In general, individuals of these species were smaller and more abundant in the tidal tributary than in the nontidal tributaries (Fig. 5). We did not detect significant differences between tributary types in the size distribution of two selected freshwater species (largemouth bass, brook silverside), but bluegill length-frequency distributions differed between the tributary types (KS test; D = 0.237, p < 0.05), which was attributable to substantial differences in the abundances of the smallest size classes between tidal and nontidal tributaries.
Length-frequency of selected species by tributary. Marine species include common snook, Centropomus undecimalis, red drum, Sciaenops ocellatus, and spot, Leiostomus xanthurus. Freshwater species include bluegill, Lepomis macrochirus, largemouth bass, Micropterus salmoides, and brook silverside, Labidesthes sicculus. SL = standard length
Discussion
The driving forces that shape communities within tributaries can be dynamic and influenced by local conditions or short-term, large-scale disturbances (Paperno and Brodie 2004; Paperno et al. 2006). Nekton communities within this study appeared to differ between the tidal and nontidal tributaries despite relatively small differences in the measured physical conditions between the tributary types. While mean salinity levels were significantly lower in nontidal tributaries during wet seasons, the influence of salinity on community composition may be muted as they were within tolerance ranges for many euryoecious estuarine species (Gunter and Hall 1963; Crocker et al. 1981; Gilmore Jr et al. 1983a, b). Regardless, nontidal tributaries supported a greater abundance of typical freshwater and marsh fishes (centrarchids, cichlids, poeciliids, and atherinids) than was found in the tidal tributary in this study. In contrast, the species composition within the tidal tributary contained many euryoecious estuarine species including seasonal juvenile recruits of several sciaenids, gerreids, centropomids, and lutjanids. The tidal tributary in this study is near Sebastian Inlet and contained salinity in the bottom waters associated with the presence of a salt wedge. This difference likely enables estuarine and coastal-spawning species to take greater advantage of the tidal habitat (Paperno and Brodie 2004). This is also supported by an analysis of adult fishes in the IRL by Kupschus and Tremain (2001) that indicated the distance to coastal egress/ingress points was a major factor structuring communities of larger mobile fish within the estuary. Studies in other estuaries have also indicated that the distance a habitat is from the mouth of an estuary is a key determinant of community composition (Lucas et al. 1998; Wagner and Austin 1999; Franco et al. 2006). The results presented suggest that similar effects may be involved in structuring communities in adjacent tributaries.
Tidal and nontidal tributaries within estuarine systems may function very differently regarding the ability to provide critical nursery habitat for sectors of the associated estuarine fish community (Guindon and Miller 1995; Ross 2003). Proximity (to inlets) alone may not be the sole determinant of whether a habitat will be effectively used by the estuarine community (Martino and Able 2003). Connectivity to other habitats (e.g., seagrass beds, mangrove forests) and hydrodynamic processes have also been shown to have a pronounced effect on structuring estuarine communities (Jelbart et al. 2007; Kang and King 2013). Similar to portions of estuaries around the world (Etherington and Eggleston 2003; Jenkins et al. 2010; Pereira et al. 2015), much of the northern portion of the IRL is nontidal (0–5 cm tidal amplitude) with wind stress effects being the primary source of water movement (Pitts 1989; Smith 1993). Therefore, the extent of saltwater intrusion into the nontidal tributaries in the northern IRL becomes more a function of episodic discharge rates and wind-driven water movement than tidal forcing (Liu et al. 1997). The relative stability of the physical conditions in nontidal tributaries may lead to reduced seasonal signals that could further limit the opportunities for estuarine recruitment. The combined influence of these differences in physical characteristics, ocean proximity, and inconsistent tidal transport should be reflected in differences in abundance and length-frequency of newly recruited individuals of coastal spawning species. In the present study, juveniles of red drum, common snook, and spot were found to recruit to the nontidal tributaries later in the year, with lower CPUE, and at larger sizes than to the tidal tributary.
Seagrass, mangrove marsh, and tidal tributary habitats near inlets serve as primary nurseries for many estuarine-dependent coastal species (Brown-Peterson et al. 1993; Taylor et al. 1998; Faunce and Paperno 1999). Nontidal tributaries that are distant from these habitats, and therefore more difficult for larvae to reach due to the lack of tidal forcing, may function as secondary nursery grounds during years when prevailing wind conditions are particularly favorable for recruitment. A secondary function of the nontidal tributaries may be to mitigate impacts due to density-dependent mortality of young-of-the-year during an exceptional recruitment year, loss of primary estuarine habitats, or periods of high predator abundance. None of these impacts were recorded in the northern IRL estuary during the period of this study so the corresponding role of nontidal tributaries during such conditions could not be confirmed. Long-term monitoring efforts within these nontidal tributaries are necessary to identify conditions under which their importance as secondary nursery areas may be demonstrated.
The results suggest that while instantaneous physical conditions within tidal and nontidal tributaries in the northern IRL might not differ greatly from each other (particularly in the low-salinity reaches), nekton communities that use the tributaries may differ substantially. Proximity of the tributary to ocean habitats may be the greatest determinant of habitat value to species that differentiate between seasons. The tidal SSR, closest to Sebastian Inlet, included species that were encountered only in this tributary (e.g. bonefish, Albula vulpes, weakfish-sand seatrout, scaled sardine, Harengula jaguana). During the dry season, several estuarine species extended farther upstream (Paperno and Brodie 2004) indicating that utilization of available habitats was affected by ambient physical conditions (e.g., salinity, current), biological influences (e.g., recruitment timing, habitat availability), or a combination of each. Increasing demands for freshwater and potential withdrawals from tidal and nontidal tributaries can alter the timing and magnitude of local physical conditions, which can affect habitat availability to the nekton community. Identifying previously undervalued nursery habitats is becoming increasingly important in the conservation of estuarine habitats (Sheaves et al. 2015). Many large-scale field studies, including the present study, suffer from a lack of replication (pseudoreplication), which can be logistically difficult to achieve (Hurlbert 1984; Millar and Anderson 2004). Therefore, our interpretation of the results provides a compelling and informative narrative, but may not be definitive, and future work comparing other tidal and nontidal tributaries are needed to validate these findings. Still, our results are valuable for promoting effective management and conservation of smaller tidal and nontidal tributaries, as well as adjacent riparian areas that serve as key habitats for economically and ecologically important fishes and invertebrates.
References
Able, K.W., D.M. Nemerson, R. Bush, and P. Light. 2001. Spatial variation in Delaware Bay (USA) marsh creek fish assemblages. Estuaries 24 (3): 441–452.
Anderson, M.J., R.N. Gorley, and K.R. Clarke. 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth: PRIMER-E.
Arnold, W.S., T.M. Bert, I.R. Quitmeyer, and D.S. Jones. 1998. Contemporaneous deposition of annual growth bands in Mercenaria mercenaria (Linnaeus), Mercenaria campechiensis (Gmelin), and their natural hybrid forms. Journal of Experimental Marine Biology and Ecology 223 (1): 93–109.
Ayvazian, S.G., L.A. Deegan, and J.T. Finn. 1992. Comparison of habitat use by estuarine fish assemblages in the Acadian and Virginian zoogeographic provinces. Estuaries 15 (3): 368–383.
Bergman, M. J., and L. J. Donnangelo. 1998. Simulation of freshwater discharge to the Sebastian River using regional parameters. Technical Memorandum No. 25. Palatka, Florida: Department of Water Resources, St. John’s Water Management District.
Bray, J.R., and J.T. Curtis. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs 27 (4): 325–349.
Brown-Peterson, N.J., M.S. Peterson, D.A. Rydene, and R.W. Eames. 1993. Fish assemblages in natural versus well-established recolonized seagrass meadows. Estuaries 16 (2): 177–189.
Chernoff, B., J. Conner, and C. Bryan. 1981. Systematics of the Menidia beryllina complex (Pisces: Atherinidae) from the Gulf of Mexico and its tributaries. Copeia 1981 (2): 319–336. https://doi.org/10.2307/1444221.
Chittenden, M.E. 1971. Status of the Striped Bass, Morone saxatilis, in the Delaware River. Chesapeake Science 12 (3):131.
Christensen, R.F. 1965. An ichthyological survey of Jupiter Inlet and Loxahatchee River, Florida. Unpubl. M.S. Thesis, 318. Fla. State Univ. Tallahassee, FL.
Clarke, K.R., and R.N. Gorley. 2015. PRIMER v7: user manual/tutorial. Plymouth: PRIMER-E.
Clarke, K.R., R.N. Gorley, P.J. Somerfield, and R.M. Warwick. 2014. Change in marine communities: an approach to statistical analysis and interpretation. 3rd ed. Plymouth: Natural Environment Research Council, Plymouth Marine Laboratory.
Colwell, R. K. 2013. EstimateS: statistical estimation of species richness and shared species from samples. Version 9. User’s Guide and application published: http://viceroy.eeb.uconn.edu/estimates. Accessed 2017 December 7.
Colwell, R.K., A. Chao, N.J. Gotelli, S.-Y. Lin, C.X. Mao, R.L. Chazdon, and J.T. Longino. 2012. Models and estimators linking individual-based and sample-based rarefaction, extrapolation, and comparison of assemblages. Journal of Plant Ecology 5 (1): 3–21.
Crocker, P.A., C.R. Arnold, J.D. Holt, and J.A. DeBoer. 1981. Preliminary evaluation of survival and growth of juvenile red drum (Sciaenops ocellata) in fresh and salt water. Journal of the World Mariculture Society 12 (1): 122–134. https://doi.org/10.1111/j.1749-7345.1981.tb00249.x.
Dahlberg, M.D. 1970. Atlantic and Gulf of Mexico menhaden’s, genus Brevoortia (Pisces: Clupeidae). Bulletin of the Florida State Museum 15 (3): 91–162.
Daniels, R.A., K.E. Limburg, R.E. Schmidt, D.L. Strayer, and R.C. Chambers. 2005. Changes in fish assemblages in the tidal Hudson River, New York. American Fisheries Society Symposium 45: 471–503.
Engle, V.D., and J.K. Summers. 1999. Latitudinal gradients in benthic community composition in western Atlantic estuaries. Journal of Biogeography 26 (5): 1007–1023.
Etherington, L.L., and D.B. Eggleston. 2003. Spatial dynamics of large-scale, multistage crab (Callinectes sapidus) dispersal: determinants and consequences for recruitment. Canadian Journal of Fisheries and Aquatic Sciences 60 (7): 873–887.
Faunce, C.H., and R. Paperno. 1999. Tilapia-dominated fish assemblages within an impounded mangrove ecosystem in east-central Florida. Wetlands 19 (1): 126–138.
Franco, A., P. Franzoi, S. Malavasi, F. Riccato, and P. Torricelli. 2006. Fish assemblages in different shallow water habitats of the Venice lagoon. Hydrobiologia 555 (1): 159–174.
Freeman, M.C., C.M. Pringle, E.A. Greathouse, and R.J. Freeman. 2003. Ecosystem-level consequences of migratory faunal depletion caused by dams. American Fisheries Society Symposium 35: 255–266.
Gibson, R.N. 2003. Go with the flow: tidal migration in marine animals. Hydrobiologia 503 (1-3): 153–161.
Gilmore, R.G. 1995. Environmental and biogeographic factors influencing ichthyofaunal diversity: Indian River Lagoon. Bulletin of Marine Science 57: 153–170.
Gilmore, R.G. 2001. The origin of Florida fish and fisheries. Proceedings of the Gulf and Caribbean Fisheries Institute 52: 713–731.
Gilmore, R. G., Jr, C. J. Donohoe, D. W. Cooke, and D. J. Herrema. 1981. Fishes of the Indian River Lagoon and adjacent waters, Florida. Technical Report 41. Ft. Pierce, Florida: Harbor Branch Oceanographic Foundation.
Gilmore, R.G., Jr., C.J. Donohoe, and D.W. Cooke. 1983a. Observations on the distribution and biology of east-central Florida populations of the common snook, Centropomus undecimalis (Bloch). Florida Scientist 46: 313–336.
Gilmore, R.G., Jr., P.A. Hastings, and D.J. Herrema. 1983b. Ichthyofaunal additions to Indian River Lagoon and adjacent waters, east-central Florida. Florida Scientist 46: 22–30.
Guindon, K.Y., and J.M. Miller. 1995. Growth potential of juvenile southern flounder, Paralichthys lethostigma, in low salinity nursery areas of Pamlico sound, in North Carolina, USA. Netherlands Journal of Sea Research 34 (1-3): 89–100.
Gunter, G., and G.E. Hall. 1963. Biological investigations of the St. Lucie estuary (Florida) in connection with Lake Okeechobee discharge through the St. Lucie canal. Gulf Research Reports 1 (5): 189–307.
Harris, J.E., R.S. McBride, and R.O. Williams. 2007. Life history of hickory shad in the St. Johns River, Florida. Transactions of the American Fisheries Society 136 (6): 1463–1471.
Hurlbert, S.H. 1984. Pseudoreplication and the design of ecological field experiments. Ecological Monographs 54 (2): 187–211.
Jelbart, J.E., P.M. Ross, and R.M. Connolly. 2007. Fish assemblages in seagrass beds are influenced by the proximity of mangrove forests. Marine Biology 150 (5): 993–1002.
Jenkins, G.P., S.D. Conron, and A.K. Morison. 2010. Highly variable recruitment in an estuarine fish is determined by salinity stratification and freshwater flow: implications of a changing climate. Marine Ecology Progress Series 417: 249–261.
Kang, S.-R., and S.L. King. 2013. Effects of hydrologic connectivity and environmental variables on nekton assemblage in a coastal marsh system. Wetlands 33 (2): 321–334.
Kirby-Smith, W.W., M.E. Lebo, and R.B. Hermann. 2001. Nekton variations in tributaries along a hydrologically modified North Carolina estuary. Estuaries 24 (1): 59–68.
Kupschus, S., and D. Tremain. 2001. Associations between fish assemblages and environmental factors in nearshore habitats of a subtropical estuary. Journal of Fish Biology 58 (5): 1383–1546.
Liu, J.T., G.A. Zarillo, and C.R. Surak. 1997. The influence of river discharge on hydrodynamics and mixing in a subtropical lagoon. Journal of Coastal Research 13: 1016–1034.
Love, J.W., A.K. Johnson, and E.B. May. 2006. Spatial and temporal differences of Atlantic Menhaden (Brevoortia tyrannus) recruitment across major drainages (1966ל2004) of the Chesapeake Bay watershed. Estuaries and Coasts 29 (5): 794–801.
Love, J.W., P. Chigbu, and E.B. May. 2009. Environmental variability affects distributions of coastal fish species (Maryland). Northeastern Naturalist 16 (2): 255–268.
Lucas, M.C., T. Mercer, E. Batley, P.A. Frear, G. Peirson, A. Duncan, and J. Kubecka. 1998. Spatio-temporal variations in the distribution and abundance of fish in the Yorkshire Ouse system. Science of the Total Environment 210: 437–455.
Lyons, J. 1996. Patterns in the species composition of fish assemblages among Wisconsin streams. Environmental Biology of Fishes 45 (4): 329–341.
Malavasi, S., R. Fiorin, A. Franco, P. Franzoi, A. Granzotto, F. Riccato, and D. Mainardi. 2004. Fish assemblages of Venice lagoon shallow waters: an analysis based on species, families and functional guilds. Journal of Marine Systems 51 (1-4): 19–31.
Martino, E.J., and K.W. Able. 2003. Fish assemblages across the marine to low salinity transition zone of a temperate estuary. Estuarine, Coastal and Shelf Science 56 (5-6): 969–987.
Millar, R.B., and M.J. Anderson. 2004. Remedies for pseudoreplication. Fisheries Research 70 (2-3): 397–407.
Nicolas, D., J. Lobry, M. Lepage, B. Sautour, O. Le Pape, H. Cabral, A. Uriarte, and P. Boët. 2010. Fish under influence: a macroecological analysis of relations between fish species richness and environmental gradients among European tidal estuaries. Estuarine, Coastal and Shelf Science 86 (1): 137–147.
Paperno, R., and R.B. Brodie. 2004. Effects of environmental variables upon the spatial and temporal structure of a fish community in a small, freshwater tributary of the Indian River Lagoon, Florida. Estuarine, Coastal and Shelf Science 61 (2): 229–241.
Paperno, R., D.M. Tremain, D.H. Adams, A.P. Sebastian, J.T. Sauer, and J. Dutka-Gianelli. 2006. The disruption and recovery of fish communities in the Indian River Lagoon, Florida, following two hurricanes in 2004. Estuaries and Coasts 29 (6): 1004–1010.
Pereira, H.H., L.M. Neves, M.R. Costa, and F.G. Araújo. 2015. Fish assemblage structure on sandy beaches with different anthropogenic influences and proximity of spawning grounds. Marine Ecology 36 (1): 16–27.
Peterson, M.S. 2003. A conceptual view of environment-habitat-production linkages in tidal river estuaries. Reviews in Fisheries Science 11 (4): 291–313.
Peterson, M.S., and S.T. Ross. 1991. Dynamics of littoral fishes and decapods along a coastal river-estuarine gradient. Estuarine, Coastal and Shelf Science 33 (5): 467–483.
Pitts, P.A. 1989. Upwind return flow in a coastal lagoon: seasonal-scale barotropic transport. Estuaries 12 (2): 92–97.
Ross, S.W. 2003. The relative value of different estuarine nursery areas in North Carolina for transient juvenile marine fishes. Fishery Bulletin 101: 384–404.
Schultz, E.T., K.M.M. Lwiza, M.C. Fencil, and J.M. Martin. 2003. Mechanisms promoting upriver transport of larvae of two fish species in the Hudson River estuary. Marine Ecology Progress Series 251: 263–267.
Sheaves, M., R. Baker, I. Nagelkerken, and R.M. Connolly. 2015. True value of estuarine and coastal nurseries for fish: incorporating complexity and dynamics. Estuaries and Coasts 38 (2): 401–414.
Smith, N.P. 1983. Tidal and low-frequency net displacement in a coastal lagoon. Estuaries 21: 180–189.
Smith, N.P. 1987. An introduction to the tides of Florida’s Indian River Lagoon. I. Water levels. Florida Scientist 50: 49–61.
Smith, N.P. 1993. Tidal and nontidal flushing of Florida’s Indian River Lagoon. Estuaries 16 (4): 739–746.
Snelson, F.F., Jr. 1983. Ichthyofauna of the northern Indian River Lagoon system, Florida. Florida Scientist 46: 187–206.
Springer, V. G. 1960. Ichthyological surveys of the lower St. Lucie and Indian rivers, Florida east coast. Report No. 60–19: 1–20, appendix 1. St. Petersburg, Florida: Florida State Board of Conservation Marine Laboratory.
Steward, J. S., and J. A. Van Arman. 1987. Indian River Lagoon joint reconnaissance report. Final report to Department of Environmental Regulation and OCRM/NOAA, contract no. CM-137. Palatka, Florida: St. John’s Water Management District and South Florida Water Management District.
Stewart, C.B., and F.S. Scharf. 2008. Estuarine recruitment, growth, and first-year survival of juvenile red drum in North Carolina. Transactions of the American Fisheries Society 137 (4): 1089–1103.
Suphunvorranop, T., and D. A. Clapp. 1984. A preliminary study of runoff hydrographs and pollutant concentrations for Turkey Creek basin. Technical Publication SJ 84–11. Palatka, Florida: St. Johns River Water Management District.
Taylor, D.S., G.R. Poulakis, S.R. Kupschus, and C.H. Faunce. 1998. Estuarine reconnection of an impounded mangrove salt marsh in the Indian River lagoon, Florida: short-term changes in fish fauna. Mangroves and Marshes 2 (1): 29–36.
Tremain, D.M., and D.H. Adams. 1995. Seasonal variation in species diversity, abundance, and composition of fish communities in the northern Indian River Lagoon, Florida. Bulletin of Marine Science 57: 171–192.
Tringali, M.D., S. Seyoum, E. Wallace, and M. Higham. 2004. The distribution of weakfish (Cynoscion regalis), sand seatrout (C. arenarius), and their hybrids in Florida Atlantic Waters. A special report to the Florida Fish and Wildlife Conservation Commission. June 2004. Florida Fish and Wildlife Research Institute. Report Number IHR2004-018.
USFWS (U.S. Fish and Wildlife Service). 1999. Multi-species recovery plan for South Florida. Hydric pine flatwoods. https://www.fws.gov/verobeach/MSRPPDFs/HydricPineFlat.pdf. Accessed 13 July 2017.
Wagner, C.M., and H.M. Austin. 1999. Correspondence between environmental gradients and summer littoral fish assemblages in low salinity reaches of the Chesapeake Bay, USA. Marine Ecology Progress Series 177: 197–212.
Acknowledgments
We thank the staff of the FWRI Melbourne Freshwater Fisheries Laboratory for their assistance and logistic support that made this study possible. We greatly appreciate the efforts of staff from the FWRI Indian River Laboratory for their assistance in the field, with special thanks to S. Landers for data entry support and Ted Switzer for statistical advice. We thank M. Clark, B. Crowder, D. Gandy, G. Huston, P. Stevens, B. Yeiser, and three anonymous reviewers for providing useful editorial comments that improved this manuscript. This study was supported in part by Florida’s State Wildlife Grants Program of the Florida Fish and Wildlife Conservation Commission and in part by recreational fishing license funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Mark S. Peterson
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Paperno, R., Dutka-Gianelli, J. & Tremain, D. Seasonal Variation in Nekton Assemblages in Tidal and Nontidal Tributaries in a Barrier Island Lagoon System. Estuaries and Coasts 41, 1821–1833 (2018). https://doi.org/10.1007/s12237-018-0389-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-018-0389-4