Abstract
The Mediterranean endemic seagrass Posidonia oceanica is generally regarded as a stenohaline species, highly sensitive to salinity increments; however, in a few particular cases, natural populations can grow under salinity levels above its normal threshold of tolerance. One such case is a population of P. oceanica in the southeastern coastal region of Spain, which is able to survive under the fluctuating influence of hypersaline waters coming from an adjacent coastal lagoon (Mar Menor). The present work examines the physiological mechanisms underlying the species’ ability to overcome this hypersaline stress and persist in the long term. To this end, the physiological, morphological, and population statuses of plants from the site of influence were compared to those from a reference site where plants grew under normal conditions. P. oceanica leaves from the influenced sites showed more negative water potentials than those from the reference sites as a response to maintain a positive water balance under hypersaline conditions. However, these lower water potentials were not explained by the accumulation of intracellular solutes since their osmotic potential were similar to reference leaves and their ionic content generally lower. In addition, these leaves also accumulated higher concentrations of proline and soluble sugars but these organic osmolytes are more likely acting as osmo-protectants, rather than as osmoticums. These responses indicated that plants growing at the influenced site have developed physiological strategies to maintain lower ion concentration in their leaf tissues in order to avoid the alteration of ion homeostasis (i.e., ionic ratios), which can be toxic for plant metabolism. Photosynthesis and photochemistry were not adversely affected in leaves exposed to hypersalinity; in fact, these processes showed a tendency to become enhanced, possibly to support the assimilation of anthropogenic nitrogen coming from the lagoon waters. At the individual and population levels, P. oceanica plants growing under the influence of hypersaline waters exhibited a marked reduction in shoot size compared to those at the reference site, while shoot density and population growth rates were similar to those of the reference site and remained stable over time. This shoot size reduction involves a lower demand of metabolic resources necessary to maintain vegetative structures, which is mainly required for metabolic adjustments at the physiological level. We propose that this morphological adaptation serves as a stress-coping mechanism, helping the species to inhabit this unfavorable environment, as has been widely described for terrestrial plants subjected to long-term environmental stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seagrasses are marine clonal plants that have successfully colonized the infralittoral zones of tropical and temperate coastal waters worldwide, where they form extensive and highly productive meadows that are recognized as one of the most ecologically relevant marine coastal habitats (Kuo and Den Hartog 2000). This group of plants has adapted to living completely submersed in a saline medium through the evolutionary development of physiological, ultrastructural, and morphological properties that allow them to grow in a wide range of coastal environments with contrasting salinity regimes (e.g., from estuaries to hypersaline coastal lagoons) (Arber 1920; Touchette 2007; Tyerman 1989, Jagels 1983; Iyer and Barnabas 1993). However, salinity increments above their natural ranges have the potential to alter important biochemical and physiological processes, which in turn can influence their metabolism, growth, development, and reproduction (Touchette 2007). Salinity is therefore considered—together with other primary factors such as light, temperature, and nutrients—a major environmental factor influencing seagrass structure, productivity, and distribution (McMillan and Moseley 1967; Zieman 1974; Walker and McComb 1990; Vermaat et al. 2000; Fernández-Torquemada et al. 2005; Ruiz et al. 2009a, b). In spite of the central role played by salinity in the ecology and evolution of seagrasses, basic knowledge regarding adaptation/acclimation mechanisms in response to hypersaline conditions is somewhat lacking, as compared to other aspects of seagrass biology (Larkum et al. 2006; Olsen et al. 2016).
Increases in the salinity of coastal waters can occur not only as a consequence of human activities but also through the changes brought about by global climate change (Fourqurean et al. 2003; Short and Neckles 1999). Currently, the most frequent anthropogenic cause of salinity increments in marine coastal waters is the discharge of brine from seawater reverse osmosis desalination plants—an industry that has experienced rapid growth worldwide during the last decade (Schiermeier 2008), particularly in the Mediterranean region (Dreizin et al. 2007; Palomar and Losada 2010).
All around the Mediterranean, the endemic seagrass Posidonia oceanica L. (Delile) forms dense meadows between the surface and depths of 30–40 m, constituting the climax stage of infralittoral zones (Ruiz et al. 2009a, b). These meadows are key habitats for the functioning of the Mediterranean coastal ecosystem, and their loss can result in complete devastation of the associated biological community and associated ecological services, entailing severe socioeconomic consequences (Boudouresque et al. 2009). Early studies on P. oceanica provided experimental evidence of its reduced capacity to withstand chronic salinity increments caused by brine discharges from desalination plants (Fernández-Torquemada and Sánchez-Lizaso 2005; Sánchez-Lizaso et al. 2008; Ruiz et al. 2009a). More recently, the physiological stress-response mechanisms induced by chronic hypersalinity in this species have been studied through mesocosm approaches employing different stress conditions (i.e., intensity and duration; Marín-Guirao et al. 2011, 2013a; Sandoval-Gil et al. 2012a) and plant ecotypes (e.g., shallow and deep populations; Sandoval-Gil et al. 2014a). Among other plant dysfunctions, salt-stressed P. oceanica leaves undergo photosynthetic inhibition and respiratory enhancement, which reduces their internal carbon (C) stores and growth rates, and increases plant mortality. The culmination of these experimental studies is confirmation that the salinity tolerance threshold of this species is very close to its ambient mean salinity. This conclusion agrees with the biological and ecological attributes of a stenohaline species, as well as the natural distribution of P. oceanica populations in open coastal waters with stable salinity levels (Boudouresque et al. 2009).
Despite the known sensitivity of this seagrass species to even very minor salinity increments, a few cases have demonstrated the existence of persistent P. oceanica populations confined to natural hypersaline environments, albeit with certain growth and reproductive constraints (Pergent et al. 2002; Tomasello et al. 2009). One particularly interesting case has been found in a locality along the southeastern coast of Spain, where P. oceanica has persisted over several decades while under the influence of hypersaline waters (i.e., salinity levels up to 42–43, practical salinity scale) originating from an adjacent hypersaline coastal lagoon named Mar Menor. Hypersaline waters flow from the lagoon into the Mediterranean Sea through the El Estacio channel—a natural inlet that allows water exchange between both water bodies (Terrados and Ros 1991). This channel was enlarged for navigation in the 1970s (Pérez-Ruzafa et al. 1991), increasing the level of water exchange and, hence, the outflow of hypersaline waters towards the Mediterranean Sea. Consequently, compared to the situation before the construction of the channel, the P. oceanica meadow situated in front of El Estacio has been experiencing higher salinity levels since that time.
To date, the response mechanisms developed by P. oceanica to cope with hypersaline stress—at the physiological, morphological, and population levels—has largely been addressed by means of short- and medium-term experiments performed in laboratory-based mesocosm systems. While this kind of experimental approach has been demonstrated to be very efficient and useful for such purposes, its interpolation to actual natural conditions has obvious limitations. For instance, mesocosm systems are unable to simulate the natural variability of environmental characteristics within the infralittoral zone where meadows grow. Furthermore, this kind of ex situ experimental approach limits our capacity to maintain a viable population of the species over periods longer than several months. Therefore, knowledge on how hypersalinity coping mechanisms allow Mediterranean seagrasses to survive and persist in natural environments is quite scarce. Accordingly, the discovery of P. oceanica meadows that have persisted in confined hypersaline environments represents an excellent opportunity to further our understanding of the tolerance mechanisms to hypersaline conditions that this seagrass species possesses in its natural environment, along with the consequences for plant fitness and long-term survival. To this end, we investigated a set of selected plant traits at the physiological [photosynthesis, respiration, water relations, nutrient content, and C and nitrogen (N) stable isotopes], individual shoot (leaf growth and morphology and shoot size), and population (shoot density and net population growth rate) levels in a P. oceanica meadow under the influence of the hypersaline outflows of Mar Menor and in plants of the same meadow growing under typical coastal waters with normal and stable salinity levels.
Materials and Methods
Study Area and Sampling Design
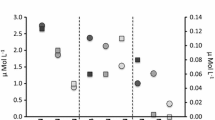
Mar Menor is one of the biggest coastal lagoons in the Mediterranean Sea. It is isolated by a 22-km-long sandbar (La Manga) and its waters have salinity levels (42–47, practical salinity scale) greater than the adjacent Mediterranean Sea (37–38) due to the low rates of precipitation and high rates of evaporation that are characteristic of the semi-arid climate of the region. On the other hand, the shallowness of Mar Menor means the lagoon waters experience faster seasonal temperature changes and wider annual temperature ranges than the adjacent Mediterranean Sea (Mas 1994). Historically, the exchange of waters between the lagoon and the Mediterranean was low, taking place through a few shallow inlets originally created by storms causing the breakdown of La Manga. However, in the early 1970s, the influx of Mediterranean waters into the lagoon was increased when the El Estacio inlet was dredged and widened to create a navigable artificial channel (Pérez-Ruzafa et al. 1991). Since then, most of the water exchange between the lagoon and the open sea occurs through this channel, wherein the intensity and direction of the water flow are affected by irregular and fluctuating processes, driven themselves by differences in the sea level between the water bodies, and mainly regulated by wind forces, atmospheric pressure, and tidal cycles (Arevalo 1988). Consequently, the P. oceanica meadow growing in the proximity of the channel mouth experiences intermittent exposure to lagoonal hypersaline waters of varying intensity and duration, with an annual mean salinity level of 39.2 ± 1.3 SD (Fig. 2), which is higher than in areas of the meadow away from the influence of the lagoonal waters (37.6 ± 0.2) (Fig. 2; Ruiz et al. 2009a).
Measurements of seagrass traits were performed at two randomly selected sites of influence (I-1 and I-2) close to the El Estacio inlet and at two other reference sites (R-1 and R-2) at Isla Grosa (Fig. 1). Water depths at all of the sites were similar, at 4–5 m. Vertical (ortotrophic) P. oceanica shoots were sampled at each site during the following two sampling periods: March 2012 (sampling I) and April 2012 (sampling II). In each sampling period and at each site, a number of samples were randomly collected within a circular area 25 m in diameter for subsequent analyses (see further details in the following sections). For each sample, vertical shoots were collected and transported (<2 h) in large coolers filled with ambient seawater collected at the time of sampling. Once in the laboratory, some shoots were kept in the dark and aerated in their ambient seawater at the temperature of sampling until the following day, while other shoots were immediately processed and/or stored for subsequent analysis.
Environmental Characterization of Sites
Underwater sensors for recording temperature, salinity, and irradiance (photosynthetic active radiation (PAR)) were installed to characterize the variability of these environmental factors in the P. oceanica meadows at both sites. Salinity and temperature were measured with a CT-Compact model sensor (Alec Electronics, Japan; resolution 0.001 °C, 0.001 mS cm−1), while irradiance was recorded using submersible photometers (MDS-Mk V/L PAR, Alec Electronics, Japan; resolution 1 μmol quanta m−2 s−1). At each site, both sensors were installed at 10 cm above the sea bed, anchored with steel rods in small meadow clearings to prevent shading by the meadow canopy. The sensors were programmed to acquire data every 10 min. Photometers were cleaned every week to avoid the possibility of shading effects as a result of fouling. Measurements were obtained throughout the year preceding the study (i.e., 2011), as well as in the 2 weeks preceding each sampling survey (i.e., the last 2 weeks in February 2012 and March 2012, respectively).
Leaf–Water Relations
Leaf–water variables [i.e., water potential (Ψ w ), osmotic potential (Ψ π ), and turgor pressure (P)] were determined in mature leaves of nine different shoots randomly selected from each site and sampling time (n = 9). Measurements of leaf-tissue osmolality (mmol kg−1 FW) were performed using a Wescor Vapor Pressure Osmometer 5520 (Logan, Utah, USA), following the method described in Sandoval-Gil et al. (2012a) and according to Tyerman et al. (1984). Osmolality was measured both in fresh and frozen blotted leaf segments to obtain the Ψ w and Ψ π for each shoot, and expressed in megapascals (MPa), using the van’t Hoff relation (Tyerman 1982). The P was then calculated as the absolute difference between Ψ w and Ψ π . Ambient seawater osmolality was also determined through measurements of seawater in 6.5-mm sample disks, following the standard protocol (Wescor Inc., Paris, France).
Inorganic and Organic Osmolytes and Elemental Composition of Leaves
Leaf osmolyte concentration was determined in nine samples of leaf tissues randomly selected from each site and sampling time (n = 9). Each sample comprised the first and second mature leaves of at least three individual shoots, avoiding old and necrotic parts of the leaves. Leaf tissues were scraped and cleaned with paper tissue soaked in distilled water to remove epiphytes and salts; then, samples were oven-dried at 60 °C for at least 48 h and ground to a fine powder. The amino acid proline was analyzed on fresh leaf tissues kept at −80 °C in sulfosalicylic acid (3%), using the acidic ninhydrin reagent colorimetric assay (Bates 1973), as described in Sandoval-Gil et al. (2012a).
Leaf inorganic cation (Na+, K+, Ca2+) and anion (Cl−) concentrations (mg g−1 DW) were determined in each sample using ionic chromatography (Metrohm 850 ProfIC AnCat-MCS, Herisau, Switzerland), as described in Marín-Guirao et al. (2013a).
The leaf content of non-structural carbohydrates (soluble fraction and starch; %DW) was determined using the anthrone assay protocol according to the methods also detailed in Marín-Guirao et al. (2013a) and based on Invers et al. (2004). Non-structural carbohydrates were also analyzed in the apical (2 cm) section of rhizomes (at least three rhizomes per sample, n = 9).
Total C and N content and the signatures of isotopic C (δ13C) and N (δ15N) were determined in six dried and grounded leaf samples (n = 6). An Elemental Analyzer FlashEA1112 (ThermoFinnigan, Waltham, USA) connected to an isotopic relation mass spectrometer (Thermo Finnigan, Waltham, USA) was used for the analytical process. Elemental C and N compositions are expressed as percentages per unit of dry weight and as the molar ratio C/N. Isotopic values are expressed in δ notation, as parts per thousand deviations from atmospheric N2 and Vienna Pee Dee Belemnite (VPDB), the international standards for C and N, respectively, according to the equation δX = (R sample / R standard − 1) × 1000, where X = 13C or 15 N and R = 13C:12C or 15N:14N.
Chlorophyll-a Fluorescence
Measurements of chlorophyll-a fluorescence emissions were performed following the protocol described in Marín-Guirao et al. (2013a, b), using a diving-PAM portable fluorometer (Walz, Germany). Saturation pulse measurements were undertaken in leaves maintained in laboratory conditions the day after each field sampling. Measurements were carried out at a fixed position on the first mature leaf of nine shoots per site (n = 9). The maximum quantum yield of PSII (F v /F m ) was measured in leaves adapted to the dark throughout the night. Rapid light-response curves of variable chlorophyll fluorescence were measured in the same leaf area after being exposed for 2 h to a homogenous irradiance of 100–110 μmol quanta m−2 s−1. The coefficient of photochemical quenching (qP) and the relative maximum electron transport rate (r-ETRmax) were obtained from the PAM WinControl program (Walz, Germany), and non-photochemical quenching (NPQ) was calculated according to the method of Maxwell and Johnson (2000). Finally, absolute ETRmax was calculated by multiplying the r-ETRmax by the leaf absorptance (the fraction of light absorbed by leaf pigments; see below).
Photosynthetic and Respiratory Rates
Determination of the maximum photosynthetic and respiratory rates was carried out on mature leaves of six shoots from each site and sampling time (n = 6) using an incubation chamber with a Clark-type O2 electrode (Hansatech, UK) connected to a controlled temperature circulating bath. From each shoot, a leaf segment with a surface area of approximately 2 cm2 from the middle part of the first mature leaf was used. Leaf pieces were first incubated in darkness for 10 min to determine dark respiration rates (R d ) and then exposed to 20, 80, and 250 μmol quanta m−2 s−1 to determine maximum photosynthetic rates (net-P max). Gross photosynthesis (gross-Pmax) was then calculated as the sum of net-P max and R d , and the ratio of gross-P max:R d was used as a proxy of the leaf metabolic C balance.
Pigment Content and Leaf Absorptance
Leaf pigment content and leaf absorptance (A) were determined in mature leaves of six shoots per site and sampling time (n = 6), following the methodology described in Marín-Guirao et al. (2013a) and Sandoval-Gil et al. (2014a). Light absorption was measured according to the opal glass technique (Shibata 1959), in which the absorbance (D) of intact leaf segments was measured between 380 and 750 nm with a spectrophotometer, and bleached leaves were used as a reference to correct for leaf absorption by non-pigmented structures. Leaf absorptance was calculated using A = 1–10−D. Following this measurement, leaf pigments were extracted from half of each leaf segment (∼1 cm2) in 10 ml of 80% acetone and examined spectrophotometrically at 470, 646, and 663 nm. The concentrations of chlorophyll-a and -b along with total carotenoids were calculated from the readings using the equations of Lichtenthaler and Wellburn (1983).
Vegetative Variables
A set of vegetative variables (number of leaves per shoot, maximum leaf width, maximum leaf length, shoot size, percentage of leaf necrotic tissue, relative leaf growth rate, and net shoot change) were considered in order to address the acclimative and adaptative consequences of ecophysiological responses at the level of plant fitness and survival. Ten vertical shoots were marked at each site in the first week of April, following the methods described in Ruiz et al. (2009a). Marked shoots were harvested 1 month later to determine relative leaf growth rates and morphology by measuring in each shoot (n = 10) the number of leaves per shoot, the length and width of each leaf, and the newly formed leaf segments (Dennison 1990). The relative growth rate was then estimated as the ratio of leaf material produced before marking to that produced after marking divided by the time interval and expressed as leaf surface produced by day (cm2 d−1).
Shoot density and net population growth were estimated by the changes in the total number of shoots within three fixed quadrats (40 × 40 cm) placed at each site from the year 2012, from the start of sampling I, following the methodology described in Ruiz et al. (2015). Counts of total shoot numbers were then repeated every year, at the same time, until 2015. Final and initial counts were normalized to the initial shoot number and expressed as percentages. Negative values of net shoot change indicate a net decline of the shoot population from the initial shoot number due to imbalances between shoot recruitment (i.e., shoot division or rhizome branching) and mortality.
Statistical Analysis
In order to detect significant differences in plant traits between P. oceanica growing at the I and R sites, two-level nested ANOVA was used, in which the “condition” (i.e., I or R) was the fixed factor and the “site” (i.e., 1 or 2) was the nested factor within the condition. Owing to the marked natural changes that plants experience in terms of most of the selected variables at this time of the year (i.e., from late winter to early spring; Alcoverro et al. 1995, 1998), and to the lack of independence between the sampling times, the differences between conditions were examined in two different nested-ANOVA analyses for each sampling time. Changes in shoot density and net population growth were examined by univariate repeated measures ANOVA (one-factor rmANOVA; Crowder and Hand 1990). Before the analyses, data were checked for the assumptions of normality and homocedasticity and transformed when necessary. In the case of rmANOVA, the assumption of sphericity was assessed using Mauchly’s sphericity test. A post hoc mean comparison test (Student-Newman-Keuls (SNK); Zar 1984) was performed when significant differences were found (p < 0.05). All data are represented as mean values ± standard error.
Results
Salinity levels measured at the R sites were almost stable, annually ranging between 37 and 38. This was in contrast to the high-salinity fluctuations (37–42.5) recorded at the I sites (Fig. 2 and Table 1). Seawater salinity at the I sites showed mean and maximum levels for the 2-week period preceding sampling I of 38.3 ± 0.7 and 41.2, respectively. At the R sites, these values were lower (37.9 ± 0.05 and 38, respectively) (Table 1). As reflected in Fig. 2, sampling I was a period of low lagoonal water outflow, and the salinity at the I sites did not exceed 38.5 throughout the 5 days preceding the sampling, showing a third quartile value of 38.2. The influence of hypersaline waters was clearly more intense during sampling II, when the third quartile rose to a salinity of 40.1 (Table 1 and Fig. 2). The salinity at the time of plant collection was ∼40.2, and mean and maximum salinity levels for the two previous weeks were 39.4 ± 1.5 and 42.3, respectively. Indeed, during this period, high levels of salinity fluctuation at the I sites were observed, where salinity reached levels higher than 40.0, at least during the 10 days preceding plant sampling (Fig. 1). As shown in Table 1, the standard deviation of mean salinity at the I sites was one order of magnitude higher than at the R sites for sampling I and up to two orders of magnitude higher for sampling II. The influence of the lagoonal waters was also reflected in temperature and irradiance, albeit to a lesser extent than in salinity. During both sampling periods, temperature at the I sites were around 1 °C higher compared to at the R sites, while irradiance were around 4.3 mol quanta m−2 d−1 lower (Table 1), which involves a reduction in light availability of ca. 30%.
A summary of the statistical analysis results of the different plant traits is provided in Table S1 of the online supplementary material. P. oceanica leaves from the I sites showed significantly lower water potential (Ψ w ; Fig. 3a) than those from the R sites during both samplings, although greater differences were found during sampling II, characterized by higher outflow of hypersaline waters from the lagoon (Fig. 3). Also, turgor pressure (P; Fig. 3c) was significantly lower in leaves from the I sites, while no differences were observed in the leaf osmotic potential (Ψ π ; Fig. 3b) of leaves from both sites.
Leaf tissue water relations (expressed in MPa) of P. oceanica shoots from the reference (R-1 and R-2) and lagoon-influenced (I-1 and I-2) sites during both sampling periods (I and II). a Water potential (Ψ w ). b Osmotic potential (Ψ π ). c Turgor pressure (P). Bars represent the mean values and standard errors. Black dots inside the bars represent the seawater osmolality at each site. Different letters indicate the groups of homogeneous means obtained in the post hoc SNK test (p < 0.05)
Leaves from the I sites had lower ionic content (except for Ca2+) than R leaves at both sampling times (Fig. 4a–d). Specifically, for K+ (Fig. 4c), greater differences corresponded to sampling I when the K+:Na+ molar ratio was significantly lower (Fig. 4e). On the contrary, the leaf content of Ca2+ was higher in leaves from the I sites, but uniquely at sampling I. In both sampling periods, the Ca2+:Na+ molar ratio remained significantly higher in leaves at the I sites compared to those from the R sites (Fig. 4f).
Leaf ionic content (Cl−, Na+, K+, and Ca2+) and ionic molar ratios (K+:Na+ and Ca2+:Na+) of P. oceanica leaves from the reference (R-1 and R-2) and lagoon-influenced (I-1 and I-2) sites during both sampling periods (I and II). Bars represent the mean values and standard errors. Different letters indicate the groups of homogeneous means obtained in the post hoc SNK test (p < 0.05)
P. oceanica leaves from the I sites showed twofold to fourfold higher proline concentrations than R leaves, for both sampling periods (Fig. 5a). Leaf soluble sugar content in shoots from the I sites was around double that in those from the R sites in both sampling periods (Fig. 5b). However, rhizomes from the I sites showed lower concentrations of soluble sugars, while the starch content was similar between the rhizomes from both sites (Fig. 5c, d).
Leaf tissue content of a the amino acid proline and b soluble sugars and the rhizome tissue content of c soluble sugars and d starch for P. oceanica shoots from the reference (R-1 and R-2) and lagoon-influenced (I-1 and I-2) sites during both sampling periods (I and II). Bars represent the mean values and standard errors. Different letters indicate the groups of homogeneous means obtained in the post hoc SNK test (p < 0.05)
Leaves from the I sites exhibited much higher N content (70% on average) than leaves from the R sites during both sampling periods; and as a consequence, their C/N ratio was almost 40% lower (Fig. 6a, c). Additionally, more positive values of δ15N were detected in leaves from the I sites (Fig. 6b), while less negative values of δ13C were measured in these leaves, but uniquely during sampling II (Fig. 6d). The δ13C enrichment observed in leaves from the I sites was in the order of 2‰, which is similar in magnitude to its seasonal variation in P. oceanica leaves (Vizzini et al. 2003).
The a leaf tissue content of N, b leaf δ15N isotope signature, c leaf C:N molar ratio, and d leaf δ13C signature for P. oceanica shoots from the reference (R-1 and R-2) and lagoon-influenced (I-1 and I-2) sites during both sampling periods (I and II). Bars represent the mean values and standard errors. Different letters indicate the groups of homogeneous means obtained in the post hoc SNK test (p < 0.05)
Chlorophyll-a fluorescence parameters (i.e., F v /F m , NPQ, qP, and absolute-ETRmax) did not show any significant differences between sites during sampling I (Fig. 7). However, significant differences were found during sampling II, and leaves from the I sites displayed significantly increased (26%) photochemical capacity and higher electron transport rates (30%) in relation to the R leaves (Fig. 7c, d). Gross and net maximum photosynthetic rates were similar to those determined in the R leaves during both sampling periods (Fig. 7e, g), although during sampling II, leaves from the I sites had higher gross-P rates (albeit not statistically significant) than leaves from the R sites. Also, P. oceanica leaves from the I sites increased their respiratory activity (52%, but not statistically significant) during sampling I and reached rates that were twofold higher than in the R leaves during sampling II (Fig. 7f). The daily leaf metabolic C balance (gross-P max:R d) did not differ between shoots from both sites (Fig. 7h).
Chlorophyll-a fluorescence parameters and descriptors derived from P-E curves of P. oceanica leaves from the reference (R-1 and R-2) and lagoon-influenced (I-1 and I-2) sites during both sampling times (I and II). a Maximum quantum yield of PSII. b Thermal energy dissipation. c Photochemical quenching coefficient. d Absolute electron transport rate. e Maximum net photosynthesis. f Respiratory activity. g Maximum gross photosynthetic activity. h Estimated C balance. Bars represent the mean values and standard errors. Different letters indicate the groups of homogeneous means obtained in the post hoc SNK test (p < 0.05)
Leaf pigment content (chlorophyll-a and -b and total carotenoids) and their molar ratios (Chl-b:a, carotenoids:Chl-a) were generally similar in both sites and during both sampling periods (Fig. S1 of the online supplementary material).
Plants under both conditions showed high and significant differences in their leaf morphology. Compared to the R sites, shoots from the I sites were fourfold smaller and their leaves were significantly narrower (20%) and three times shorter (Fig. 8a, b). These leaves also showed a significant increment in the percentage of necrotic leaf marks (Fig. 8c), while the number of leaves per shoot was similar among both sites (Fig. 8d). Relative leaf growth rates were significantly higher in I sites.
Vegetative descriptors of P. oceanica shoots from the reference (R-1 and R-2) and lagoon-influenced (I-1 and I-2) sites. a Maximum leaf width. b Shoot size. c Percentage of leaf necrotic area. d Number of leaves per shoot. e Relative leaf growth rate. Bars represent the mean values and standard errors. Significant differences between shoots at both sites are represented with asterisks: ** = p < 0.01, *** = p < 0.001
Shoot density and net population growth were similar between sites and did not change during the study period or the following 3 years (i.e., 2012–2015; Fig. 9).
The a net-shoot change and b shoot density determined in P. oceanica meadows at the reference (R-1 and R-2) and lagoon-influenced (I-1 and I-2) sites and determined annually from 2012 to 2015. Bars represent the mean values and standard errors. Dashed lines in a represent the accumulative net-shoot change between 2012 and 2015
Discussion
P. oceanica plants growing in the vicinity of the channel that connects the Mar Menor coastal lagoon with the Mediterranean Sea are intermittently exposed to outflows of hypersaline waters from the Mar Menor coastal lagoon. Plants at these sites influenced by hypersaline waters (i.e., the I sites) also experience higher temperatures and lower irradiance than plants from the same meadow outside such influence (i.e., the R sites). However, compared to these factors, salinity was the environmental parameter that experienced the greater deviations from normal conditions. At the I sites, 57 and 35% of salinity observations (on an annual basis) exceeded salinity levels of 38.5 and 40, respectively; these values were well above those measured at the R sites and clearly surpassed critical levels established previously for this seagrass species under chronic hypersaline conditions (Sánchez-Lizaso et al. 2008; Fernández-Torquemada and Sánchez-Lizaso 2005; Ruiz et al. 2009a; Marín-Guirao et al. 2011; Sandoval-Gil et al. 2012a, b, 2014a). In fact, some of the observed effects on the I site plants are consistent with effects previously reported for the species under hypersaline stress (Ruiz et al. 2009a; Marín-Guirao et al. 2011, 2013a; Sandoval-Gil et al. 2012a, b, 2014a; Garrote-Moreno et al. 2015). The temperature increases were very small and unable to explain such effects (Lee et al. 2007; Olsen et al. 2012), and the light reduction reported at the I sites (30%) was well below the levels needed to cause any significant alteration in physiology, growth, or meadow structure in shallow P. oceanica meadows (ca. 80%; Ruiz and Romero 2001; Serrano et al. 2011). But, unexpectedly, despite these levels of hypersaline stress, the P. oceanica population studied at the I sites had shoot densities equal to those measured at the reference site and were stable over time. As discussed below, our results suggest that plants have developed acclimative homeostatic mechanisms to cope with the huge salinity fluctuations at the I sites, although interactions with other factors (i.e., nutrients) are involved.
P. oceanica shoots at the I sites exhibited reduced (i.e., more negative) values of leaf Ψ w compared to shoots at the R sites during both sampling periods (I and II). This is a primary osmo-acclimative response of this and other seagrass species to cope with hypersaline stress, because it allows for the maintenance of positive water balances under hypersaline conditions (Tyerman 1989; Murphy et al. 2003; Koch et al. 2007; Sandoval-Gil et al. 2012a). Previous experimental studies have demonstrated that the reduction in Ψ w is mainly accomplished via osmo-regulation processes in P. oceanica leaves exposed to hypersaline stress, which implies the active accumulation of osmolytes (ions and organic solutes) and a decrease in Ψ π (Sandoval-Gil et al. 2012a; Marín-Guirao et al. 2013a, 2014a). Our results strongly contrast with these previous observations. Firstly, we found similar mean values of Ψ π in leaves from both sampling conditions. Secondly, ions of Cl− and Na+, which typically function as osmoticums during osmo-regulation, were detected in lower concentrations in leaves from the I sites. And thirdly, both Ψ π and ion concentrations were stable across time regardless the degree of influence of hypersaline waters, as indicated by the lack of significant differences of these variables between sampling times. These results suggest that P. oceanica plants growing near the El Estacio inlet and under the fluctuating influence of the Mar Menor hypersaline waters during decades possess an osmo-acclimation strategy different to that documented in previous mesocosm experiments, where plants growing under natural salinity levels (∼37–38) were exposed to persistent hypersalinity (up to 43) during time periods from days to months (Sandoval-Gil et al. 2012a; Marín-Guirao et al. 2013a, 2014a). Thus, it can be inferred that, at longer time scales, the species has been able to develop physiological and/or structural properties to support this particular strategy and that allow plants to resist fluctuating salinities in the long term.
The maintenance of reduced concentrations of ions may help in sustaining ionic homeostasis (i.e., higher K+/Na+ and Ca2+/Na+) in leaves at the I sites, which is considered a primary factor for plant tolerance to hyperionic stress (Hasegawa et al. 2000; Cramer 2002; Marín-Guirao et al. 2013a; Garrote et al. 2014a). It is likely that P. oceanica plants at the I sites have a certain capacity to exclude (or pump out) toxic ions from young leaf tissues (i.e., the leaf tissues analyzed here) and transport them to old leaf tissues (or rhizomes; Garrote-Moreno et al. 2015). Also, the increments in Ca2+ detected in the leaves of stressed plants can be related to the regulation of these ions, since it can mediate the activation of salt stress signaling for the control of ion homeostasis (i.e., influx and efflux systems) (Kirst 1989; Niu et al. 1995; Hasegawa et al. 2000). On the other hand, shoots growing at the I sites exhibited higher concentrations of organic osmolytes (proline and soluble sugars) in their leaves compared to shoots from the R sites. However, since this higher accumulation was not reflected in more negative values of leaf osmotic potential (Ψ π ), it is very likely that these compatible solutes are acting as osmo-protectants more than as osmoticums, thereby helping to stabilize and protect intracellular structures and metabolic processes (Touchette 2007), and being possible sinks for excess energy and reductants (Lambers et al. 2006).
The avoidance of toxic ion accumulation and the active accumulation of osmo-protectants are also consistent with the lack of photosynthetic inhibition and pigment alterations in leaves from the I sites, which again is contrary to previous findings regarding P. oceanica and other seagrasses exposed to hypersalinity (Ogata and Matsui 1964; Biebl and McRoy 1971; Kerr and Strother 1985; Fernández-Torquemada et al. 2005; Kahn and Durako 2006; Marín-Guirao et al. 2011, 2013a; Sandoval-Gil et al. 2014a). These two responses, however, may involve a large amount of metabolic energy (Munns 2002), which is consistent with the increased respiratory activity found in leaves at the I sites, as compared to those from the R sites (Greenway and Munns 1980; Hasegawa et al. 2000). Higher respiration rates can impair the plant C balance, giving rise to C starvation and plant death in the long term (Alcoverro et al. 2001; Ruiz et al. 2009a; Marín-Guirao et al. 2013a). However, we found no evidence of an exhaustion of internal C stores.
Furthermore, interactions between hypersalinity and increased N availability could also be involved in these unexpected responses. P. oceanica leaves at the I sites showed a more enriched signal of δ15N and higher N content in leaf tissues—an indicator of anthropogenic nutrient enrichment (McClelland and Valiela 1998; Fry et al. 2003; Ruiz et al. 2010). This is consistent with the huge amounts of anthropogenic N that have entered into the Mar Menor Sea during recent decades (Velasco et al. 2006; Marín-Guirao et al. 2008). In fact, large amounts of dissolved inorganic N (DIN, mostly NH4 +) coming from the Mar Menor have been measured near the El Estacio channel (i.e., the I sites) (ca. 12 μM; Navarro et al. 2007). To assimilate DIN into organic N compounds (free amino acids) takes a large amount of metabolic energy, as well as the utilization of the C skeletons of carbohydrates (Invers et al. 2004; Touchette and Burkholder 2007). This can be provided by photosynthesis and plant C reserves, as documented for Zostera marina exposed to increasing external NH4 + concentrations (Villazán et al. 2015). In this study, P. oceanica plants from the I sites showed higher photosynthetic capacities (i.e., higher ETR and qP) and leaf tissues more enriched in 13C, as compared to the R plants, during periods of strong hypersaline influence (i.e., sampling II); also, these plants exhibited lower concentrations of soluble sugars in rhizome tissues. Thus, it seems that these plants stimulate photosynthesis and mobilize C stored in non-photosynthetic tissues as compensation mechanisms to overcome the enhanced metabolic demands induced by external N enrichment. On the other hand, the incorporation of supplementary NO3 − might also be helping the plants to avoid the toxic effects of increased salinity, because it can function as an osmoticum and osmo-protectant by replacing ions, as demonstrated in salt marsh species (e.g., Spartina alterniflora) (Colmer et al. 1996; Kaya and Higgs 2003; Hessini et al. 2009). In addition, these results are consistent with the lack of any extensive decline in natural P. oceanica meadows exposed for several years to hypersaline brines with high DIN concentrations from a desalination plant (Gacia et al. 2007).
Another striking result was the reduced turgor pressure, P, in leaves from the I sites compared to those from the R sites. Although it is unfortunate that our results cannot provide a conclusive explanation of this response, it is more likely to be the result of modifications of the cell wall properties through the so-called cell wall hardening processes. As have been described in other seagrass species under constant (Sandoval-Gil et al. 2012a, b) or fluctuating (Sandoval-Gil et al. 2014b) saline increments, leaves from the I sites may have developed thicker or more rigid cell walls, which is one of the main dehydration avoidance mechanisms in plants (Verslues et al. 2006). Rigid cell walls enable the rapid adjustment of the leaf water potential as external salinity changes through the reduction in turgor pressure promoted by small intracellular water losses. This osmo-acclimative strategy is more energy-conservative than osmo-regulation and allows, at the same time, a more rapid response to changes in external salinity, which can be beneficial for P. oceanica plants living in a highly fluctuating saline regime. However, the fact that these leaves have displayed similar turgor pressures under two contrasting conditions of hypersaline influence (sampling I vs sampling II) may alternatively be suggesting that they have developed more elastic cell walls as described in some macroalgae growing in estuaries with large saline fluctuations (Bisson and Kirst 1995). Elastic walls lead to lower turgor pressures but enable to keep them constant under fluctuating salinities thanks to their rapid adjustment to potential changes in the cell volume. Modifications of the cell wall properties have not been previously evidenced in P. oceanica plants exposed to constant hypersaline levels during weeks and months (Sandoval-Gil et al. 2012a, 2014a; Marín-Guirao et al. 2013a; Garrote et al. 2014a), and targeted experiments are needed to discern which of the two suggested alternatives is the one followed by the species. However, it is possible that the intermittent stress produced during decades by hypersaline lagoon waters could have facilitated the development of modified cell walls in the species most probably through environmental-induced epigenetic modifications (e.g., Dodd and Douhovnikoff 2016; Latzel et al. 2016).
At the vegetative level, the reduced plant size together with increased leaf necrosis observed in the I site plants are considered common symptoms of hypersaline stress in P. oceanica (Fernández-Torquemada and Sánchez-Lizaso 2005; Gacia et al. 2007; Ruiz et al. 2009a; Marín-Guirao et al. 2013a). Nonetheless, contrary to expectation, plants at the I sites grew faster than the R plants. This result, together with the absence of any severe alterations in the main physiological functions (e.g., photosynthesis and metabolic C balance) and the maintenance of stable shoot densities equal to those measured in the R meadow, conflicts with the increased metabolic demands necessary to maintain ionic homeostasis (i.e., ionic transport), osmo-acclimation mechanisms, or N assimilation. This anomalous situation can only be explained by the considerable reduction in plant size observed at the I sites, which is probably the result of reduced leaf turgor pressures (Cosgrove 1993), as it has been proposed as a stress coping mechanism at the whole plant level to survive under continuous stress constraints (Lichtenthaler 1996). Thus, these undersized plants could potentially reduce their metabolic energy consumption for maintaining vegetative structures, and hence, it should be considered as a key morphological adaptation of this species in overcoming the stressful hypersaline environment.
In summary, contrary to expectation based on previous experiments that used short- and medium-term expositions to chronic hypersaline conditions, the results obtained in this study show that this species of seagrass is able to cope with fluctuating hypersalinity stress, and persist over time, in a location influenced by the hypersaline waters of a coastal lagoon (Mar Menor). For the first time, the physiological basis of this particular type of hypersalinity tolerance is revealed, which also involves key morphological adaptations. These are, for instance, the ability to avoid toxic ion accumulation in plant cells; enhance photosynthesis, which may compensate the enhancement of respiration; and accumulate higher concentrations of organic osmolytes that allow osmotic stability. Also, we propose that this specific genotype/ecotype of P. oceanica manages to survive in this long-term unfavorable environment by limiting its size, as typically documented in terrestrial plants subjected to long-term environmental stress (Lichtenthaler 1996). This notion is also consistent with the existence of undersized P. oceanica shoots in other potentially unfavorable environments, such as the semi-enclosed lagoon of Stagnone di Marsala, Sicily, where hypersalinity can reach levels of 48‰ (Tomasello et al. 2009). Therefore, while P. oceanica is certainly a stenohaline species, it also, however, demonstrates a certain capacity to resist and survive under fluctuating hypersaline stress in natural environments. Finally, it is important to acknowledge that we ignored whether this particular case might have resulted from phenotypic plasticity mediated by epigenetic variation (Dodd and Douhovnikoff 2016; Latzel et al. 2016) or by selection of particular genotypes. Also, other factors, such as N availability, are necessary for the explanation of the metabolic and morphological adjustments adopted by P. oceanica to persist at the particular site studied here, and hence, caution should be applied if directly extrapolating the present results to other situations. Finally, these particular populations could serve as an experimental model, at the Mediterranean scale, for what might happen under predicted environmental changes associated with global climate change.
References
Alcoverro, T., C.M. Duarte, and J. Romero. 1995. Annual growth dynamics of Posidonia oceanica: contribution of large-scale versus local factors to seasonality. Marine Ecology Progress Series 120: 203–210.
Alcoverro, T., T. Manzanera, and J. Romero. 1998. Seasonal and age-dependent variability of Posidonia oceanica (L.) Delile photosynthetic parameters. Journal of Experimental Marine Biology and Ecology 230: 1–13.
Alcoverro, T., T. Manzanera, and J. Romero. 2001. Annual metabolic carbon balance of the seagrass Posidonia oceanica: the importance of carbohydrate reserves. Marine Ecology Progress Series 211: 105–116.
Arber, A. 1920. Water plants. A Study of Aquatic Angiosperms: Cambridge University Press, Cambridge.
Arevalo, L. 1988. El Mar Menor como sistema forzado por el Mediterráneo. Control hidráulico y agentes de fuerza. Boletín Instituto Español de Oceanografía 5: 63–96.
Bates, L. 1973. Rapid determination of free proline for water-stress studies. Plant and Soil 39: 205–207.
Biebl, R., and C.P. McRoy. 1971. Plasmatic resistance and rate of respiration and photosynthesis of Zostera marina at different salinities and temperatures. Marine Biology 8: 48–56.
Bisson, M.A., and G.O. Kirst. 1995. Osmotic acclimation and turgor pressure regulation in algae. Naturwissenschaften 82: 461–471.
Boudouresque, F.C., G. Bernard, G. Pegent, A. Shili, and M. Verlaque. 2009. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: a critical review. Botanica Marina 52: 395–418.
Colmer, T.D., W.M. Teresa, F.A. Läuchli, and R.M. Higashi. 1996. Interactive effects of salinity, nitrogen and sulphur on the organic solutes in Spartina alterniflora leaf blades. Journal of Experimental Botany 47: 369–375.
Cosgrove, D.J. 1993. How do plant cell walls extend? Plant Physiology 102: 1–6.
Cramer, G.R. 2002. Sodium-calcium interactions under salinity stress. In Salinity: Environment e plants e molecules, ed. A. Läuchli and U. Lüttge, 205–229. Dordrecht: Kluwer Academic Publishers.
Crowder, M.J., and D.J. Hand. 1990. Analysis of repeated measures. New York: Chapman & Hall.
Dennison, W.C. 1990. Leaf production. In Seagrass research methods, ed. R.C. Phillips and P. McRoy. Paris: Unesco 81-85 pp.
Dodd, R.S., and V. Douhovnikoff. 2016. Adjusting to global change through clonal growth and epigenetic variation. Frontiers in Ecology and Evolution 4: 86. doi:10.3389/fevo.2016.00086.
Dreizin, Y., A. Tenne, and D. Hoffman. 2007. Integrating large scale seawater desalination plants within Israel’s water supply system. Desalination 220: 132–149.
Fernández-Torquemada, Y., and J.L. Sánchez-Lizaso. 2005. Effects of salinity on leaf growth and survival of the Mediterranean seagrass Posidonia oceanica (L.) Delile. Journal of Experimental Marine Biology and Ecology 320: 57–63.
Fernández-Torquemada, Y.F., J.L. Sánchez-Lizaso, and J.M.G. González-Correa. 2005. Preliminary results of the monitoring of the brine discharge produced by the SWRO desalination plant of Alicante (SE Spain). Desalination 182: 579–590.
Fourqurean, J.W., J.N. Boyer, M.J. Durako, L.N. Hefty, and B.J. Peterson. 2003. Forecasting responses of seagrass distributions to changing water quality using monitoring data. Ecological Applications 13: 474–489.
Fry, B., A. Gace, and J.V. McClelland. 2003. Chemical indicators of anthropogenic nitrogen loading in four Pacific estuaries. Pacific Science 57: 77–101.
Gacia, E., O. Invers, M. Manzanera, E. Ballesteros, and J. Romero. 2007. Impact of the brine from a desalination plant on a shallow seagrass (Posidonia oceanica) meadow. Estuarine Coastal and Shelf Science 72: 579–590.
Garrote-Moreno, A., J.M. Sandoval-Gil, J.M. Ruiz, L. Marín-Guirao, J. Bernardeau-Esteller, R. García, and J.L. Sánchez-Lizaso. 2015a. Plant water relations and ion homoeostasis of Mediterranean seagrasses (Posidonia oceanica and Cymodocea nodosa) in response to hypersaline stress. Marine Biology 162 (1): 55–68.
Garrote-Moreno, A., Y. Fernández-Torquemada, and J.L. Sánchez-Lizaso. 2014. Salinity fluctuation of the brine discharge affects growth and survival of the seagrass Cymodocea nodosa. Marine Pollution Bulletin 81 (1): 61–68.
Garrote-Moreno, A., A. McDonald, T.D. Sherman, J.L. Sánchez-Lizaso, K.L. Heck, and J. Cebrian. 2015b. Short-term impacts of salinity pulses on ionic ratios of the seagrasses Thalassia testudinum and Halodule wrightii. Aquatic Botany 120: 315–321.
Greenway, H., and R. Munns. 1980. Mechanisms of salt tolerance in non halophytes. Annual Review of Plant Physiology 31: 149–190.
Hasegawa, P.M., R.A. Bressan, J.K. Zhu, and H.J. Bohnert. 2000. Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology 51: 463–499.
Hessini, K., J.P. Martínez, M. Gandour, A. Albouchi, A. Soltani, and C. Abdelly. 2009. Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environmental and Experimental Botany 67: 312–319.
Invers, O., G.P. Kraemer, M. Pérez, and J. Romero. 2004. Effects of nitrogen addition on nitrogen metabolism and carbon reserves in the temperate seagrass Posidonia oceanica. Journal of Experimental Marine Biology and Ecology 303: 97–114.
Iyer, V., and A.D. Barnabas. 1993. Effects of varying salinity on leaves of Zostera capensis Setchell. I. Ultrastructural changes. Aquatic Botany 46: 141–153.
Jagels, R. 1983. Further evidence for osmoregulation in epidermal leaf cells of seagrasses. American Journal of Botany 70: 327–333.
Kahn, A.E., and M.J. Durako. 2006. Thalassia testudinum seedling responses to changes in salinity and nitrogen levels. Journal of Experimental Marine Biology and Ecology 335: 1–12.
Kaya, C., and D. Higgs. 2003. Supplementary potassium nitrate improves salt tolerance in bell pepper plants. Journal of Plant Nutrition 26: 1367–1382.
Kerr, E.A., and S. Strother. 1985. Effects of irradiance, temperature and salinity on photosynthesis of Zostera muelleri. Aquatic Botany 23: 177–183.
Kirst, G.O. 1989. Salinity tolerance of eukaryotic marine algae. Annual Review of Plant Physiology and Plant Molecular Biology 40: 21–53.
Koch, M.S., S.A. Schopmeyer, C. Kyhn-Hansen, C.J. Madden, and J.S. Peters. 2007. Tropical seagrass species tolerance to hypersalinity stress. Aquatic Botany 86: 14–24.
Kuo, J., and C. Den Hartog. 2000. Seagrasses: a profile of an ecological group. Biologia Marina Mediterranea 7: 3–17.
Lambers, H., F.S. Chapin, and T.L. Pons. 2006. Plant physiological ecology. New York: Springer-Verlag.
Larkum, A.W.D., E.A. Drew, and P.J. Ralph. 2006. Photosynthesis and metabolism in seagrasses at the cellular level. In Seagrasses: Biology, ecology and conservation, ed. A.W.D. Larkum, R.J. Orth, and C.M. Duarte, 323–345. The Netherlands: Springer.
Latzel, V., A.P. Rendina Gonzalez, and J. Rosenthal. 2016. Epigenetic memory as a basis for intelligent behavior in clonal plants. Frontiers in Plant Science 7: 1354. doi:10.3389/fpls.2016.01354.
Lee, K.-S., S.R. Park, and Y.K. Kim. 2007. Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: a review. Journal of Experimental Marine Biology and Ecology 350: 144–175.
Lichtenthaler, H.K. 1996. Vegetation stress: an introduction to the stress concept in plants. Plant Physiology 148: 4–14.
Lichtenthaler, H.K., and A.R. Wellburn. 1983. Determination of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochemical Society Transactions 603: 591–592.
Marín-Guirao, L., J. Lloret, and A. Marín. 2008. Carbon and nitrogen stable isotopes and metal concentration in food webs from a mining-impacted coastal lagoon. Science of the Total Environment 393: 118–130.
Marín-Guirao, L., J.M. Sandoval-Gil, J.M. Ruiz, and J.L. Sánchez-Lizaso. 2011. Photosynthesis, growth and survival of the Mediterranean seagrass Posidonia oceanica in response to simulated salinity increases in a laboratory mesocosm system. Estuarine, Coastal and Shelf Science 92: 286–296.
Marín-Guirao, L., J.M. Sandoval-Gil, J. Bernardeau-Esteller, J.M. Ruiz, and J.L. Sánchez-Lizaso. 2013a. Responses of the Mediterranean seagrass Posidonia oceanica to hypersaline stress duration and recovery. Marine Environmental Research 84: 60–75.
Marín-Guirao, L., J.M. Ruiz, J.M. Sandoval-Gil, J. Bernardeau-Esteller, C.M. Stinco, and A.J. Meléndez-Martínez. 2013b. Xanthophyll cycle-related photoprotective mechanism in the Mediterranean seagrasses Posidonia oceanica and Cymodocea nodosa under normal and stressful hypersaline conditions. Aquatic Botany 109: 14–24.
Mas, J. 1994. El Mar Menor: Relaciones, diferencias y afinidades entre la Laguna costera y el Mar Mediterráneo adyacente. PhD thesis, Autonomous University of Madrid.
Maxwell, K., and G.N. Johnson. 2000. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany 345: 659–668.
McClelland, J.W., and I. Valiela. 1998. Linking nitrogen in estuarine producers to land-derived sources. Limnology and Oceanography 43: 577–585.
McMillan, C., and F.N. Moseley. 1967. Salinity tolerances of five marine spermatophytes of Redfish Bay, Texas. Ecology 48: 503–506.
Munns, R. 2002. Comparative physiology of salt and water stress. Plant, Cell and Environment 25: 239–250.
Murphy, L.R., S.T. Kinsey, and M.J. Durako. 2003. Physiological effects of short-term salinity changes on Ruppia maritima. Aquatic Botany 75: 293–309.
Navarro G, Prieto L & Ruiz J. 2007. Estudio de la dinámica de circulación de la Manga del Mar Menor. Informe parcial de los resultados obtenidosen la campaña oceanográfica en el Mar Menor. Universidad de Granada y CEAMA-UGR, Granada, Spain, 150 pp.
Niu, X., R.A. Bressan, P.M. Hasegawa, and J.M. Pardo. 1995. Ion homeostasis in NaCl stress environments. Plant Physiology 109: 735–742.
Ogata, E., and T. Matsui. 1964. Photosynthesis in several marine plants of Japan as affected by salinity, drying and pH, with attention to their growth habitat. Botanica Marina 8: 199–217.
Olsen, Y.S., M. Sánchez-Camacho, N. Marbà, and C.M. Duarte. 2012. Mediterranean seagrass growth and demography responses to experimental warming. Estuaries and Coasts 35: 1205–1213.
Olsen, J.L., P. Rouzé, B. Verhelst, Y.-C. Lin, T. Bayer, J. Collen, E. Dattolo, E. De Paoli, S. Dittami, F. Maumus, G. Michel, A. Kersting, C. Lauritano, R. Lohaus, M. Töpel, T. Tonon, K. Vanneste, M. Amirebrahimi, J. Brakel, C. Boström, M. Chovatia, J. Grimwood, J.W. Jenkins, A. Jueterbock, A. Mraz, W.T. Stam, H. Tice, E. Bornberg-Bauer, P.J. Green, G.A. Pearson, G. Procaccini, C.M. Duarte, J. Schmutz, T.B.H. Reusch, and Y. Van de Peer. 2016. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 530: 331–335. doi:10.1038/nature16548.
Palomar, P., and I.J. Losada. 2010. Desalination in Spain: recent developments and recommendations. Desalination 225: 97–106.
Pérez-Ruzafa, A., C. Marcos, and J. Ros. 1991. Environmental and biological changes related to recent human activities in the Mar Menor. Marine Pollution Bulletin 23: 747–751.
Pergent, G., A. Djellouli, A.A. Hamza, K.S. Ettayeb, A.A. El Mansouri, F.M. Talha, M.A. Hamza, C. Pergent-Martin, and F. Platini. 2002. Characterization of the benthic vegetation in the Farwà Lagoon (Libya). Journal of Coastal Conservation 8: 119–126.
Ruiz, J.M., and J. Romero. 2001. Effects of in situ experimental shading on the Mediterranean seagrass Posidonia oceanica. Marine Ecology Progress Series 215: 107–120.
Ruiz, J.M., L. Marín-Guirao, and J.M. Sandoval-Gil. 2009a. Responses of the Mediterranean seagrass Posidonia oceanica to in situ simulated salinity increase. Botanica Marina 52: 459–470.
Ruiz, J.M., C.F. Boudouresque, and S. Enríquez. 2009b. Mediterranean seagrasses. Botanica Marina 52: 369–381.
Ruiz, J.M., C. Marcos, and J.L. Sánchez-Lizaso. 2010. Remote influence of off-shore fish farm waste on Mediterranean seagrass (Posidonia oceanica) meadow. Marine Environmental Research 69: 118–126.
Ruiz, J.M., J. Bernardeau, R. García Muñoz, and A. Ramos Segura. 2015. Monitoring network of Posidonia oceanica meadows and climate change in the region of Murcia: period 2004–2015. Seagrass Ecology Group, Spanish Institute of Oceanography, Oceanography Center of Murcia, Murcia, Spain, 152 pp. http://hdl.handle.net/10508/10139
Sánchez-Lizaso, J.L., J. Romero, J. Ruiz, E. Gacia, J.L. Buceta, O. Invers, Y. Fernández-Tormenada, J. Mas, A. Ruíz-Mateo, and M. Manzanera. 2008. Salinity tolerance of the Mediterranean seagrass Posidonia oceanica: recommendations to minimise the impact of brine discharges from desalination plants. Desalination 221: 602–607.
Sandoval-Gil, J.M., L. Marín-Guirao, and J.M. Ruiz. 2012a. Water relations and osmolyte concentrations in Mediterranean seagrasses (Posidonia oceanica and Cymodocea nodosa) in response to simulated salinity increase. Marine Biology 159: 1129–1141.
Sandoval-Gil, J.M., L. Marín-Guirao, and J.M. Ruiz. 2012b. Effect of salinity increase on photosynthesis, growth and survival of the seagrass Cymodocea nodosa. Estuarine, Coastal and Shelf Science 115: 260–271.
Sandoval-Gil, J.M., J.M. Ruiz, L. Marín-Guirao, J. Bernardeau-Esteller, and J.L. Sánchez-Lizaso. 2014a. Ecophysiological plasticity of shallow and deep populations of the Mediterranean seagrasses Posidonia oceanica and Cymodocea nodosa in response to hypersaline stress. Marine Environmental Research 95: 39–61.
Sandoval-Gil, J.M., I. Barrote, J. Silva, I. Olivé, M.M. Costa, J.M. Ruiz, L. Marín-Guirao, J.L. Sánchez-Lizaso, and R. Santos. 2014b. Plant-water relations of intertidal and subtidal seagrasses. Marine Ecology. doi:10.1111/maec.12230.
Schiermeier, Q. 2008. Water: purification with a pinch of salt. Nature 452: 260–261.
Serrano, O., M.A. Mateo, and P. Renom. 2011. Seasonal response of Posidonia oceanica to light disturbances. Marine Ecology Progress Series 423: 29–38.
Shibata, K. 1959. Spectrophotometry of translucence biological materials e opal glass transmission method. Methods of Biochemical Analysis 7: 77–109.
Short, F.T., and H. Neckles. 1999. The effects of global climate change on seagrasses. Aquatic Botany 63: 169–196.
Terrados, J., and J. Ros. 1991. Production dynamics in a macrophyte-dominated ecosystem: the Mar Menor coastal lagoon (SE Spain). Oecologia Aquatica 10: 255–270.
Tomasello, A., G. Di Maida, S. Calvo, M. Pirrota, M. Borra, and G. Procaccini. 2009. Seagrass meadows at the extreme of environmental tolerance: the case of Posidonia oceanica in a semi-enclosed coastal lagoon. Marine Ecology 30: 288–300.
Touchette, B.W. 2007. Seagrass-salinity interactions: physiological mechanisms used by submersed marine angiosperms for a life at sea. Journal of Experimental Marine Biology and Ecology 350: 194–215.
Touchette, B.W., and J.M. Burkholder. 2007. Carbon and nitrogen metabolism in the seagrass, Zostera marina L.: environmental control of enzymes involved in carbon allocation and nitrogen assimilation. Journal of Experimental Marine Biology and Ecology 350: 216–233.
Tyerman SD. 1982. Stationary Volumentric elastic modulus and osmotic pressure of the leaf cells of Halophilla ovalis, Zostera capricornii and Posidonia australis. Plant Physiology 69: 957–965.
Tyerman SD, Hatcher AI, West RJ, Larkum AWD. 1984. Posidonia australis growing in altered salinities: leaf growth, regulation of turgor and the development of osmotic gradients. Australian Journal of Plant Physiology 11: 35–47.
Tyerman, S.D. 1989. Solute and water relations of seagrasses. In Biology of Seagrasses: A treatise on the Biology of Seagrasses with special reference to the Australian region, ed. A.W.D. Larkum, A.J. Mc Comb, and S.A. Sheperd, 723–759. Amsterdam: Elsevier.
Velasco, J., J. Lloret, A. Millán, A. Marín, J. Barahona, P. Abellán, and D. Sánchez-Fernández. 2006. Nutrient and particulate inputs into the Mar Menor lagoon (SE Spain) from an intensive agricultural watershed. Water, Air and Soil Pollution 176: 37–56.
Vermaat, J.E., F.C.A. Verhagen, and D. Lindenburg. 2000. Contrasting responses in two populations of Zostera noltii Hornem. To experimental photoperiod manipulation at two salinities. Aquatic Botany 67: 179–189.
Verslues, P.E., M. Agarwal, S. Katiyar-Agarwal, J. Zhu, and J.K. Zhu. 2006. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant Journal 45: 523–539.
Villazán, B., T. Salo, F.G. Brun, J.J. Vergara, and M.F. Pedersen. 2015. High ammonium availability amplifies the adverse effect of low salinity on eelgrass Zostera marina. Marine Ecology Progress Series 536: 149–162.
Vizzini, S., G. Sarà, M.A. Mateo, and A. Mazzola. 2003. δ13C and δ15N variability in Posidonia oceanica associated with seasonality and plant fraction. Aquatic Botany 76: 195–202.
Walker, D.I., and A.J. McComb. 1990. Salinity response of the seagrass Amphibolis antarctica (Labill.) Sonder et Aschers: an experimental validation of field results. Aquatic Botany 36: 359–366.
Zar, J.H. 1984. Statistical significance of mutation frequencies, and the power of statistical testing using the Poisson distribution. Biometrical Journal 26: 83–88.
Zieman, J.C. 1974. Methods for the study of the growth and production of turtlegrass Thalassia testudinum König. Aquaculture 4: 139–143.
Acknowledgements
This research was funded by the Ministry of Science and Innovation of the Spanish Government through the project OSMOGRASS II (project ref. CTM2009-08413MAR) and RECCAM (project ref. CTM2013-48027-C3-2-R). During the writing of the paper, L. Marín-Guirao was supported by a Marie-Curie Fellowship (FP7-PEOPLE-IEF-2013; HEATGRASS Project). We express our gratitude to Arantxa Ramos-Segura for their field and laboratory support. This study was also supported by the project Monitoring network of P. oceanica meadows and global climate change of the Murcia Region funded by the Autonomous Government of the Murcia Region (General Directorate of Livestock and Fishery) and the European Fishery Fund (EFF 2007–2013). We would also like to thank the General Directorate of Fishery Resources and Aquaculture of the Spanish Ministry of the Environment and the General Directorate of the Environment of the Regional Government for their support in the field sampling performed in the declared Zone of Special Bird Protection Isla Grosa (ZEPA ES0000200) of the Natura 2000 Network.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ken Dunton
Rights and permissions
About this article
Cite this article
Marín-Guirao, L., Sandoval-Gil, J.M., García-Muñoz, R. et al. The Stenohaline Seagrass Posidonia oceanica Can Persist in Natural Environments Under Fluctuating Hypersaline Conditions. Estuaries and Coasts 40, 1688–1704 (2017). https://doi.org/10.1007/s12237-017-0242-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-017-0242-1