Abstract
Halodule wrightii is an ecologically important seagrass; however, little is known about the adaptation of this species in the context of environmental change, particularly changes arising from alterations in salinity of coastal ecosystems. This study aimed to determine the effects of different salinities on growth, morphology, leaf ultrastructure, and cell viability of H. wrightii. To accomplish this, plants were cultivated for 21 days in salinities of 25, 35, and 45. More hydropotens were observed in samples exposed to salinity of 45 with increased invagination of the plasma membrane and cell wall. These invaginations were also observed in other epidermal cells of the leaf blade. In particular, a significant retraction of plasma membrane was seen in samples exposed to salinity of 45, with possible deposition of compounds between the membrane and cell wall. Osmotic stress in samples exposed to salinity of 45 affected the chloroplasts through an increase in plastoglobules and thylakoids by granum in the epidermal chloroplasts of the leaf and decrease in the number of chloroplasts. Overall, this study showed that H. wrightii can survive within salinities that range between 25 and 45 without changing growth rate. However, the plant did have higher cell viability at salinity of 35. Salt stress in mesocosms, at both salinity of 25 and 45, decreased cell viability in this species. H . wrightii had greater changes in salinity of 45; this showed that the species is more tolerant of salinities below this value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Halodule wrightii Ascherson is a seagrass found on every continent, except the Antarctic (den Hartog 1970; Dawes 1998). In Brazil, it is the most common seagrass. Widely distributed on the coast (Oliveira Filho et al. 1983; Fonseca & Fisher 1986), it is usually found in the intertidal zone to about 10 m in depth (Marques and Creed 2008), and this species usually occupies warmer areas with approximate salinity of 35 (Oliveira Filho et al. 1983). However, this species is also found at higher salinities (Koch et al. 2007), and despite its preference for saline areas, H. wrightii can also be found in estuarine environments with lower salinity of 30 (Ferreira 2012).
Saline environments have significantly affected the morphology and physiology of seagrasses (Kuo and den Hartog 2006; Taiz and Zeiger 2009). Among the resources of morphological adaptation, the following characteristics stand out: leaves with small epidermal cells, thick cell walls, thin cuticle and large concentrations of chloroplasts, absence of stomata, a vast aerenchyma system, and reduced xylem (Kuo and den Hartog 2006). The general physiological mechanisms include deleting, compartmentalizing and controlling ion entrance, osmoprotection, activation of antioxidants, and other genetically derived controls (Brilhante 2006; Esteves and Suzuki 2008), including changes in the rate of cell wall synthesis (Fricke and Peters 2002). Projections in the anticlinal cell walls and external periclinal walls of leaf epidermal cells can be seen in some seagrasses (Doohan and Newcomb 1976; Kuo and den Hartog 2006; Ferreira et al. 2015). Jagel (1973), indicating that this type of wall expansion may be associated with an osmoregulation similar to that found in the secretory cells of mangrove plants. By studying the salt glands of Amarix aphylla, Bosabalidis (2010) noticed that numerous projections of the cell wall were always followed by a large increase in the number of mitochondria and microvacuoles in its vicinity.
Hydropotens containing many mitochondria and projections of the cell wall are found in the epidermis of some seagrasses, including H. wrightii (Ferreira et al. 2015). Metcalfe and Chalk (1979) described the important role of such structures in the transport of water and salts into and out of aquatic plants, absorbing two to three times more than the remaining cells of the epidermis. These structural peculiarities, which are similar to salt glands, suggest that hydropotens are active in the transport of minerals.
Altered salinity regimes have contributed to the disappearance of seagrasses (Zieman et al. 1999; Rudnick et al. 2005). Clearly, salinity is a major component of the environment that may influence the structure and function of seagrass communities (Montague and Ley 1993). Studies of these angiosperms suggest that most species have optimum growth between salinity of 30 and 40, although some species have higher growth in brackish water (Touchette 2007).
Seagrass exposed to changes in salinity undergoes osmotic stress (Zhu 2001), which, in turn, will affect photosynthetic rate (Khalafallah et al. 2013), metabolic processes (Hasegawa et al. 2000; Parvaiz and Satyawati 2008), ultrastructure (Jagels and Barnabas 1989; Iyer and Barnabas 1993), and growth (Walker and McComb 1990; Fernández-Torquemada and Sánchez-Lizaso 2005), as well as development and survival (Vermaat et al. 2000).
Although H. wrightii is one of the most common seagrasses in the world with a correspondingly large amount of ecological data (Barros 2008), few studies have reported on the adaptation of this species in the face of changes in salinity of coastal ecosystems. Thus, this study utilized controlled mesocosm conditions to determine the effects of different salinities on growth, morphology, ultrastructure, and cell viability of H. wrightii.
Materials and methods
Collection site and plant material
The Lagoa da Conceição Channel has been continuously open since 1982, an event which, according to Soriano-Sierra (1999), has begun to dramatically change the characteristics of this environment, as determined by a gradual increase of salinity and changes in biotic communities. In 2010 and 2011, Ferreira (2012) recorded salinities in this channel between 22 and 28. In the present study, salinities from 25 to 38 were recorded from November 2013 to August 2014, and these values varied with rainy and dry seasons. Taking into consideration the expected increases and observed frequency of intense marine heat waves (IPCC 2014), this area is expected to show further increase in salinity. In August 2014, H. wrightii specimens were collected at a depth of approximately 1 m in the Lagoa da Conceição Channel, which is located in Florianopolis, Santa Catarina, Brazil (27° 34′ 47″ S and 48° 25′ 47″ W). Vegetative structures of H. wrightii individuals were used in the treatments. Specimens were carefully removed from the substrate, using a garden spade, and placed in containers with water from the site. The sediment was also collected and stored in another container. All the collected material was taken to the laboratory. Specimens were washed in seawater previously sterilized by filtration, and contaminants and epiphytes were removed. All sediments were autoclaved to prevent the introduction of unknown variables.
Culture conditions in the laboratory
For cultivation in the laboratory, H. wrightii specimens were placed in aquaria with 4 cm of sediment and 5 l of seawater previously sterilized at salinity of 35 (= salinity of collection site). The roots and rhizomes were then fixed in the sediment, with the leaves out of the sediment and fully submerged in the saline water. The specimens were divided into 12 tanks, which were transferred to the culture room with controlled ambient conditions of temperature, lighting, and aeration. Throughout the period of acclimation and treatment, the temperature was maintained at 24 °C (±2), continuous aeration, 100 (±10) μmol photons m−2 s−1 (fluorescent lamps, Philips C-5 Super 84, 16 W), and 12 h photocycle (starting at 8 h) at 100 (±10) μmol photons m−2 s−1. Light intensity was measured with a Solar Light PMA 2100 quantometer (Glenside, PA, USA). The acclimation period was 14 days. After acclimation, with the same culture conditions, four tanks were subjected to salinity of 25, four to salinity of 45, and four remained in salinity of 35 (control). Concentrations to salinity of 25 and 35 were based on salinity variations naturally occurring at the collection site. Salinity of 45 took into account the planned increases in the IPCC 2014and the experiments carried out by Lirman and Cropper (2003). The duration of the experiment was 21 days for each treatment, allowing for the time required to develop new adult leaves. Low salinity was obtained by dilution with distilled water, while high salinity was obtained by addition of seawater with salinity of 90 obtained by boiling of the seawater. Throughout the experimental period, pH measurements, irradiance, temperature, and salinity were performed weekly to monitor cultivation conditions. Aquarium water was renewed twice a week, maintaining the same salinity.
Growth rate
Growth rates (GRs) were calculated according to the methods of Magalhães (1985), with modifications for percentage, using the following equation: GR [% day−1] = [(Wt/Wi)1/t − 1] × 100, where Wi = fresh weight in grams of the whole plant (root + rhizome + leaf) at the initial time, Wf = fresh weight in grams of the whole plant (root + rhizome + leaf) at the final time, and t = experimental time in days.
Morphological studies
External morphological measurements were taken for root (diameter), rhizome (diameter), and leaf blade (width and thickness). Internal measurements of leaf blade included thickness of the epidermis, mesophyll and aerenchyma, and number of cells in the mesophyll. For these measurements, permanent histological slides were made with samples sectioned transversely. Measurements were made from the median region of adult root, rhizome (internode region), and leaf blade of samples from the environment (hereinafter termed field samples) and treatment samples (at the end of experimentation). This material was fixed using 2.5% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.2) for 24 h. Subsequently, the samples were dehydrated in increasing series of aqueous ethanol solutions and infiltrated with Historesin (Leica Historesin, Heidelberg, Germany). Then, sections 5 μm in length were stained with toluidine blue (TB) (Gordon and McCandless 1973). The material was analyzed and photographed with a light microscope (Olympus, model BX41, Japan), using a digital color 3.3 Mpx QImaging camera equipped with QCapture Pro 5.1, which has a system of measures in micrometers. Twenty individuals from each treatment were analyzed, and for the analysis of epidermal thickness, five measurements of each section were made, totaling 100 measurements per treatment.
Transmission electron microscope (TEM)
The material was processed according to the method of Bouzon et al. (2006). Samples of the median and mature regions of leaf blade from field and treatment samples post-experiment were prefixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) plus 2% sucrose for 12 h. The material was postfixed with 1% osmium tetroxide for 12 h, dehydrated in a graded acetone series, and embedded in Spurr’s resin. Thin sections were stained with aqueous uranyl acetate followed by lead citrate. The samples were examined under JEOL JEM-2010 TEM (Model JEM 101, Japan) at 80 kV.
Analysis of cell viability by MTT assay
Mitochondrial viability and, consequently, cell viability was quantitated indirectly by the reduction of the yellow dye salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to its insoluble formazan, which has a purple color, by the activity of dehydrogenases (Mosmann 1983; Freimoser et al. 1999). The analysis of MTT reduction was based on procedures described by Mendes et al. (2013). At the end of experimentation, adult samples (6 mg fresh weight) of root, rhizome, and leaf blade for both field and treatment specimens were placed in separate test tubes containing 3 mL of water of the culture and 30 μL of MTT solution (MTT 0.017 g in 1 mL of sodium phosphate buffer 0.1 M). The test tubes were maintained in the greenhouse at 37 °C for 3 h under stirring. After this period, samples were washed in distilled water, transferred to Eppendorf tubes containing 1.0 mL of analytical grade dimethylsulfoxide (DMSO), and centrifuged for 4 min (4000 rpm). The supernatant was placed in microplates and then analyzed at 570 nm in an Infinite M200 Tecan plate reader. Analyses were performed in quadruplicate, and to compare data, the average values of absorbance of MTT degradation were used.
Data analysis
Statistical analysis of the growth rate of the whole plant (root + rhizome + leaf); external morphology of the root (diameter), rhizome (diameter), and leaf blade (width and thickness); internal measurements of leaf blade (thickness of the epidermis, mesophyll and aerenchyma, and number of cells in the mesophyll); and cell viability by MTT assay were all evaluated by analysis of variance (ANOVA), followed by Tukey’s test, when parametric, or analysis by Kruskal-Wallis followed by Dunn’s test, when nonparametric, at the 5% significance level, using Excel 2010 and BioEstat 5.0. For microscopic analysis (TEM, CLSM), four samples of each treatment were used to verifying qualitative aspects.
Results
Growth rate and morphological analysis
The specimens of H. wrightii grown and developed in culture in the different treatments. The maximum growth occurred in salinity of 35 (0.12% day−1), however no statistical differences comparing the three treatments (Table 1). The diameter of the rhizome also showed no significant differences among treatments (Table 2). However, the samples showed a thickening of root diameter in the treatments with salinity of 25 and 45 (Table 2).
Field samples achieved the highest average value of thickness of the leaf blade with significant differences relative to treatments, but no significant differences were noted among samples (Table 3). Field samples reached the lowest average values of leaf blade width. Treatments with salinity of 25 and 45 obtained the highest mean values, with no significant differences between these two treatments (Table 3).
The number of cells in the mesophyll presented statistically significant differences where the largest number was obtained from field samples, followed by treatment with salinity of 25 and 45. Treatment with salinity of 35 had the lowest number (Table 4). Based on an analysis of the thickness of mesophyll and epidermal cells, field samples showed the highest average values with statistically significant differences relative to treatments in different salinities, but among the treatments, no statistical differences were found (Table 4). No significant variation among treatments, including no differences in field samples, was observed in thickness of main aerenchyma (Table 4).
Ultrastructure of the leaf blade
In all samples, TEM of leaf blade transverse sections revealed epidermal cells with large expansion of cell wall followed by invaginations of the plasma membrane (Fig. 1a–l). This phenomenon was not as noticeable in samples exposed to salinity of 25 (Fig. 1d, e). These expansions were found in the anticlinal cell walls, sometimes reaching the external and internal periclinal walls. Next to these expansions, mitochondria and small vesicles were found with a greater number of mitochondria observed in samples exposed to salinity of 25 (Fig. 1d, e). No such expansions were found in mesophyll cells, even those in direct contact with the epidermis.Beyond these expansions, large retraction of the plasma membrane was found in samples exposed to salinity of 45 (Fig. 1j, k) with apparent deposition of compounds in the space created between the plasma membrane and the cell wall (Fig. 1k). This retraction takes up much of the cytoplasm. In the epidermis, hydropotens were observed to contain many mitochondria, vesicles and large projections of the cell wall, mainly the outer periclinal cell wall, followed by invaginations of the plasma membrane (Fig. 1c, f, i, l). In this cell, the outer periclinal cell wall was thickened threefold over that of other epidermal cells. Hydropotens were found in greater quantities in samples exposed to salinity of 45, and they also had a higher number of invaginations of the plasma membrane, which occupied almost the entire cytoplasm (Fig. 1l).
TEM micrographs of transverse sections of Halodule wrightii Aschers. leaf blade showing details of the epidermal cells. a–c Field samples. a, b Detail showing cell wall expansions with invaginations of the plasma membrane (star). c Detail of a hydropoten with cell wall expansion and invaginations of the plasma membrane (star). d–f Samples treated with salinity of 25. d, e Detail showing cell wall expansions with invaginations of the plasma membrane (star). f Detail of a hydropoten with cell wall expansion and invaginations of the plasma membrane (star). g–i Samples treated with salinity of 35. g, h Detail showing the cell wall expansions with invaginations of the plasma membrane (star). i Detail of a hydropoten with cell wall expansion and invaginations of the plasma membrane (star). j–l Samples treated with salinity of 45. j, k Detail showing significant retraction of the plasma membrane with a possible deposition of compounds in the space created between the membrane and the cell wall (star). l Detail of a hydropoten with large expansions of cell wall and plasma membrane invaginations (star). C chloroplast, CW cell wall, M mitochondria, N nucleus, Ve vesicle
Many chloroplasts were found in the epidermal cells distributed throughout the cytoplasm, with higher concentration at the outer peripheral region (Fig. 2a, d, g, j). Epidermal chloroplasts have an elliptical shape with organized thylakoids and without starch grains (Fig. 2a–l). Compared to mesophyll chloroplasts, they are much larger and have larger quantities. Samples exposed to salinity of 45 showed the least amount of chloroplasts in the epidermis (Fig. 2j), and they were also smaller. However, these chloroplasts had a higher number of thylakoids by granum (Fig. 2l). Plastoglobules were seen in the epidermal chloroplasts of all samples with higher amounts in field samples (Fig. 2b, c) and samples exposed to salinity of 45 (Fig. 2k).
TEM micrographs of transverse sections of Halodule wrightii Aschers. leaf blade showing detail of the chloroplasts of the epidermis. a–c Field samples. a Epidermis with cell containing many chloroplasts scattered throughout the cytoplasm. b Detail of a chloroplast with the presence of plastoglobules (arrow). c Detail of the thylakoids of a chloroplast; plastoglobules (arrow). d–f Samples treated with salinity of 25. d Epidermis with cell containing many chloroplasts scattered throughout the cytoplasm. e Detail of a chloroplast. f Detail of the thylakoids of a chloroplast. g–i Samples treated with salinity of 35. g Epidermis with cell containing many chloroplasts scattered throughout the cytoplasm; presence of vacuoles. h Detail of a chloroplast. i Detail of the thylakoids of a chloroplast. j–l Samples treated with salinity of 45. j Epidermis with cells containing a smaller number of chloroplasts. k Detail of a chloroplast with the presence of plastoglobules (arrow). l Detail of the thylakoids of a chloroplast. Ch chlorenchyma, C chloroplast, CW cell wall, Ep epidermis, H hydropoten, N nucleus, T thylakoids, Va vacuole
Analysis of cell viability by MTT assay
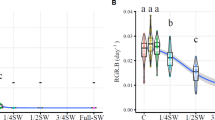
The highest values of absorbance of MTT degradation were found in the leaf blade (Table 5). Field samples had the lowest average values of absorbance of MTT degradation in all vegetative organs (root, rhizome, and leaf) (Table 5). Between root and rhizome, no significant differences were observed based on MTT degradation among treatments (Table 5). However, for leaf blade, statistical differences were noted between samples exposed to salinity of 25 and 45 relative to 35, which had the highest average value of degradation of MTT (Table 5).
Discussion
Our results show that H. wrightii plants were able to maintain their growth, even under hypersaline conditions. Tolerance of this plant to variations in salinity was also noted by Lirman and Cropper (2003) who analyzed the influence of variation in salinity (5 to 45) in Thalassia testudinum König, Syringodium filiforme Kützing, and H. wrightii with respect to growth rate and leaf elongation. Of the three species tested by these authors, H. wrightii showed the greatest tolerance to salinity variations, and changes in the growth rates did not vary much among treatments, with the highest average rate of extension of the leaf blade recorded at salinity of 35 and the lowest at salinity of 45.
Parida and Das (2005) found that increased salinity affected leaf anatomy, leading to increased thickness of the epidermis and mesophyll and decrease of the intercellular spaces. However, in the present study, variations in the intercellular spaces of specimens treated with salinity of 45 were not observed, and the diameter of the aerenchyma showed no differences between field samples and those subjected to different salinity treatments. The greatest thickness of leaf blades and their constituent structures was only observed in field samples where the main regulatory factor is light, not salinity, since more ambient light is available compared to laboratory culture. According to Dickison (2000), the greater exposure of leaf to the light increases the thickness of the leaf blade and mesophyll, whereas a lower exposure results in increased leaf area with enlargement of the leaf to increase the capture of light, as observed in plants cultured in the laboratory.
Samples of treatment with salinity of 45 had higher mean value of the root diameter, caused by the increase of cell layers in the cortex and the thickening of exodermis (unpublished data). The exodermis of the plant roots represents an apoplastic barrier with variable resistance radial flow of water and ions and makes an essential contribution to the plant’s resistance to environmental stress conditions (Hose et al. 2001, Hartung et al. 2002). The increase of cell layers of the cortex can also be serving as a barrier to prevent the flow of ions to the aerial parts of the plant.
We asked how H. wrightii could tolerate such differences in salinity without also undergoing changes in growth rate and morphology. Most likely, this plant has the ability to present ultrastructural alterations that may favor such tolerance to saline stress. For example, under TEM, we saw the formation of specialized cells called hydropotens. Hydropotens are involved in the transport of water and salts, and they are able to retain more mineral ions (Castro and Machado 2006). Samples treated with higher salinity (salinity of 45) showed the highest amount of hydropotens in the epidermis in response to the increase in concentration of salts in the water. In the hydropotens of aquatic plants, Metcalfe and Chalk (1979) noted the presence of numerous vesicles, mitochondria, and many projections of cell wall and plasma membrane in the cytoplasm. According to Pang and Wang (2008), mitochondria are specialized organelles that also combat reactive oxygen species (ROS) through their own enzymatic antioxidant systems. These features were found in all samples, and in samples exposed to salinity of 45, we saw an increase in the invaginations of the plasma membrane and cell wall expansion, which occupied most of the cytoplasm. Increase in the number of hydropotens and invaginations of the plasma membrane and cell wall expansion of these cells are apparently one of the strategies that enables H. wrightii to tolerate salinity of 45 with increase in surface area it facilitates the exchange with the environment.
The projections of the cell wall and cell membrane were viewed in the other epidermal cells of the leaf blade of samples exposed to all treatments, albeit to a lesser extent, and always accompanied by mitochondria and vesicles. Kuo and den Hartog (2006) also viewed these wall expansions in the epidermis of the leaf blades of seagrass, terming them as wall ingrowth. Doohan and Newcomb (1976) studied the anatomy of three species of seagrasses (Cymodocea rotundata (Ehrenb.) Aschers., Cymodocea serrulata (R. Brown) Aschers. & Magnus and T. hemprichii (Ehrenb.) Aschers.), and they found these expansions in the epidermal cell wall, ascribing to them functions similar to transfer cells which would indicate that they could be involved in short-distance transport and absorption of solutes from the surrounding water. In addition to their function in adaptation to a saline environment, expansion of cell wall and plasma membrane allows seagrass species to transport water and nutrients through the leaves.
Distinct differences in leaf ultrastructure were found in samples of H. wrightii exposed to lower salinity at 25, including fewer invaginations in plasma membrane and cell wall. On the other hand, a significant retraction of the plasma membrane with possible deposition of compounds between the region of the plasma membrane and cell wall was seen in samples subjected to salinity of 45. Iyer and Barnabas (1993) analyzed Zostera capensis Setchell in salinity of 2, 12, 40, and 60, and they also found an increase in invaginations of the plasma membrane and wall ingrowth with increasing salinity. Interestingly, at salinity of 60, these characteristics were disorganized in a manner similar to that found in samples exposed to salinity of 45 in the present study. These changes were also observed in Ruppia maritima Linnaeus growing in low and high water salinity (Jagels and Barnabas 1989). Leaf epidermal cells of low-salinity plants showed a reduced area of plasma membrane as a result of smaller extension of invaginations, whereas at higher salinities, plants exhibited a deeply invaginated plasma membrane with most prominent interdigitated. Fibrillar material is freely distributed in the space between the plasma membrane and the cell wall in high-salinity plants.
In addition to the presence of sodium, salt stress alters the ionic composition of the stroma of chloroplasts, which affects the loads on the membrane surface contributing to their disorganization (Marcondes and Garcia 2009). However, in the present study, seagrass samples submitted to different salinity treatments showed no disorganization of the thylakoids, not even at salinity of 45. An increase in the amount of thylakoids by granum in the chloroplasts of the leaf epidermis of samples exposed to salinity of 45 was the only ultrastructural variation observed. The presence of a larger number of plastoglobules was also observed in these samples. According to Bréhélin et al. (2007), plastoglobules are lipoprotein particles located in the plastids and are directly related to the synthesis and storage of tocopherols. Under conditions of oxidative stress, tocopherols stored in plastoglobules are sent to thylakoid membranes to eliminate ROS. Thus, the number of plastoglobules increases during stress.
The increase in the number of thylakoids may be related to some sort of compensatory system since quantity of chloroplasts in epidermal cells decreased in samples exposed to salinity of 45. Iyer and Barnabas (1993) also saw a decrease of epidermal chloroplasts in samples of Z. capensis exposed to higher levels of salinity (60). Our findings showing that the osmotic stress affects chloroplast structure in the samples of H. wrightii submitted to a salinity of 45.
MTT assay is an indirect way to assess mitochondrial viability and, consequently, cell viability, since it is based on the colorimetric quantification of mitochondrial dehydrogenase activity (Mosmann 1983). Using MTT in the present work, the highest values were found in leaf blade, followed by roots, sometimes reaching levels ten times higher in the leaf blade than the rhizome. These results indicate the degree of metabolic activity in the leaves. In addition, under salt stress, both increasing and decreasing, MTT results show that cell viability decreased in the studied seagrass, indicating susceptibility of mitochrondria to variations in salinity. On the other hand, field samples showed low values of MTT degradation at the root, rhizome, and leaf blade. These results are expected since field plants are subjected to frequent variations of salinity in the channel, as a result of tidal changes and rainfall, in addition to other abiotic factors.
To establish the effects of varying salinity on seagrasses, it is critical to understand its occurrence, distribution, abundance, and performance in estuaries. Over a period of at least 3 weeks, our results demonstrated that salinity of 25 and 45 reduced the cell viability in H. wrightii. Salinity of 45 also had the greatest impact on leaf ultrastructure, especially with regard to the amount of chloroplasts and the disorganization of invaginations of the plasma membrane of leaf epidermal cells. This indicates that H. wrightii best tolerates salinity below, but not above, 35.
References
Barros KVS (2008) Efeitos da variação sazonal do ecossistema Halodule wrightii Ascherson sobre comunidades bentônicas associadas, na praia das Goiaberas. Dissertation, Federal University of Ceara, Brazil, Fortaleza-CE, p. 153p
Bosabalidis AM (2010) Wall protuberance formation and function in secreting salt glands of Tamarix aphylla L. Acta Bot Bras 69:229–235
Bouzon ZL, Ouriques LC, Oliveira EC (2006) Spore adhesion and cell wall formation in Gelidium floridanum (Rhodophyta, Gelidiales). J Appl Psychol 18:287–294
Bréhélin C, Kessler F, van Wijk KJ (2007) Plastoglobules: versatile lipoprotein particles in plastids. Trends Plant Sci 12(6):260–266
Brilhante JCA (2006) Contribuição de solutos orgânicos e inorgânicos no potencial osmótico de folhas de Atriplex nummularia submetidas ao NaCl, seca e PEG. Federal University of Ceara, Brazil, Dissertation, p. 194p
Castro MM, Machado SR (2006) Células e Tecidos Secretores. In: Appezzato-da-Gloria B, Carmello-Guerreiro SM (eds) Anatomia Vegetal, 2rd edn. UFV, Viçosa, pp. 179–203
Dawes CJ (1998) Marine Botany, 2rd edn. Wieley, New York, p. 408p
den Hartog C (1970) The sea-grasses of the world. North-Holland Publishing Company, Amsterdam, p. 275p
Dickison WC (2000) Integrative plant anatomy. Harcourt/Academic Press, Massachusetts, p. 533p
Doohan ME, Newcomb EH (1976) Leaf ultrastructure and δ13C values of three seagrasses from the Great Barrier Reef. Aust J Plant Physiol 3:9–23
Esteves BS, Suzuki MS (2008) Efeito da salinidade sobre as plantas. Oecol Bras 12:662–679
Fernández-Torquemada Y, Sánchez-Lizaso JL (2005) Effects of salinity on leaf growth and survival of the Mediterranean seagrass Posidonia oceanica (L.) Delile. J Exp Mar Biol Ecol 320(1):57–63
Ferreira C (2012) Aspectos morfoanatômicos e ultraestruturais de gramas marinhas do litoral brasileiro. Dissertation, Federal University of Santa Catarina, Brazil, p. 158p
Ferreira C, Horta PA, Almeida GM, Zitta CS, Oliveira EDM, Gueye MB, Rodrigues AC (2015) Anatomical and ultrastructural adaptations of seagrass leaves: an evaluation of the southern Atlantic groups. Protoplasma 252(1):3–20
Fonseca MS, Fisher JS (1986) A comparison of canopy friction and sediment movement between 4 species of seagrass with reference to their ecology and restoration. Mar Ecol-Prog Ser 29:15–22
Freimoser FM, Jakob CA, Aebi M, Tour U (1999) Use of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide for rapid detection of rifampin-resistant Mycobacterium tuberculosis. Appl Environ Microbiol 65:3727–3729
Fricke W, Peters WS (2002) The biophysics of leaf growth in salt-stressed barley. A study at the cell level. Plant Physiol 129:374–388
Gordon EM, McCandless EL (1973) Ultrastructure and histochemistry of Chondrus crispus stack. P N S I Sci 27:111–133
Hartung W, Sauter A, Hose E (2002) Abscisic acid in the xylem: where does it come from, where does it go to? J Exp Bot 53(366):27–32
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol Plant Mol Biol 51:463–499
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W (2001) The exodermis: a variable apoplastic barrier. J Exp Bot 52(365):2245–2264
IPCC (2014) Summary for policymakers. In: Stocker TF, Qin D, Plattner G–K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013:the physical science basis. Contribution of working group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York
Iyer V, Barnabas AD (1993) Effects of varying salinity on leaves of Zostera capensis Setchell. I Ultrastructural changes Aquat Bot 46(2):141–153
Jagel RH (1973) Studies of a marine grass Thalassia testudinum. I. Ultrastructure of osmoregulatory leafcefts. Am J Bot 60:1003–1009
Jagels R, Barnabas A (1989) Variation in leaf ultrastructure of Ruppia maritima L. Along a salinity gradient. Aquat Bot 33(3):207–221
Khalafallah AA, Geneid YA, Shaetaey SA, Shaaban B (2013) Responses of the seagrass Halodule uninervis (Forssk.) Aschers. to hypersaline conditions. Egypt J Aquat R 39(3):167–176
Koch MS, Schopmeyer SA, Kyhn-Hansen C, Madden CJ, Peters JS (2007) Tropical seagrass species tolerance to hypersalinity stress. Aquat Bot 63:169–196
Kuo J, den Hartog C (2006) Seagrass morphology, anatomy, and ultrastructure. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht, pp. 51–87
Lirman D, Cropper WP (2003) The influence of salinity on seagrass growth, survivorship, and distribution within Biscayne Bay, Florida: field, experimental, and modeling studies. Estuaries 26(1):131–141
Magalhães ACN (1985) (1985) Análise quantitativa do crescimento. In: Fisiologia vegetal. 2. ed. São Paulo: EDUSP v. 1:333–350
Marcondes J, Garcia AB (2009) Aspectos citomorfologicos do estresse salino em plantulas de arroz (Oryza sativa L.). Arq Inst Biol (Sao Paulo) 76(2):187–194
Marques LV, Creed JC (2008) Biologia e ecologia das fanerógamas marinhas do Brasil. Oecol Bras 12:315–331
Mendes LF, Zambotti-Villelav L, Colepicolo P, Marinho-Soriano E, Stevani CV, Yokoya NS (2013) Metal cation toxicity in the alga Gracilaria domingensis as evaluated by the daily growth rates in synthetic seawater. J Appl Phycol 25:1939–1947
Metcalfe CR, Chalk L (1979) Anatomy of the dicotyledons, vol 1, 2nd edn. Claredon Press, Oxford
Montague CL, Ley JA (1993) A possible effect of salinity fluctuation on abundance of benthic vegetation and associated fauna in Northeastern Florida Bay. Estuaries 16:703–717
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Oliveira Filho EC, Pirani JR, Giulietti AM (1983) The Brazilian seagrass. Aquat Bot 16:251–267
Pang CA, Wang B (2008) Oxidative stress and salt tolerance in plants. Prog Bot 69:231–246
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotox Environ safe 60(3):324–349
Parvaiz A, Satyawati S (2008) Salt stress and phyto-biochemical responses of plants—a review. Plant Soil Environ 54(3):89–99
Rudnick DT, Ortner PB, Browder JA, Davis SM (2005) A conceptual model of Florida Bay. Wetlands 25:870–883
Soriano-Sierra EJ (1999) Ecossistemas de marismas da Lagoa da Conceição. III. A Produção Primária. In: Sierra de Ledo B, Soriano-Sierra EJ (eds) O Ecosistema da Lagoa da Conceição. NEMAR/CCB/UFSC, SDM/FEPEMA. Florianopolis, Brasil, pp 185–198
Taiz L, Zeiger E (2009) Fisiologia vegetal, 4th edn. Artmed, Porto Alegre
Touchette BW (2007) Seagrass-salinity interactions: physiological mechanisms used by submersed marine angiosperms for a life at sea. J Exp Mar Biol Ecol 350(1):194–215
Vermaat JE, Verhagen FC, Lindenburg D (2000) Contrasting responses in two populations of Zostera noltii Hornem. to experimental photoperiod manipulation at two salinities. Aquat Bot 67(3):179–189
Walker DI, McComb AJ (1990) Salinity response of the seagrass Amphibolis antarctica (Labill.) Sonder et Aschers.: an experimental validation of field results. Aquat Bot 36(4):359–366
Zieman JC, Fourqurean JW, Frankovich TA (1999) Seagrass die-off in Florida Bay: long-term trends in abundance and growth of turtle grass, Thalassia testudinum. Estuaries 22(2):460–470
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Acknowledgements
We are grateful to the staff of the Laboratory of Biochemistry and Morphogenesis Plant (LMBV-UFSC), Multiuser Laboratory of Studies in Biology (LAMEB-UFSC), and Central Laboratory of Electron Microscopy (LCME-UFSC) for their assistance and for space and equipment available in these laboratories. This study is part of the first author’s doctoral thesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling Editor: Néstor Carrillo
Rights and permissions
About this article
Cite this article
Ferreira, C., Simioni, C., Schmidt, É.C. et al. The influence of salinity on growth, morphology, leaf ultrastructure, and cell viability of the seagrass Halodule wrightii Ascherson. Protoplasma 254, 1529–1537 (2017). https://doi.org/10.1007/s00709-016-1041-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-1041-4