Abstract
Planktonic larvae combine directed swimming and functional sensory systems to locate benthic habitats. Some adult marine fishes use chemical cues for orientation to specific habitats, but olfactory function for estuarine fish larvae has received little research attention. This laboratory study quantified behavioral responses of red drum (Sciaenops ocellatus) larvae to estuarine chemical cues to examine the role of water chemistry as an orientation cue for locating or remaining in settlement habitat. Spontaneous activity (kinesis) was measured for pre-settlement-size larvae exposed to artificial sea water (as a negative control) and one of six treatments (sterilized sea water, sea water from a channel at ebb tide, sea water from a channel at flood tide, sea water from seagrass habitat, tannic acid dissolved in sterilized sea water, or lignin dissolved in sterilized sea water). Larvae that reached a size of competency to settle (approximately 10 mm standard length) swam faster when exposed to lignin dissolved in sterilized sea water than in other treatments; smaller larvae showed no response. Olfactory preference (taxis) was tested using a paired-choice experiment. Settlement-size larvae preferred water from seagrass beds to artificial sea water. The observed chemokinesis and chemotaxis in response to lignin dissolved in sterilized sea water and sea water from a seagrass bed demonstrate that red drum larvae can distinguish and respond to different water masses and suggest that chemical stimuli from seagrass settlement habitat may aid in orientation and movement to or retention in suitable settlement sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish use chemical cues for communication, foraging, mating, predator detection, avoidance, and navigation (Bélanger et al. 2004; Dixson et al. 2012; Døving et al. 1994; Gerlach et al. 2007; Hubbard et al. 2003). They respond to olfactory cues that range from simple amino acids to complex mixtures of biologically and environmentally produced molecules and are sensitive to these compounds at different concentrations (Døving et al. 1994; Gerlach et al. 2007). For example, Atlantic salmon (Salmo salar) respond to testosterone concentrations in sea water as low as 10−14 mol−1, but much higher concentrations of alcohols (Hara 1994; Moore and Scott 1991). In general, responses such as turning rate and swimming speed can increase with increasing concentration of a chemical cue (Døving et al. 1994).
Responses to chemical cues vary among species, chemical compounds, time, and space. Some chemical cues elicit an innate response (Arvedlund et al. 1999; Dixson et al. 2012; Miller-Sims et al. 2011), and others are learned (Odling-Smee and Braithwaite 2003). Certain species can distinguish a specific coral reef or a host anemone based on learning or imprinting, and others innately respond to chemical alarm cues from confamilial species (Arvedlund and Kavanagh 2009; Arvedlund et al. 1999; Miller-Sims et al. 2011; Mitchell et al. 2012). Since olfactory imprinting is common in marine fishes (e.g., Arvedlund et al. 1999; Hasler and Scholz 1983) and chemosensory morphology develops early, olfaction likely functions early in life (Døving et al. 1994; Kingsford et al. 2002; Lara 2008). However, there have been few studies of the development of the olfactory system and chemically mediated behavioral responses in these organisms.
Fishes commonly use chemical cues for orientation, and many studies suggest olfaction may be the most important cue for locating habitats on both large and small spatial scales (Baird et al. 1996; Dittman and Quinn 1996; Lecchini et al. 2005). In Japanese red rockfish (Sebastes inermis), olfaction is more important than vision for locating specific habitats, and olfaction operates over greater distances than vision in certain larvae (Lecchini et al. 2005; Mitamura et al. 2005). Settlement-size reef fishes are capable of discerning different chemical cues associated with benthic habitats, preferring water located near islands or reefs over water from the open ocean or other unsuitable habitats (Atema et al. 2002; Dixson et al. 2008). Some species use chemical cues to distinguish between their natal habitat and a different vicinity, which contributes to retention of larvae in specific sites (Gerlach et al. 2007; Mitamura et al. 2005). They can also use olfaction to determine habitat quality. Coppock et al. (2013) found that three species of reef fishes in Papua New Guinea are attracted to the olfactory cues from live coral and actively avoid odors from degraded coral. In addition to the chemical compounds associated with the habitat structure itself (e.g., oyster reefs, seagrass beds, coral reefs), many settlers respond to predators, prey, and conspecifics in the area (Døving et al. 2006; Lecchini et al. 2005; Lecchini et al. 2007; Sweatman 1985).
Most research on olfactory settlement cues has been conducted on coral reef fishes; much less is known about the chemical cues associated with settlement in other environments. It has been suggested that larvae depend on olfactory cues to locate estuaries (Boehlert and Mundy 1988), yet few studies have tested the effects of chemical compounds on settlement behavior. Both Radford et al. (2012) and James et al. (2008) found that settlement-stage sparid larvae (Rhabdosargus holubi and Pagrus auratus) orient toward water sourced from their typical nursery habitats. Radford et al. (2012) also discovered that larvae prefer water collected from seagrass beds to artificial sea water in which seagrass blades had been soaking, suggesting that chemical compounds derived from sources other than the seagrass (e.g., conspecifics or prey) are involved in attracting the larvae to the seagrass beds.
If seagrasses are an important source of chemical information for larvae of other species, it is possible that seagrass phenolic compounds, which are aromatic molecules that can leach into the environment as a result of structural damage or senescence (reviewed by Arnold and Targett 2002), are involved. Seagrasses contain phenols such as condensed tannins and lignins at concentrations ranging from 3 to 11 % and <1–5 % dry mass (DM), respectively, and these concentrations can vary greatly across species and over time (Arnold and Targett 2002). Emergent vegetation, including mangroves and salt marshes, also produce these compounds, and brown algae produces tannins but not lignins. Salt marsh plants (measured as humic substances, Filip and Alberts 1989) and mangroves contain even greater concentrations of these compounds than seagrasses (mangrove tannin concentrations of 8.8–40.8 % DM, Basak et al. 1998). Terrestrial plants also produce phenolic compounds and, when located near rivers and streams, can be transported to nearby estuaries as well (Benner and Opsahl 2001). Collectively, these phenolic sources may serve as a coastal signal to larvae searching for estuarine settlement sites.
Chemoreception may be especially important for finding settlement habitat in areas where estuaries do not provide a reliable signal through other physical or environmental variables. For instance, postlarval and juvenile flounder (Pleuronectes flesus) prefer water of low salinity (Bos and Thiel 2006). In regions of the western Gulf of Mexico where salinity gradients are not only unstable but sometimes result in reverse estuaries, salinity is not a dependable signal for navigation. Thus, it is even more likely that chemoreception would be important for locating nursery habitats within these estuaries. The present study tested the hypothesis that larvae of the estuarine-dependent fish, red drum (Sciaenops ocellatus), respond to estuarine-derived olfactory cues when they are competent to settle, helping them locate and remain in their preferred nursery habitat, seagrass beds.
Methods
To test the hypothesis, laboratory studies were conducted to quantify changes in activity (kinesis) and preference (taxis) of red drum larvae exposed to a variety of natural olfactory cues. Environmental flow rates were measured in the field to determine an appropriate velocity to use for the taxis experiment. After analyzing the responses, water samples collected from pre-settlement and settlement habitats were analyzed for specific chemical (lignin) concentrations that could serve as a reliable estuarine olfactory cue.
Environmental Flow Rates
To determine estuarine flow rates, 20-cm SeaHorse tilt current meters (OkeanoLog, Woods Hole, MA) were installed in Redfish Bay near Harbor Island, TX (27° 53′ N, 97° 7′ W). One tilt current meter was installed above the canopy of a seagrass (Thalassia testudinum) bed at a depth of approximately 1 m, and another tilt current meter was installed over bare substrate at a similar depth, approximately 20 m from where water samples were taken for laboratory experiments. The meters were set out at 1600 hours on September 20, 2012, and retrieved the next day at 1330 hours. The tilts from the zenith on each of the three planes were converted into horizontal velocity (in cm s−1) with MATLAB software (Mathworks, Natick, MA).
Study Species
Red drum inhabit temperate and subtropical waters from the Gulf of Mexico to Massachusetts in the Western Atlantic and are an important recreational fishery, contributing to Texas’s $3.2 billion per year fishing industryFootnote 1 (Atlantic States Marine Fisheries Commission 2009; Hoese and Moore 1998). In Texas, they spawn offshore or along the coast in late summer and fall, and larvae reach the estuaries in approximately 3 weeks (Holt et al. 1983; Rooker et al. 1998). Larvae become competent to settle at 10 mm standard length (SL), and newly settled individuals are most common in Halodule wrightii and T. testudinum seagrass beds, but will occupy marsh edges or unvegetated bottoms when seagrass is not available (Stunz et al. 2002). Red drum remain in the estuaries until they reach maturity (3 years for males and 5 years for females), after which they move offshore during the spawning season (Pattillo et al. 1997).
Larval Care
Larvae were raised from eggs released from broodstock maintained at the University of Texas Marine Science Institute’s Fisheries and Mariculture Laboratory (FAML) in Port Aransas, TX. On the day following a spawn, approximately 5000 (5 ml) viable (floating) eggs were placed into 150-l conical tanks filled with UV-sterilized sea water maintained at 27 °C and a salinity of 35. An airstone was placed into each tank to provide a continuous supply of oxygen. On 1–11 days posthatching (dph), larvae were fed approximately 400,000 rotifers (Brachionus sp.) enriched with Algamac 3050 (Aqua-fauna Bio-Marine, Hawthorne, CA) for 45 min each morning. On mornings 10 and 11, larvae were also fed approximately 10,000 1-day-old Artemia sp. nauplii. From 12 dph until testing, larvae were fed twice each morning approximately 60,000 2-day-old Artemia sp. nauplii enriched with Algamac 3050. The hatchery was kept on a 12:12 light/dark cycle. Larvae were starved 24–30 h prior to testing.
Kinesis Experiment
This experiment measured the activity of red drum larvae 4–10 mm SL exposed to five different treatments and two controls: (1) natural sea water collected from a seagrass bed and from a tidal inlet on (2) ebb tide and on (3) flood tide, (4) the water source used for rearing larvae (FAML hatchery water), (5) FAML hatchery water mixed with lignin or (6) tannic acid, and (7) artificial sea water (Instant Ocean®, Spectrum Brands Holdings, Madison, WI). Artificial sea water and FAML hatchery water were used as a negative control and control, respectively. The ebb and flood tide water were collected from the Aransas Pass Ship Channel adjacent to the Marine Science Institute during the last quarter of the incoming or outgoing tide. The seagrass treatment water was collected at the seagrass blade/water interface in a H. wrightii bed in Redfish Bay. Redfish Bay is a well-mixed estuary, with H. wrightii beds located in shallower water adjacent to T. testudinum beds in the bay. Samples were taken over H. wrightii beds because those beds are located closer to shore and were therefore easier from which to collect water. Hatchery water, which was pumped from the Corpus Christi Ship Channel, was held in outdoor ponds for 1–3 weeks to allow particulates to settle out, then filtered through a pressurized sand filter and kept in dark outdoor holding containers for at least 1 week. Before use, this hatchery water was then UV-sterilized. While the chemical composition of the water was not tested for this experiment, other olfaction studies have found that processes similar to these remove biologically active molecules and create water with a consistent chemical composition (Chiussi et al. 2001; Rittschof et al. 1983).
The concentrations (c) of lignin (67 μg l‑1) and tannic acid (148 μg l−1) used in the experiments were calculated from the mean dry weight of a T. testudinum blade (w = 0.092 g blade−1; Mumby et al. 1997), the mean density of T. testudinum in Redfish Bay (d = 1698 blade m−2; Rooker et al. 1998), the amount of tannin and lignin in a seagrass blade (P = 11 and 5 % DW, respectively; Arnold and Targett 2002), the average volume of water over T. testudinum in Redfish Bay (v = 580 l; Rooker et al. 1998), and the leaching rate of dissolved organic carbon (DOC) from seagrass (r = 0.5 % DOC leached day−1; Maie et al. 2006), where
These values do not take into account degradation (photo- or biogenic) but serve as a rough estimate of potential concentrations. The calculated value for lignin was on the same order of magnitude as previous studies on the nearby Nueces River (10.6 μg l−1; Louchouarn et al. 2000). There are no published data on tannin concentrations in sea water for the local area.
The evening before testing, larvae were transferred from the hatchery to the experimental room and kept overnight in individual 600-ml beakers filled with 300-ml of hatchery water or artificial sea water (negative control treatment only). The overnight acclimation was to ensure plasma cortisol concentrations (which increase as a stress response during transfer) returned to basal levels (Robertson et al. 1988). The beakers were placed in a water bath to maintain water temperatures of 27 °C. For the negative control, lignin, and tannic acid treatments, water samples were mixed and stored in glass aquaria kept at 27 °C with underwater heaters. On the morning of the experiment, water was collected from various field sources and taken to the laboratory for same-day use. These water samples were also stored in glass aquaria and maintained at 27 °C with underwater heaters. Two randomly selected treatments were tested each day.
Larvae were tested in a 15 × 10 × 35-cm (length × width × height) glass aquarium filled with one of the five treatments or the negative control or control samples. Tests were conducted in a window-less room with two 60-W incandescent bulbs placed 60 cm above the tank 30 cm apart to allow for the observer to remain unseen during the trial. The larva was given 5 min to acclimate to the testing chamber, after which its behavior was recorded for 1 min with a Casio High Speed EXILIM HS EX-FH25 video camera (Casio Computer Company, Ltd., Tokyo, Japan). Preliminary time course studies on red drum settlement demonstrated that larvae exhibit similar behavior over the course of the first hour of testing. Therefore, the 6-min testing period was chosen to allow for acclimation while capturing settlement behavior. Every fish was sacrificed with an overdose of tricaine methanesulfonate (MS-222) immediately following the trial and photographed under a dissecting microscope to measure SL using ImageJ software (US National Institutes of Health, Bethesda, MD).
Video recordings were converted to image stacks with QuickTime Pro (Apple, Inc., Cupertino, CA), and two-dimensional swimming behavior was tracked with ImageJ software. The original framing rate of the camera was 30 frames s−1, and the fish were tracked using every tenth frame in the stack (three frames s−1). From the tracking data, settlement (mean distance from the bottom of the tank, cm) and activity (mean speed, cm s−1) were calculated.
Taxis Experiment
Two sets of taxis experiments were conducted to test the olfactory preference for potential settlement cues in larval red drum. In one set, the experimental design included two size classes (pre-settlement [5 mm SL] and settlement-size [10 mm SL]) and three treatments (artificial sea water mixed with 67 μg l−1 lignin, sea water collected from a H. wrightii seagrass bed in Redfish Bay, and sea water collected from the Aransas Pass Ship Channel on flood tide). In the other set of experiments, settlement-size larvae were tested in FAML hatchery water mixed with 67 μg l−1 lignin or prey (Artemia sp. nauplii) + artificial sea water. In all trials, artificial sea water was paired with one of the treatments (above) as a negative control. The water collected from the seagrass bed, the Aransas Pass Ship Channel, and the water with the prey were all filtered through a 73-μm filter before experimentation. Water collected from all field sources was used within 24 h of collection, and all of the other treatments were prepared at least 12 h before trials.
The experimental setup consisted of a 20 × 4 × 2.5-cm (length × width × height) Plexiglas Y-maze (modified from Gerlach et al. 2007, for similar diagram see supplemental information in Gerlach et al. 2007; Y-maze used in this study had fine mesh placed at outflow end) fed by a peristaltic pump and silicone tubing from two 2-l beakers: one containing one of the treatments and one with the negative control (artificial sea water). Water flowed at a rate of 90 ml min−1 (linear velocity of 0.167 cm s−1) through the chamber, and dye tests were performed at the beginning of each day to ensure smooth flow. A 30.5 × 30.5-cm mirror was placed above the Y-maze at a 45° angle for the researcher to observe the fish from above. Based on the location where laminar flow broke down and mixing between the two treatments began, three areas of the Y-maze were identified: a “no decision” area (40 cm2) at the downstream end of the maze (where the fish did not actively swim toward one of the treatments) and treatment and control areas (28 cm2 each) at the upstream end on either side of the chamber.
Larvae were transferred from the hatchery to the experimental room on the evening before trials and placed into individual 600-ml beakers filled halfway with artificial sea water (negative control). The beakers were maintained at a constant 27 °C in a water bath. To test the larvae, an individual was placed into the center of the Y-maze and allowed to acclimate for 5 min with both treatments flowing. The section of the maze in which the fish was located (control, treatment, or “no decision”) was then recorded every 10 s for 2 min, after which the treatment and control supply tubes were each moved to the other arm of the maze (i.e., water sources were reversed). This switch controlled for side bias. The fish were then given another 5 min to acclimate, followed by recording of their position every 10 s for an additional 2 min. In total, each trial took 15 min (10 min for acclimation, 4 min for testing, 1 min for switching of tubes) and resulted in 24 observations per individual. Each larva was tested only once and was sacrificed with an overdose of MS-222 after the trial. A photo of each fish was taken under a dissecting microscope, and its size was measured using ImageJ software.
Statistical Analyses
Statistical analyses were performed using the R statistical package (R 3.0.2, The R Foundation for Statistical Computing, http://www.R-project.org). Analysis of covariance (ANCOVA) was used in the kinesis experiment to test the effects of water sample (treatment) on activity (mean swimming speed) and settlement (mean distance from the bottom of the experimental aquarium) with SL as a covariate. Assumptions of the parametric statistical methods were tested by graphical representation for normality of residuals and variance comparisons. Tukey contrasts (using the “multcomp” statistical package) were applied for post-hoc analyses (P < 0.05, Hothorn et al. 2008) and were confirmed by comparing confidence intervals of linear regressions against SL for each treatment.
For each combination of water treatment and size class in the taxis experiment, a Pearson’s chi-square test with sequential Bonferroni correction (Rice 1989) was used to determine whether the time spent by larvae in the “no decision” section of the Y-maze differed from that expected by chance (uniform distribution), based on area. Since the “no decision” area was 41 % of the testing chamber, the null expectation was 10 out of a possible tally of 24. If the chi-square test showed that larvae spent less time in the “no decision” section than expected, a paired t test with sequential Bonferroni correction (Rice 1989) was used to determine whether there was a difference in the time spent in the treatment vs. the control areas.
A two-factor ANOVA, with size class (pre-settlement and settlement-size) and water treatment was used to compare time spent in the treatment area from only those water treatments for which both pre-settlement and settlement-size larvae were tested (lignin + artificial sea water, seagrass water, and shipping channel water). Visual inspection of the residuals and variances were performed to ensure assumptions were met. Tukey post-hoc tests were performed to identify significant differences among treatments (P < 0.05).
Lignin Analysis
The concentration of dissolved lignin in water samples collected from seagrass beds and the tidal inlet was measured for comparison with the lignin treatment used in the experiments. Water samples (10–14 l) were collected from seagrass beds in Redfish Bay on November 6, 2012 (the end of the spawning season, when seagrass beds are in decline), and July 29, 2013 (immediately preceding red drum spawning, when seagrasses have the highest densities and shoot heights, Rooker et al. 1998). In addition, 5 l of water were collected from the Aransas Pass Ship Channel at 1100 hours on May 31, 2014 (peak of high tide), and kept frozen until lignin analysis. Samples were filtered through a 0.7-μm Whatman® glass fiber filter (Sigma-Aldrich, St. Louis, MO) to remove particulates. Solid-phase extraction (SPE) through octadecyl carbon moieties (C18) (Empore, 3M Company, St. Paul, MN) isolated the dissolved organic matter. Cartridges were pretreated with 20 ml of methanol and 10 ml of Milli-Q Plus UV water to activate the discs. Water samples were acidified with 12 N HCl to pH 2 and then pumped through the cartridges using a peristaltic pump with silicone tubing connected to an Erlenmeyer flask with headspace. After extraction, the cartridges were rinsed with 7 ml of methanol three times, and the methanol elution was stored in the freezer until processing.
The eluted samples were evaporated to 2 ml in a Hei-VAP rotary evaporator (Heidolph Instruments, Schwabach, Germany) and then sparged to dryness with N2 at a temperature of 80 °C in 6-ml square Teflon vials (Savillex Corp., Eden Prairie, MN). Lignin oxidation and phenolic compound extraction followed the procedure of Sun et al. (2015). The following were added to the vials containing the dry samples: 0.5 g CuO (Fisher Scientific, Fair Lawn, NJ), 0.1 g Fe(NH4)2(SO4)2.6 H2O (Acros Organics, Fair Lawn, NJ), 10 mg glucose (Sigma-Aldrich, St. Louis, MO), and 5 ml 2 N NaOH (8 %, w/w, sparged with Ar and sonicated for 30 min to remove O2). The mixture was then sparged for 30 min with Ar and quickly capped to minimize mixing with air. The capped Teflon vials were heated for 3 h in a 150 °C oven. Once cooled, the internal standard (ethyl vanillin) was added.
HCl (12 N) was added to the oxidized samples to bring acidity to pH 2. The samples were put in the dark to let particulates settle out, then centrifuged (Model 5810 R, Eppendorf International, Hamburg, Germany) and the supernatant was saved for further analysis. The samples were pushed through C18 cartridges (Analtech Inc., Newark, DE) treated with 3 ml methanol and water at a flow rate of 4–5 ml min−1. The column was dried with N2 for 5 min, and then, 2 ml of ethyl acetate was pushed through the column to elute the lignin oxidation products. The ethyl acetate was evaporated with N2 while in a 45 °C water bath, and the dried solvent was dissolved in 2 ml 10 % (v/v) methanol/water and sonicated for 1 min. The solutions were analyzed by a Shimadzu Prominence HPLC (Shimadzu Scientific Instruments, Columbia, MD) in an Alltima 5 μm, 250 × 4.6 mm C18 column (Alltech Associates, Inc., Deerfield, IL) at room temperature with a 150-μl injection volume. The separation was performed according to methods of Sun et al. (2015).
Results
Environmental Flow Rates
Water velocity inside the seagrass bed ranged from 0.2 to 15.3 cm s−1, with a mean flow of 2.4 ± 0.02 cm s−1. Water velocity over the sandy bottom outside the seagrass bed ranged from 0.03 to 23.0 cm s−1, with a mean flow of 6.0 ± 0.06 cm s−1. These values were used to set the flow rate for the taxis experiment.
Kinesis Experiment
In this experiment, 19–43 individuals (4–10 mm SL) were tested in each of the seven treatments (193 individuals in total). According to the overall ANCOVA for swimming speed, there was a significant size × treatment interaction (P = 0.001, F 6 = 3.79), where swimming speed increased sharply with SL in the lignin treatment compared to a more gradual increase in artificial sea water (P = 0.021, SE = 0.28, t = −3.25). The rate of increase in speed with SL for lignin was also significantly greater than that for flood tide (P = 0.002, SE = 0.34, t = −3.99), ebb tide (P = 0.008, SE = 0.29, t = −3.56), and tannic acid treatments (P = 0.019, SE = 0.30, t = 3.28, Fig. 1). For the latter three treatments, the rate of increase in speed with SL was not significantly different from zero (linear regressions; P > 0.05; adjusted R 2 = −0.032, F 21 = 0.32; adjusted R 2 = 0.022, F 41 = 1.94; adjusted R 2 = −0.015, F 17 = 0.74, respectively). Swimming speed also increased significantly with SL for artificial sea water (P = 0.031, adjusted R 2 = 0.145, F 24 = 5.25), FAML sea water (P = 0.017, adjusted R 2 = 0.162, F 27 = 6.43), and seagrass water (P = 0.014, adjusted R 2 = 0.167, F 28 = 6.81). There were no significant effects of water treatment on distance from the bottom of the tank (P > 0.05, F 6 = 1.78); mean distance from the bottom was 14.4 cm (Fig. 2).
Results from the kinesis experiment. Swimming speed for larva spanning a range of sizes in a flood tide, b ebb tide, c control, d lignin, e tannic acid, and f seagrass water. Black points and regression line represent treatment; gray points and regression line represent artificial sea water (negative control)
Results from the kinesis experiment. Mean distance from the bottom (with SE bars) was not significantly affected by size, treatment, or the interaction between them (ANCOVA, P > 0.05). N = 26, 43, 23, 23, 29, 30, and 19 for control, ebb tide, flood tide, lignin, negative control, seagrass, and tannic acid, respectively
Taxis Experiment
In each taxis treatment, 17–29 individuals were tested, 171 larvae in total. Pre-settlement larvae averaged 4.7 ± 0.5 mm SL, and settlement-size larvae averaged 11.0 ± 1.2 mm SL.
When all size × treatment combinations (the six used in the ANOVA plus settlement-size larvae in prey and lignin + FAML hatchery water) were considered, only settlement-size larvae in the prey (Χ 2 = 79.7, df = 16, P < 0.001), lignin + artificial sea water (Χ 2 = 114.8, df = 17, P < 0.001), and seagrass treatments (Χ 2 = 68.5, df = 19, P < 0.001) spent significantly more time than expected out of the “no decision” area (P < 0.006, sequential Bonferroni correction). Larvae of both size classes from all other treatments spent more time in the “no decision” area than expected. In preference tests that followed positive results, time spent in the seagrass treatment was significantly greater than time spent in the negative control (P = 0.014, t 19 = 2.8) but there was no preference in prey (P = 0.371, t 16 = 0.9) or lignin (P = 0.102, t 17 = 1.7) + artificial sea water trials (Table 1).
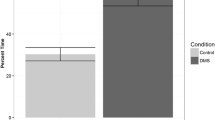
For the trials that included both pre-settlement and settlement-size larvae (lignin + artificial sea water, seagrass water, and channel water), the size × treatment interaction was not significant (P > 0.05, F 2 = 0.8) but both size (P < 0.001, F 1 = 55.5) and treatment (P = 0.026, F 2 = 3.8) had an effect on the amount of time spent in the treatment area of the Y-maze (Fig. 3). Settlement-size larvae spent more time in the treatment area than pre-settlement-size larvae (average difference of 6.7 tallies, P < 0.001), and larvae spent more time in the lignin + artificial sea water treatment than the channel water (average difference of 2.7 tallies, P = 0.038).
Results from the taxis experiment. Mean preference (tallies in treatment area) for treatment (with SE bars) was a greater for lignin than channel water (P = 0.038) and b greater for settlement-size larvae than pre-settlement larvae (P < 0.001, ANOVA with Tukey post-hoc comparison). N = 54, 38, 39, 64, and 67 for channel, lignin, seagrass, pre-settlement, and settlement larvae, respectively
Lignin Analysis
The lignin concentration was 1.0 μg lignin l−1 in the Aransas Pass Ship Channel, 1.1 μg l−1 in Redfish Bay in 2012 during seagrass decline, and 1.3 μg l−1 in Redfish Bay in 2013 during the height of seagrass production. These values were measured assuming 53.1–65.6 % recovery from the HPLC analysis.
Discussion
Time spent in an environment is regularly used as a proxy for preference (Atema et al. 2002; Dixson et al. 2008; Gerlach et al. 2007). Therefore, our results from the taxis experiment indicate that settlement-size red drum larvae prefer the olfactory cues of water from the estuary over those of artificial sea water. Results from both the kinesis and taxis experiments indicate that when red drum larvae are competent to settle, they respond to sea water taken from seagrass beds and water to which lignin was added. Based on these results, larvae should navigate (by either swimming or controlling their water column position to take advantage of currents) away from the oceanic environment and toward the estuaries, which contain both allochthonous (terrigenous plants) and autochthonous (seagrass and marsh plants) sources of lignin (Louchouarn et al. 2000; Mannino and Harvey 2000).
Pre-settlement larvae did not respond to any of the treatments tested, suggesting that they respond to these cues in ways other than the behavioral traits measured in this study, they perceive the cues but do not or cannot respond to them, they are incapable of detecting olfactory and other sensory cues (i.e., treatments not tested), or other modalities (i.e., senses) are more important during this life stage. In the taxis experiment, pre-settlement larvae spent significantly more time in the “no decision” area of the testing chamber than either the negative control or the treatment areas. This almost certainly represents a lack of choice by the larvae, since they easily could have swum against the slow flow in the Y-maze. That flow (0.167 cm s−1) is well below mean water velocities in a seagrass bed or surrounding bare substrate and similar to the lowest values measured in those habitats in this study. The maximum sustainable swimming speed (U crit) of red drum larvae, even as small as 4–5 mm SL, is approximately 5 cm s−1 (Faria et al. 2009).
Larvae are capable of olfaction at early developmental stages, yet most studies on the role of olfaction in settlement were limited to competent larvae (e.g., Dixson 2011; Huijbers et al. 2008; Leis 2010). Both temperate and tropical larvae respond to chemical cues associated with predators or prey shortly after hatching; however, the ontogeny of chemically mediated behavior has not been well studied (Dixson et al. 2010; Døving et al. 1994). Although the olfactory morphology of red drum larvae has not been studied, studies on other species suggest that red drum likely have a functional olfactory sense early in development (Dixson 2011; Lara 2008).
Red drum swimming capabilities improve during ontogeny, with average U crit values increasing from 1 to >22.2 cm s−1 for larvae approximately 2.5–18 mm SL (Faria et al. 2009). Water velocity in the tidal inlet during the spawning season ranges from 5 to 100 cm s−1, with a mean velocity of 35 cm s−1 (Faria et al. 2009). Therefore, young red drum likely rely on selective tidal stream transport (STST), and settlement-size larvae use a combination of STST and active swimming to control their spatial position and navigate toward nursery grounds (Forward et al. 1999; Holt and Holt 2000).
Surprisingly, lignin concentrations did not differ in water samples from seagrass beds collected during the annual height of seagrass, seagrass beds in decline, and the tidal inlet. Lignin levels measured in this study were ten times greater than those in the Gulf of Mexico (90.2 ng l−1), comparable to previous measurements in the tidal inlet (3.2 μg l−1), and approximately one order of magnitude less than the nearby Nueces River (10.6 μg l−1; Louchouarn et al. 2000). While the lignin concentration in the shipping channel matched previous findings, higher concentrations were expected in seagrass bed water (regardless of seagrass species, as they were located adjacent to each other in the bay). This expectation was based on calculations, previous studies on lignin concentrations in estuaries (Louchouarn et al. 2000; Mannino and Harvey 2000), and the larger quantities of vascular plants in estuaries than offshore environments (Arnold and Targett 2002). Instead, our results indicate that the entire nearshore environment, including the shipping channel, contains a similar lignin signal. The concentration used in the experiments was 50 times greater than that in the seagrass beds measured at peak production and 4–6.3 times greater than riverine values (Louchouarn et al. 2000; Mannino and Harvey 2000).
Caution must be used when making comparisons between the concentration of lignin (and tannic acid) used in the experiments and the environmental concentrations. Lignins and tannins are groups of related molecules comprising monomers, dimers, and polymers. Up to half of all phenols in seagrasses can be simple phenolic acids (monomers; Vergeer and Develi 1997). Fish olfactory systems are known to be sensitive to small molecules such as amino acids (reviewed in Derby and Sorensen 2008), so it is likely that the concentration of phenolic monomers is the relevant reference for larval fishes. Our measured values of lignin may be overestimates because the analytical methods we used require lignin polymers to be hydrolyzed into monomers, which would increase lignin concentrations over those found in the environment. Therefore, the concentrations of lignin monomers in the experiment could have been of the same magnitude as the actual concentration of lignin monomers in the environment. The most important conclusion is that larvae responded with both increased activity and preference to lignin, and further studies are necessary to establish the specific lignin monomers to which larvae are responding.
Larvae demonstrated a strong response to lignin but not tannin in the kinesis experiment, despite both substances being produced by nearshore plants. Both are classes of phenolic compounds, with aromatic properties that could act as potential olfactory signals (Arnold and Targett 2002). Lignins and condensed tannins are produced by terrestrial and marine vascular plants, but algae do not produce lignins (although lignins have been discovered in red algae, Calliarthron cheilosporioides [Martone et al. 2009]). Tannins, however, (in the form of phlorotannins) are produced by brown algae (Arnold and Targett 2002). More than a million tons of brown algae (Sargassum spp.) are distributed throughout the Gulf of Mexico, both nearshore and offshore (Gower and King 2009). This could make tannins, like salinity, an unreliable cue for settling fishes to Gulf of Mexico estuaries.
Responses to olfactory cues associated with settlement sites have been shown in other estuarine-dependent species and in those that settle to seagrass beds; however, the studies have been limited to quantifying taxis responses in competent (settlement-size) individuals (Huijbers et al. 2008; James et al. 2008; Radford et al. 2012). In laboratory trials, settlement-stage sparid larvae (R. holubi) spent more time in estuarine and riverine water than sea water controls (James et al. 2008). Radford et al. (2012) discovered that larval snapper (P. auratus) prefer water collected from seagrass beds but not water in which seagrass had been soaked, suggesting that snapper respond to a cue associated with seagrasses other than the seagrass blades (e.g., prey or conspecifics). In the current study, red drum larvae appeared to have responded to estuarine and seagrass cues, but may in fact have responded to contents of the seagrass blades, and lignin in particular.
This is the first study to examine changes in the olfactory-related settlement response in a subtropical and temperate estuarine-dependent fish species. It demonstrates that settlement-size larvae respond to olfactory cues that emanate from their benthic habitat and react to one particular compound within seagrass but not another. Results from this study support recent findings that estuarine vegetation produces olfactory cues that fish larvae use to locate settlement habitats (Huijbers et al. 2008; Radford et al. 2012). Future studies should address concentration gradients of different estuarine compounds, as well as address the role that conspecific olfactory cues might play in settlement. The results from the kinesis and taxis experiments (i.e., behavioral responses to lignin and to water from seagrass beds), combined with the lignin environmental data, indicate that in addition to being important refuge and foraging areas for newly settled individuals, seagrasses and marsh plants may be an important navigational aid for estuarine larvae.
References
Arnold Thomas M., and Nancy M. Targett. 2002. Marine tannins: the importance of a mechanistic framework for predicting ecological roles. Journal of Chemical Ecology 28: 1919–1934.
Arvedlund Michael, and Kathryn Kavanagh. 2009. The senses and environmental cues used by marine larvae of fish and decapod crustaceans to find tropical coastal ecosystems. In Ecological connectivity among tropical coastal ecosystems, ed. I. Nagelkerken. Dordrecht: Springer.
Arvedlund Michael, Mark I. McCormick, Daphne G. Fautin, and Mogens Bildsøe. 1999. Host recognition and possible imprinting in the anemonefish Amphiprion melanopus (Pisces: Pomacentridae). Marine Ecology-Progress Series 188: 207–218.
Atema Jella, Michael J. Kingsford, and Gabriele Gerlach. 2002. Larval reef fish could use odour for detection, retention and orientation to reefs. Marine Ecology-Progress Series 214: 151–160.
Atlantic States Marine Fisheries Commission. 2009. ASMFC stock assessment overview: red drum. http://www.asmfc.org/uploads/file/redDrumStockAssmtSummary1209.pdf (accessed 22 March 2015)
Baird Ronald C., Hamid Johari, and George Y. Jumper. 1996. Numerical simulation of environmental modulation of chemical signal structure and odor dispersal in the open ocean. Chemical Senses 21: 121–134.
Basak Uday C., Anath B. Das, and Premananda Das. 1998. Seasonal changes in organic constituents in leaves of nine mangrove species. Marine and Freshwater Research 49: 369–372.
Bélanger Andrea J., Wes J. Arbuckle, Lynda D. Corkum, Donald B. Gammon, Weiming Li, Alexander P. Scott, and Barbara S. Zielinski. 2004. Behavioural and electrophysiological responses by reproductive female Neogobius melanostomus to odours released by conspecific males. Journal of Fish Biology 65: 933–946.
Benner Ronald, and Stephen Opsahl. 2001. Molecular indicators of the sources and transformations of dissolved organic matter in the Mississippi river plume. Organic Geochemistry 32: 597–611.
Boehlert George W., and Bruce C. Mundy. 1988. Roles of behavioral and physical factors in larval and juvenile fish recruitment to estuarine nursery areas. American Fisheries Society Symposium 3: 51–67.
Bos Arthur R., and Ralf Thiel. 2006. Influence of salinity on the migration of postlarval and juvenile flounder Pleuronectes flesus L. in a gradient experiment. Journal of Fish Biology 68: 1411–1420.
Chiussi Roberto, Humberto Díaz, Dan Rittschof, and Richard B. Forward Jr.. 2001. Orientation of the hermit crab Clibanarius antillensis: effects of visual and chemical cues. Journal of Crustacean Biology 21: 593–605.
Coppock Amy G., Naomi M. Gardiner, and Geoffrey P. Jones. 2013. Olfactory discrimination in juvenile coral reef fishes: response to conspecifics and corals. Journal of Experimental Marine Biology and Ecology 443: 21–26.
Derby Charles D., and Peter W. Sorensen. 2008. Neural processing, perception, and behavioral responses to natural chemical stimuli by fish and crustaceans. Journal of Chemical Ecology 34: 898–914.
Dittman Andrew, and Thomas Quinn. 1996. Homing in Pacific salmon: mechanisms and ecological basis. Journal of Experimental Biology 199: 83–91.
Dixson Danielle L. 2011. Predation risk assessment by larval reef fishes during settlement-site selection. Coral Reefs 31: 255–261.
Dixson Danielle L., Geoffrey P. Jones, Philip L. Munday, Serge Planes, Morgan S. Pratchett, Maya Srinivasan, Craig Syms, and Simon R. Thorrold. 2008. Coral reef fish smell leaves to find island homes. Proceedings of the Royal Society Series B: Biological Sciences 275: 2831–2839.
Dixson Danielle L., Philip L. Munday, and Geoffrey P. Jones. 2010. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecology Letters 13: 68–75.
Dixson Danielle L., Morgan S. Pratchett, and Philip L. Munday. 2012. Reef fishes innately distinguish predators based on olfactory cues associated with recent prey items rather than individual species. Animal Behaviour 84: 45–51.
Døving Kjell B., M. Mårstøl, John R. Andersen, and Jan A. Knutsen. 1994. Experimental evidence of chemokinesis in newly hatched cod larvae (Gadus morhua L.). Marine Biology 120: 351–358.
Døving Kjell B., Ole B. Stabell, Sara Östlund-Nilsson, and Rebecca Fisher. 2006. Site fidelity and homing in tropical coral reef cardinalfish: are they using olfactory cues?. Chemical Senses 31: 265–272.
Faria Ana M., Alfredo F. Ojanguren, Lee A. Fuiman, and Emanuel J. Gonçalves. 2009. Ontogeny of critical swimming speed of wild-caught and laboratory-reared red drum Sciaenops ocellatus larvae. Marine Ecology-Progress Series 384: 221–230.
Filip Zdenek, and James J. Alberts. 1989. Humic substances isolated from Spartina alterniflora (Loisel.) following long-term decomposition in sea water. Science of the Total Environment 83: 273–285.
Forward Richard B. Jr., Kathleen A. Reinsel, David S. Peters, Richard A. Tankersley, James H. Churchill, Larry B. Crowder, William F. Hettler, Stanley M. Warlen, and M.D. Green. 1999. Transport of fish larvae through a tidal inlet. Fisheries Oceanography 8: 153–172.
Gerlach Gabriele, Jella Atema, Michael J. Kingsford, Kerry P. Black, and Vanessa Miller-Sims. 2007. Smelling home can prevent dispersal of reef fish larvae. Proceedings of the National Academy of Sciences of the United States of America 104: 858–863.
Gower James F.R., and Stephanie A. King. 2009. Distribution of floating Sargassum in the Gulf of Mexico and the Atlantic Ocean mapped using MERIS. International Journal of Remote Sensing 32: 1917–1929.
Hara Toshiaki J. 1994. Olfaction and gustation in fish: an overview. Acta Physiologica Scandinavica 152: 207–217.
Hasler Arthur D., and Allan T. Scholz. 1983. Olfactory imprinting and homing in salmon. Investigations in the mechanism of the imprinting process. Berlin Heidelberg: Springer.
Hoese H. Dickson, and Richard H. Moore. 1998. Fishes of the Gulf of Mexico: Texas, Louisiana, and adjacent waters, Second edn. College Station: Texas A&M University Press.
Holt G. Joan, and Scott A. Holt. 2000. Vertical distribution and the role of physical processes in the feeding dynamics of two larval sciaenids Sciaenops ocellatus and Cynoscion nebulosus. Marine Ecology-Progress Series 193: 181–190.
Holt Scott A., Christopher L. Kitting, and Connie R. Arnold. 1983. Distribution of young red drum among different sea-grass meadows. Transactions of the American Fisheries Society 112: 267–271.
Hothorn Torsten, Frank Bretz, and Peter Westfall. 2008. Simultaneous inference in general parametric models. Biometrical Journal 50: 346–363.
Hubbard Peter C., Eduardo N. Barata, and Adelino V.M. Canário. 2003. Olfactory sensitivity of the gilthead seabream (Sparus auratus L) to conspecific body fluids. Journal of Chemical Ecology 29: 2481–2498.
Huijbers Chantal M., Eefke M. Mollee, and Ivan Nagelkerken. 2008. Post-larval French grunts (Haemulon flavolineatum) distinguish between seagrass, mangrove and coral reef water: implications for recognition of potential nursery habitats. Journal of Experimental Marine Biology and Ecology 357: 134–139.
James Nicola C., Paul D. Cowley, Alan K. Whitfield, and Horst Kaiser. 2008. Choice chamber experiments to test the attraction of postflexion Rhabdosargus holubi larvae to water of estuarine and riverine origin. Estuarine, Coastal and Shelf Science 77: 143–149.
Kingsford Michael J., Jeffrey M. Leis, Alan Shanks, Kenyon C. Lindeman, Steven G. Morgan, and Jesús Pineda. 2002. Sensory environments, larval abilities and local self-recruitment. Bulletin of Marine Science 70: 309–340.
Lara Monica R. 2008. Development of the nasal olfactory organs in the larvae, settlement-stages and some adults of 14 species of Caribbean reef fishes (Labridae, Scaridae, Pomacentridae). Marine Biology 154: 51–64.
Lecchini David, Serge Planes, and René Galzin. 2007. The influence of habitat characteristics and conspecifics on attraction and survival of coral reef fish juveniles. Journal of Experimental Marine Biology and Ecology 341: 85–90.
Lecchini David, Jeffrey Shima, Bernard Banaigs, and René Galzin. 2005. Larval sensory abilities and mechanisms of habitat selection of a coral reef fish during settlement. Oecologia 143: 326–334.
Leis Jeffrey M. 2010. Ontogeny of behaviour in larvae of marine demersal fishes. Ichthyological Research 57: 325–342.
Louchouarn Patrick, Stephen Opsahl, and Ronald Benner. 2000. Isolation and quantification of dissolved lignin from natural waters using solid-phase extraction and GC/MS. Analytical Chemistry 72: 2780–2787.
Maie Nagamitsu, Rudolph Jaffé, Toshikazu Miyoshi, and Daniel L. Childers. 2006. Quantitative and qualitative aspects of dissolved organic carbon leached from senescent plants in oligotrophic wetland. Biogeochemistry 78: 285–314.
Mannino Antonio, and H. Rodger Harvey. 2000. Terrigenous dissolved organic matter along an estuarine gradient and its flux to the coastal ocean. Organic Geochemistry 31: 1611–1625.
Martone Patrick T., José M. Estevez, Fachuang Lu, Katia Ruel, Mark W. Denny, Chris Somerville, and John Ralph. 2009. Discovery of lignin in seaweed reveals convergent evolution of cell-wall architecture. Current Biology 19: 169–175.
Miller-Sims Vanessa C., Jella Atema, Gabriele Gerlach, and Michael J. Kingsford. 2011. How stable are the reef odor preferences of settling reef fish larvae?. Marine and Freshwater Behaviour and Physiology 44: 133–141.
Mitamura Hiromichi, Nobuaki Arai, Wataru Sakamoto, Yasushi Mitsunaga, Hideji Tanaka, Yukinori Mukai, Kenji Nakamura, Masato Sasaki, and Yoshihiro Yoneda. 2005. Role of olfaction and vision in homing behaviour of black rockfish Sebastes inermis. Journal of Experimental Marine Biology and Ecology 322: 123–134.
Mitchell Matthew D., Peter F. Cowman, and Mark I. McCormick. 2012. Chemical alarm cues are conserved within the coral reef fish family Pomacentridae. PloS One 7: e47428.
Moore Andrew, and Alexander P. Scott. 1991. Testosterone is a potent odorant in precocious male Atlantic salmon (Salmo salar L.) parr. Philosophical Transactions of the Royal Society Series B: Biological Science 332: 241–244.
Mumby Peter J., Alasdair J. Edwards, Edmund P. Green, Clive W. Anderson, Angie C. Ellis, and Christopher D. Clark. 1997. A visual assessment technique for estimating seagrass standing crop. Aquatic Conservation: Marine and Freshwater Ecosystems 7: 239–251.
Odling-Smee Lucy, and Victoria A. Braithwaite. 2003. The role of learning in fish orientation. Fish and Fisheries 4: 235–246.
Pattillo Mark E., Thomas E. Czapla, David M. Nelson, and Mark E. Monaco. 1997. Distribution and abundance of fishes and invertebrates in Gulf of Mexico estuaries, volume 2: species life history summaries. Silver Spring: NOAA, NOS Strategic Environmental Assessments Division.
Radford Craig A., Carina J. Sim-Smith, and Andrew G. Jeffs. 2012. Can larval snapper, Pagrus auratus, smell their new home?. Marine and Freshwater Research 63: 898–904.
Rice William R. 1989. Analyzing tables of statistical tests. Evolution 43: 223–225.
Rittschof Daniel, Leslie G. Williams, Betsy Brown, and Melbourne R. Carriker. 1983. Chemical attraction of newly hatched oyster drills. Biological Bulletin 164: 493–505.
Robertson Lori, Peter Thomas, and Connie R. Arnold. 1988. Plasma cortisol and secondary stress responses of cultured red drum (Sciaenops ocellatus) to several transportation procedures. Aquaculture 68: 115–130.
Rooker Jay R., Scott A. Holt, Manuel A. Soto, and G. Joan Holt. 1998. Postsettlement patterns of habitat use by sciaenid fishes in subtropical seagrass meadows. Estuaries 21: 318–327.
Stunz Gregory W., Thomas J. Minello, and Phillip S. Levin. 2002. A comparison of early juvenile red drum densities among various habitat types in Galveston Bay, Texas. Estuaries 25: 76–85.
Sun Luni, Robert G.M. Spencer, Peter J. Hernes, Rachael Y. Dyda, and Kenneth Mopper. 2015. A comparison of simplified cupric oxide oxidation HPLC method with the traditional GC-MS method for characterization of lignin phenolics in environmental samples. Limnology and Oceanography: Methods 13: 1–8.
Sweatman Hugh P.A. 1985. The influence of adults of some coral reef fishes on larval recruitment. Ecological Monographs 55: 469–485.
Vergeer Luc H.T., and Akin Develi. 1997. Phenolic acids in healthy and infected leaves of Zostera marina and their growth-limiting properties towards Labyrinthula zosterae. Aquatic Biology 58: 65–72.
Acknowledgments
We thank B. Coughlan for her assistance with the choice experiments, Z. Liu, S. Liu, and L. Sun for their guidance with the chemical analyses, S. Stachelek, K. Perez, and E. Oberg for their help in the field and laboratory, the Marine Science Institute shop crew for constructing the choice chamber, and S. Wallace and W. Tan as well as the anonymous reviewers for their comments. Funds were provided from the University of Texas Marine Science Institute Perry R. Bass Chair in Fisheries and Mariculture and the National Science Foundation Research Experience for Undergraduates (REU) program (OCE-1062745). Animal protocols were approved by the University of Texas at Austin IACUC (protocol AUP-2011-00039). Contribution number 1706 of the University of Texas Marine Science Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Lawrence P. Rozas
Rights and permissions
About this article
Cite this article
Havel, L.N., Fuiman, L.A. Settlement-Size Larval Red Drum (Sciaenops ocellatus) Respond to Estuarine Chemical Cues. Estuaries and Coasts 39, 560–570 (2016). https://doi.org/10.1007/s12237-015-0008-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-015-0008-6